Abstract
Insights into immune reactions against benign and malignant melanocytes may help the development of more efficient immunotherapeutic treatments for melanoma. The interplay between an active systemic antitumor immunity and a responsive local tumor environment is crucial to achieve effective clinical responses. Increasing evidence confirms this strategy can lead to an adequate and durable immunosurveillance of melanocytes.
Keywords: haptenation, immunosurveillance, melanocyte, melanoma, regression
The immunosurveillance of benign and malignant melanocytes is a very relevant research topic as this may provide new insights in immunotherapeutic strategies for melanoma. Different aspects of the immune response against melanocytes can be investigated by in vivo models that represent a depressed (melanoma), an adequate (halo nevi) and an overactive (vitiligo) immunosurveillance against melanocytes.
Regression is microscopically detected in up to 20–30% of melanoma lesions. Spontaneous or therapy-induced vitiligo-like depigmentation has been observed in 2–7% of melanoma patients and has been linked to a better prognosis.1 Nevertheless, the prognosis of advanced stage melanoma patients remains currently poor. The different immune escape mechanisms used by tumor cells are becoming more and more clear and may reveal new targets for immunotherapy. Numerous tumor escape mechanisms have been described including the development of regulatory T cells, myeloid suppressor cells and the expression of immune-depressing markers such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1-ligand 1 (PD-L1) and indoleamine 2,3-dioxygenase (IDO).2
Despite these immunosuppressing factors, several remarkable examples of spontaneous immune responses have been reported in melanoma patients. We recently reported a case of a patient with a history of melanoma who showed regression of almost all nevi over time.3 This regression was only clinically apparent by follow up pictures as no depigmented halos around the disappearing nevi were observed. By demonstrating lesional and circulating melanocyte-specific cytotoxic T cells, we confirmed the immune-mediated elimination of these nevoid melanocytes, which suggests that this patient has spontaneously developed an efficient immunosurveillance against melanocytes. This raises the question whether an induction of melanocyte-specific T cells by vaccination could be a prevention strategy in patients with very high risk of melanoma e.g., patients who carry cyclin-dependent kinase inhibitor 2A (CDKN2A) mutation or patients with dysplastic nevus syndrome and a family history of melanoma. Regression of nevi after vaccination is however not well documented and one previous study could not find an increased immune infiltrate in nevi of glycoprotein 100 (gp100)—and tyrosinase-peptide vaccinated melanoma patients.4 Halo nevi have been proposed as a sign of immunosurveillance. Nonetheless, this phenomenon is mostly observed in young adolescents, whereas the mean age of melanoma patients ranges around 45–60 years. Despite some occasional cases of vaccinated melanoma patients, clear evidence of regression of nevi in the context of melanoma is lacking.5 However, elimination of normal epidermal melanocytes leading to vitiligo-like depigmentation is a well-known phenomenon in melanoma. Moreover, melanoma associated depigmentation is relatively frequently observed after immunotherapy and has been linked to a beneficial prognosis. Further investigation of this topic seems warranted.
In another study, we showed that a subset of patients with multiple halo nevi can develop limited ectopic depigmented areas (which are not located in regions of regressing nevi). As these lesions are not in accordance with the classic vitiligo criteria, we proposed an entity of halo nevi associated leukoderma, which is a similar phenomenon as the vitiligo-like depigmentations observed in melanoma patients. Moreover, halo nevi associated leukoderma and melanoma associated depigmentation seem to have some common clinical characteristics (mainly discrete depigmentations with a limited progressive behavior) and may be an example of collateral damage resulting from melanocyte immunosurveillance.
