Abstract
We recently described hypoxia as one of the leading mechanism for the recruitment of regulatory T cells (Treg) through CCL28 chemokine in ovarian cancer. Treg promote progression of cancer through tumor-specific immune paralysis but also reprogramming of angiogenesis. We review these mechanisms and discuss the challenges and opportunities for therapy targeting Treg.
Keywords: CCL28, angiogenesis, chemokine, hypoxia, regulatory T cells
It has long been recognized that the tumor microenvironment plays a major role in all phases of tumor genesis and progression. The role of the immune system in the tumor biology is becoming even more apparent. Its ability to either permit or restrain the tumor growth relies largely on a delicate balance between pro-inflammatory and suppressive networks. In ovarian cancer, and several other tumor types, the balance between effector and suppressor/regulatory cells may decide the fate of the tumor as well as the clinical outcome of patients.1-3 T regulatory (Treg) cells are considered pivotal regulators of immune suppression and peripheral tolerance; they are highly enriched in the tumor microenvironment and are well known for their role in tumor progression. Indeed, a low abundance of intratumoral Tregs can translate into years of added survival, highlighting the importance of these cells for tumor progression.3 Mouse models further support the role for Tregs in tumor progression, where depletion of Tregs facilitates tumor rejection and induction of antitumor immunity.4 In addition Tregs have been also demonstrated to play a key role in chronic inflammatory diseases.5 The mechanisms leading to Treg accumulation in tumors are not well understood, although recruitment by specific chemokines has been long postulated.3 We recently further characterized the pathways that drive Tregs into ovarian cancer and demonstrated a key role for CCL28 in Treg recruitment, a chemokine that we found to be directly upregulated by hypoxia.6
Tumor progression is critically linked not only to immune escape but also to the development of neovasculature or angiogenesis, required to supply oxygen and nutrients to rapidly growing tumors.7 In the absence of adequate vasculature, tumor cells undergo hypoxia and starvation, followed by apoptosis and necrosis, with release of stress signals including danger associated molecular pattern (DAMP) molecules, which could mobilize antitumor immunity. However, in the hypoxic tumor microenvironment these events fail to break tolerance to tumor associated antigens. This observation led us to postulate that the hypoxic tumor regions are actively releasing soluble factors that could recruit immunosuppressive elements to the tumor microenvironment, thereby maintaining local tolerance. Through low-density quantitative polymerase chain reaction (qPCR) microarray analysis of primary ovarian cancer cells and cancer cell lines, we discovered that CCL28 is highly upregulated under hypoxia, an effect directly mediated by the hypoxia inducible factor-1α (HIF-1α). Furthermore, we observed that overexpression of CCL28 co-localizes with upregulation of HIF-1α in human ovarian cancers, and it predicts shortened patient survival.
Recognizing that Treg cells may express CCR3 and/or CCR10, the two known receptors for CCL28, we postulated that secretion of CCL28 chemokine by hypoxic tumor cells might be acting to recruit Treg cells to the neoplasm. Using chemotaxis chambers and neutralizing antibodies against CCL28 and its two receptors, we identified the CCL28-CCR10 interaction as the primary mediator of Treg cell recruitment to tumor. To confirm the importance of CCL28 mediated Treg cell recruitment to ovarian tumors in vivo, we used the syngeneic ID8 ovarian cancer model. In this system, we showed that orthotopic (intraperitoneal) ID8 tumors, engineered to overexpress CCL28, accumulated more CD4+CD25+FOXP3+ Treg cells within the tumor and showed more aggressive phenotype than their parent ID8 counterparts. Underscoring the critical role these Treg cells play in tumor progression, mice depleted of CCR10+ Treg cells showed delayed tumor growth. Interestingly, we also noted that CCL28-overexpressing intraperitoneal tumors exhibited a higher density of blood vessels in tumors and resulted in increased levels of VEGF-A in the tumor ascites than the parent ID8 tumors. In mice inoculated with CCL28-overexpressing intraperitoneal tumors, depletion of CCR10+ Treg cells restored VEGF-A levels to those observed in mice bearing parent ID8 tumors. Taken together, these data demonstrate that recruitment of Treg cells to the tumor supports disease progression through a dual mechanism: (1) the canonical subversion of antitumor immunity and (2) through the establishment of a proangiogenic reprogramming of the tumor microenvironment. As such, depletion of Treg cells, and/or prevention of their recruitment to tumors, emerge as critical elements of antitumor therapy.
