Abstract
Pancreatic cancer is a highly aggressive and deadly disease harboring a distinct population of cancer stem cells (CSC) that is not affected by conventional therapies. A new therapeutic approach using the EpCAM/CD3-bispecific antibody MT110 is capable of activating and redirecting cytotoxic T cells to eliminate primary human pancreatic cancer stem cells, which resulted in long-term survival of preclinical xenografts models.
Keywords: CD133, EpCAM, bispecific antibody, cancer stem cells, pancreatic cancer, xenograft
Pancreatic ductal adenocarcinoma (PDAC), the most frequent form of pancreatic cancer, is the deadliest solid cancer and currently the fourth most frequent cause of cancer-related deaths.1 PDAC is characterized by late diagnosis due to lack of early symptoms, extensive metastasis, and high resistance to chemotherapy and radiation. Despite expanding research activities in the field of pancreatic tumor and vascular biology, there has been little therapeutic progress regarding clinical endpoints over the past decades. Since the 1990s, the anti-metabolite gemcitabine emerged as the gold standard for treating patients with PDAC but with a 5-y survival rate of 1–4% and a median survival period of 4–6 mo, the prognosis of patients with advanced PDAC remains extremely poor.2
Since the establishment of the cancer stem cell (CSC) hypothesis for leukemia in 1994,3 convincing evidence has also emerged for solid tumors that, like adult tissues, are sustained and promoted by cells that exhibit features of stem cells such as unlimited self-renewal capacity. We and others have recently provided conclusive evidence for a hierarchical organization of human PDAC and, even more importantly, demonstrated that pancreatic CSC at the top of the hierarchy are driving metastasis and are resistant to chemotherapy.4 If sufficient amounts of gemcitabine are delivered to the cancer cells in vivo, which can be achieved by concomitant administration of stroma-targeting agents such as inhibitors of the hedgehog pathway, the bulk of the tumor cells can indeed be successfully erased as evidenced by tumor shrinkage. However, as this initial success is only followed by relapse, a plausible explanation is that surviving CSCs are the source of treatment resistance and should at least in part account for the dismissal prognosis of these patients.
Initial studies from our groups are now providing increasing evidence that direct targeting of pancreas CSCs in combination with elimination of the more differentiated tumor cells bears therapeutic value as this significantly prolonged survival in preclinical xenograft models.5,6 While these studies were based on the inhibition of key regulatory pathways that are crucially relevant for the self-renew capacity of CSC, more recently, we have also asked about the capability of immune-based therapy to target pancreatic CSC. Immune-based therapies could bear the putative advantage of targeting CSC irrespective of potentially very diverse genetic and epigenetic alterations. Immuno-based treatment strategies are a newly emerging therapeutic modality for PDAC, where immune effector mechanism can be induced and directed toward antigens preferentially expressed by tumor cells including CSC.
Several antigens have been identified in the past years to target tumor cells. A recent study by Visus and colleagues took advantage of the ALDH activity as a marker to identify and selectively target the CSC population using several cell lines including pancreatic cancer cells.7 The authors generated in vitro ALDH1A1-specific CD8+ T cells in order to eliminate ALDHbright CSC in preclinical models of human tumor xenografts and observed growth inhibition and reduced metastasis. However, a major concern for this approach represents the fact that ALDH1A1-specific CD8+ T cells will most likely also target normal ALDHbright stem cells, which can for example be found in the hematopoietic system.
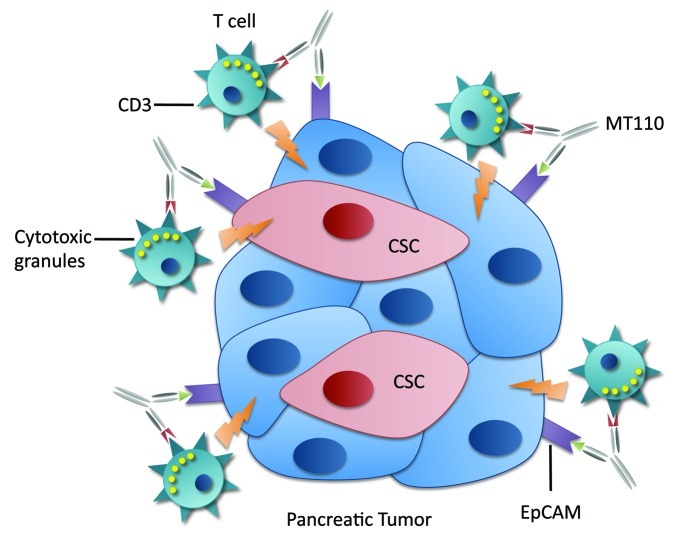
In our recent work,8 we evaluated the therapeutic value of the bispecific antibody MT110 targeting the T-cell receptor CD3 complex and Epithelial cell adhesion molecule (EpCAM; CD326). EpCAM is frequently overexpressed and functionally altered in epithelial cancer cells, including CSC,9 and therefore is becoming accessible on the surface of these cells. In contrast, in normal epithelial cells and embryonic stem cells, EpCAM is sequestered within intercellular boundaries. Therefore, EpCAM represents a promising target for immunotherapy of EpCAM-expressing cancer cells including tumorigenic CSCs.
