Abstract
We recently described that IRF-1 is important for IFNγ mediated immune surveillance in metastasis. Despite the upregulation of MHC Class I in tumor cells, IRF-1 leads to a NK cell-dependent elimination of tumor cells. This mechanism is independent on inhibitory receptors and cytotoxic granules but dependent on DNAM-1.
Keywords: DNAM-1, IFNγ, IRF-1, NK cells, metastasis
The theory of cancer immunoediting suggests three distinct phases of tumor development: elimination, equilibrium and escape.1 During the elimination phase, transformed cells are recognized and eliminated by cells of the innate and adaptive immunity. If the antitumor immunity is unable to completely eliminate transformed cells, tumor variants can survive and enter an equilibrium phase, where cells and molecules of the immune system can prevent tumor outgrowth. Due to possible mutations, new highly immunogenic tumor variants can emerge which escape the immune system and favor progression to detectable malignancies. T-, NKT- and especially NK-cells as well as Type I and Type II IFNs play a central role in the process of cancer immunoediting.2
IFNγ plays a pivotal role in antitumor responses since mice deficient in IFNγ or the IFNγ receptor show increased tumor development. IFNγ promotes direct tumor suppressive effects by inhibiting cellular proliferation, promoting apoptosis and inhibiting angiogenesis.
The transcription factor IRF-1 is critical for IFNγ-mediated effects. IRF-1 is constitutively expressed at low levels in most cell types and its transcription is induced by IFNγ, proinflammatory cytokines, dsRNA and genotoxic stress. IRF-1 exerts a variety of biological processes including inflammation, antiviral response, immune regulation and tumor suppression.3 Ectopic expression of IRF-1 inhibits proliferation and reverses e phenotype of cells transformed by various oncogenes. Deletion in the IRF-1 gene has been implicated in cancers thereby indicating its role as a tumor suppressor gene. Furthermore, the role of IRF-1 in immune responses was demonstrated by the induction of anti-tumoral effects mediated by CD4+ and CD8+ T cells.4 Tumor suppressive functions of IRF-1 are exhibited by the induction of target genes involved in cell cycle control or apoptotic processes. Interestingly, deletion of IRF-1 alone does not promote tumorigenesis but enhances the extent of tumors arising from p53 deletions.
IFNγ has been proposed to control tumor development. In Ksienzyk et al.,5 on the hypothesis of stimulation of IRF-1 upon IFNγ treatment we addressed whether IRF-1 had any role in disease progression and the mechanism of metastasis development. To this end, we administered IFNγ to mice with experimental pulmonary lung metastases. Intranasal application inhibited the development of metastases. We identified IRF-1 as a major mediator of this effect because blockage by short hairpin RNAs abolished the inhibitory effect of IFNγ on tumor metastasis. To address the mechanism of this inhibitory phenotype, we used slight ectopic expression of IRF-1 which phenocopied the inhibitory effect of IFNγ. To define the level at which inhibition takes place we induced IRF-1 expression at different stages of metastasis development and showed that IRF-1 reduces development of metastatic nodules in the lungs rather than influencing the survival of circulating cells or their entry in the lungs.
When we studied the potential mechanisms underlying this phenotype, we found that IRF-1 mediates its influence on the tumor microenvironment. By the production of the chemokine CXCL11 especially CXCR3 positive NK cells infiltrate in the lungs. Studies with mutant mice strains and depleting antibodies revealed that NK cells are responsible for the IRF-1 mediated inhibition of lung metastasis. NK cells are important mediators of antitumor immunity and mice with decreased levels of NK cells have increased susceptibility to metastasis and tumor growth.
NK cells recognize and eliminate cells through a complex set of activating and inhibiting receptors.6 Loss of MHC class I molecules leads to NK cell mediated elimination of tumor cells. However, we showed that IRF-1 expression in tumor cells induce MHC class I molecules on the surface of tumor cells. Thus, IRF-1 tips the balance between activating and inhibitory signals toward activation by other mechanisms. Other studies have indicated NK cell activation by NKG2D ligands and other activating receptors like DNAX-accessory molecule-1 (DNAM-1) in the presence of MHC class I expression.7,8 Recently it was reported that DNAM-1 together with other activating receptors induce NK cell mediated killing.9 By using DNAM-1−/− mice and TRAIL depleting antibodies we showed that IRF-1 attracts NK cells to the metastatic lungs which kill the tumor cells by DNAM-1 dependent mechanisms.