The novel findings of our work include that (1) hypoxia triggers Treg recruitment and (2) Tregs promote angiogenesis. Tumor hypoxia is known to trigger signals that promote angiogenesis, remodel the vasculature and stroma, and promote metastasis.8 In this particular instance, we found that tumor hypoxia triggers recruitment of immunosuppressive Treg cells through CCL28 upregulation. In turn, the recruited Treg cells dampen the antitumor immune response and directly increase angiogenesis, the latter being teleologically justified given their recruitment by hypoxia. These findings support the notion that tumor immune suppression and angiogenesis are parallel mechanisms operating in synchrony and synergy to render the local microenvironment conducive to tumor growth. Treg can now be added to a long list of immunosuppressive tumor-associated host cell populations which also support angiogenesis pathways, including M2 macrophages, plasmacytoid dendritic cells, myeloid derived suppressor cells, neutrophils, mesenchymal stem cells and cancer associated fibroblasts. Furthermore, additional hypoxia mechanisms synergize to enhance the effect of Tregs, including direct effects on the Treg cells9 as well as through paracrine mediators such as adenosine.10
The universality of the described mechanisms for all tumor types has yet to be established. CCL28 was the main chemokine upregulated by hypoxic ovarian cancer cells in vitro and was found to play an important role in vivo. However, other tumor types, especially those arising in extraperitoneal compartments may use alternate mechanisms. It is conceivable that each tumor type expresses a unique fingerprint of chemotactic and paracrine factors, which ultimately determine the fate and natural history of the cancer. A complete list of factors that could make up that fingerprint, and what their individual significance may be, need more in-depth investigation. The proposed scenario, however, raises some very interesting questions: Can progression be inferred through such signature? Do the chemokine fingerprints of treatable tumors differ from those resistant to therapy? Can we impact these fingerprints and change them to reverse “do not reject” to “reject” signals? Finally, although Treg cells appear to be central in ovarian cancer progression could there other suppressive populations be more important in other tumor types? By moving forward with the goal of understanding the crosstalk between the tumor and the immune system, advancements in cancer therapy will be sure to follow.
Figure 1.
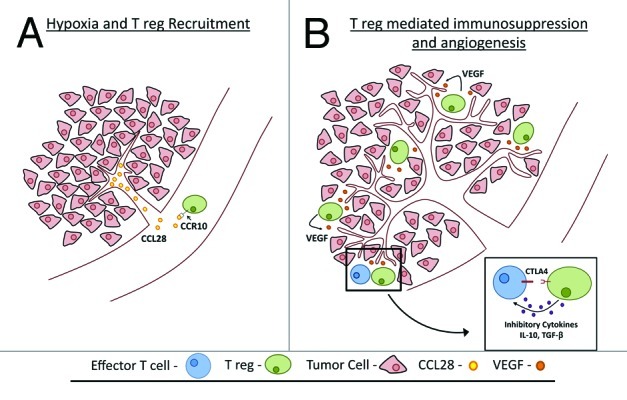
Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells: (A) Hypoxia upregulates CCL28 expression and recruit Treg (B) Once in the tumor Treg shut down the antitumor immune response and foster angiogenesis.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19401
References
- 1.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–91. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 8.Chaudary N, Hill RP. Hypoxia and metastasis. Clin Cancer Res. 2007;13:1947–9. doi: 10.1158/1078-0432.CCR-06-2971. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412–8. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 10.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–52. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]