We first evaluated the effect of MT110 using a dose escalation and time dependent approach in three different primary PDAC cells isolated from human cancer tissue samples. We observed that T cells reach maximal activity for inducing apoptosis of cancer cells at a concentration of 100 ng/ml MT110, as demonstrated by flow cytometry analysis for early T-cell activation marker CD69 and late activation marker CD25. Subsequent flow cytometry analysis for CSC markers identified by expression of CD133 and SSEA1, respectively, showed a significant reduction in the CSC population implying that they are not spared from the cytotoxic activity of activated T cells. Moreover, as a surrogate assay for the self-renewal capacity of CSCs, we examined the sphere formation capacity of the cells following treatment with MT110. As predicted, primary cancer cells exposed to MT110 for 7 d showed a significant decline in sphere formation.
The most defining feature of CSC is their ability to exclusively form tumors in vivo. Indeed, CSC treated for 7 d with MT110 and then implanted into mice had completely lost their in vivo tumorigenicity. Finally and most importantly, we then studied the treatment effects of MT110 in vivo using a model of established primary human PDAC co-implanted with healthy donor-derived PBMC. After administration of MT110 we observed a complete stall in tumor growth over the entire follow-up period indicating that the tumors were depleted for tumorigenic/tumor-promoting CSC. This was also confirmed by flow cytometry analysis of harvested tumors, which revealed a depletion for cells expressing CD133, SSEA-1, and CXCR4 as well as significantly reduced sphere-formation capacity in the MT110 group as compared with tumors harvested from mice treated with control BiTE.
Apparently, the efficacy of this treatment approach is dependent on the level of EpCAM expression. In our model system, we compared 185 cells, which were derived from a primary PDAC, and A6L cells, which were derived from a liver metastasis. Flow cytometry revealed that 185 cells were all EpCAM positive, while A6L cells also harbored an EpCAM-negative subpopulation, which could be further enriched during sphere formation. Spheres derived from EpCAM-negative cells are invasive CSC that probably underwent epithelial-to-mesenchymal transition (EMT).10 The presence of this EpCAM negative subpopulation may provide an explanation for our observation that A6L was less responsive to MT110 treatment.
MT110 is currently tested in a dose-escalating Phase I clinical trial enrolling patients with diverse epithelial cancers (lung, colon, gastric). Low toxicity and early signs of biological activity have been observed at clinically well-tolerated doses of MT110 in this first-in-human clinical trial. Our results derived from preclinical PDAC models now suggest that this treatment regimen could also represent a new opportunity for patients with PDAC. It is important to note, however, that the strong fibroblastic nature of PDAC results in poor tumor vascularization and may therefore require simultaneous targeting of the stroma, e.g., by the addition of hedgehog pathway inhibitors for sufficient delivery of MT110 to the cancer cells including the CSC subpopulation.6
Figure 1. Activation of T cells by MT110 against cancer cells and cancer stem cells.
Glossary
Abbreviations:
- PDAC
pancreatic ductal adenocarcinoma
- CSC
cancer stem cell
- EpCAM
epithelial cell adhesion molecule
- PBMC
peripheral blood-derived mononuclear cells
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19368
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Matano E, Tagliaferri P, Libroia A, Damiano V, Fabbrocini A, De Lorenzo S, et al. Gemcitabine combined with continuous infusion 5-fluorouracil in advanced and symptomatic pancreatic cancer: a clinical benefit-oriented phase II study. Br J Cancer. 2000;82:1772–5. doi: 10.1054/bjoc.1999.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–13. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 6.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–46. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8+ T cells. Clin Cancer Res. 2011;17:6174–84. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-Bispecific T-cell Engaging Antibody MT110 Eliminates Primary Human Pancreatic Cancer Stem Cells. Clin Cancer Res. 2012;18:465–74. doi: 10.1158/1078-0432.CCR-11-1270. [DOI] [PubMed] [Google Scholar]
- 9.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–9. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 10.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]