We showed that IRF-1 is a key mediator of IFN-γ mediated immune surveillance, influencing the tumor microenvironment and resulting in the attraction of immune cells which are able to recognize and eliminate tumor cells. IRF-1 is induced by proinflammatory reaction. We have shown that even low expression of IRF-1 is sufficient to inhibit metastasis and prolonged survival. Induction of IRF-1 in the tumor microenvironment by proinflammatory cytokines or small molecules could direct the immune system toward efficacious anti tumor response and could be an attractive target for cancer therapy.
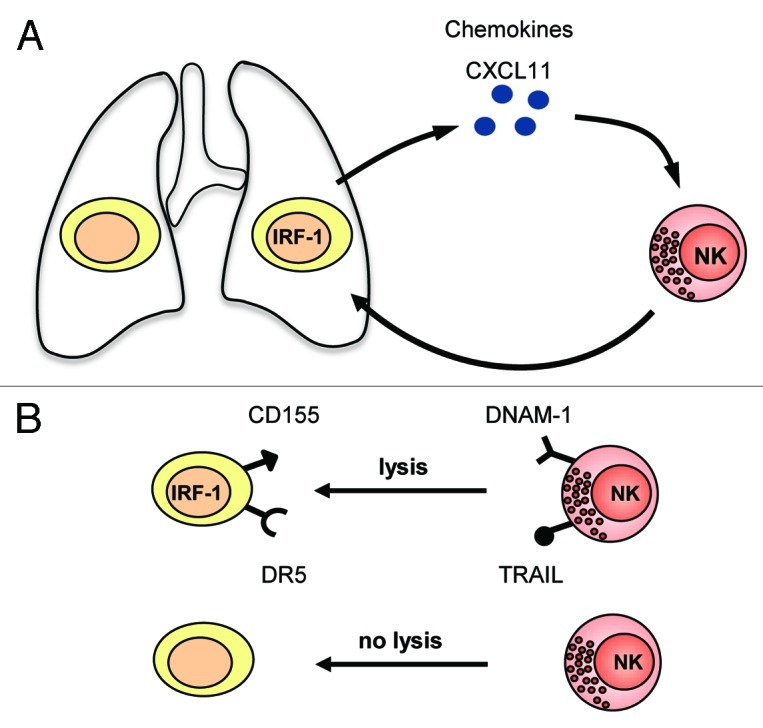
Figure 1. Schematic representation of IRF-1 mediated inhibition of metastasis. (A) Tumor cells expressing IRF-1 influence the tumor mircroenvironment by induction of CXCL11. CXCR3+ NK cells infiltrate to the site of tumor development and eliminate the tumor cells. (B) Expression of IRF-1 influence both, tumor cells and attracted NK cells. Tumor cells upregulate DR5 and CD155 whereas activated NK cells express DNAM-1 and TRAIL which leads to NK cell mediated tumor cell elimination.
Glossary
Abbreviations:
- IRF-1
interferon regulatory factor-1
- IFNγ
interferon γ
- NK
natural killer
- DNAM-1
DNAX-accessory molecule-1
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19405
References
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–55. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 4.Kröger A, Köster M, Schroeder K, Hauser H, Mueller PP. Activities of IRF-1. J Interferon Cytokine Res. 2002;22:5–14. doi: 10.1089/107999002753452610. [DOI] [PubMed] [Google Scholar]
- 5.Ksienzyk A, Neumann B, Nandakumar R, Finsterbusch K, Grashoff M, Zawatzky R, et al. IRF-1 expression is essential for natural killer cells to suppress metastasis. Cancer Res. 2011;71:6410–8. doi: 10.1158/0008-5472.CAN-11-1565. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 7.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–67. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184:902–11. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]