Previous studies have demonstrated that the induction of antigen specific T cells alone is insufficient to induce depigmentation. Local increased oxidative stress (e.g., local trauma), causing enhanced levels of H2O2, stimulates tyrosinase activity. As a consequence, increased amounts of surrogate substrates of tyrosinase are converted to ortho-quinones, which are highly immunogenic when bound to tyrosinase (= haptenation) and induce the development of antigen-specific CD8 cells (Fig. 1).6 Multiple regressing nevi and halo nevi/melanoma associated leukoderma may be due to a haptenation process linked to the oxidative stress associated with the halo phenomenon. An example of a local haptenation process leading to elimination of melanocytes at distant sites is observed with chemical depigmenting agents (e.g., monobenzone) in vitiligo. This indicates the development of a systemic melanocyte-destructive inflammatory response and monobenzone has consequently also been proposed to be effective in melanoma.7 Evidence that increased stress is crucial for developing melanocyte destruction can be found in the koebner phenomenon in vitiligo. Physical trauma elicits an immune response and depigmentation at the injured area. We recently developed an in vivo koebner induction model in vitiligo.8 As in our study all induction methods were effective, it seems that a non-specific triggering of the release of oxidative factors is sufficient to induce depigmentation in vitiligo patients. Future research with this model may determine whether the individual likelihood for koebner induced reactions resides in the degree of oxidative stress resulting from trauma. The koebner phenomenon is an example demonstrating that both the systemic immune response as local factors are necessary for melanocyte elimination. Whereas vaccination in melanoma is an attractive method to enhance systemic immune-specific responses, it is dependent on the local tumor environment to be effective. As the tumor develops several strategies to suppress active inflammation, the antitumoral lymphocytes do often not exert their potential activity at the tumor site limiting the efficacy of vaccination. Agents inducing a local, innate immune response can be crucial to overcome this hurdle. A treatment such as imiquimod—a topical immunomodulator which activates Toll-like receptor 7—has been shown to increase inflammation in melanoma lesions and may enhance the efficacy of vaccins by reversing the local tumor suppressive environment.9 Anti-PD-L1 and anti-IDO therapy seem also promising options to overcome tumoral immune escape.
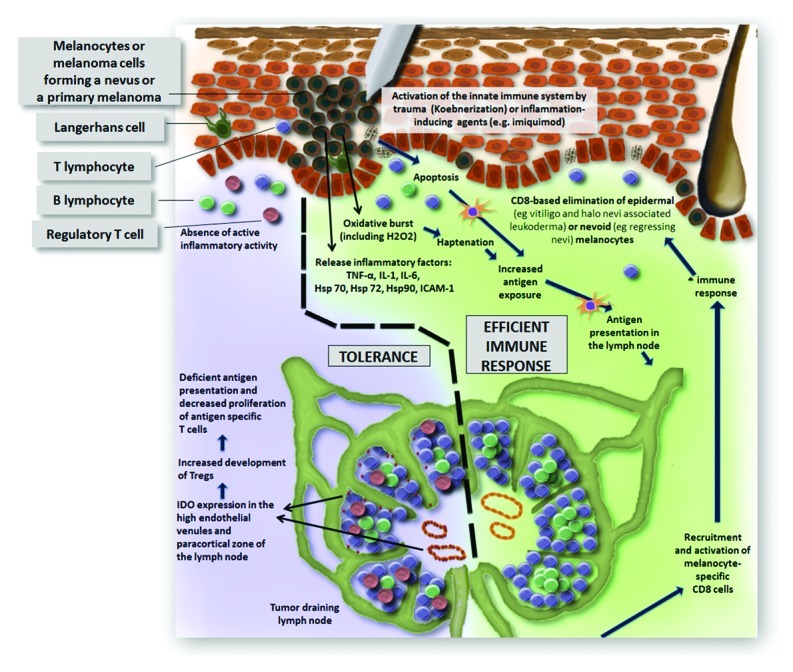
Figure 1. Presentation of an efficient immune response (right) enhanced by oxidative stress leading to haptenation. This leads to increased antigen presentation and ultimately the development of melanocyte-specific CD8 cells occurs which are recruited toward the tumor site. In the model illustrating immune tolerance of the tumor (left), no active tumor elimination is present and immune-suppressing factors at the primary melanoma and in the tumor draining lymph node maintain the tumoral immune escape.
The development of an efficient immunosurveillance of melanocytes may have a preventive effect (as illustrated by our case of a melanoma patient with regressing nevi) and is crucial for an early effective antitumoral immune response. Both halo associated leukoderma and the koebner phenomenon in vitiligo suggest the importance of oxidative stress leading to a process of haptenation. Currently, this may be an underappreciated phenomenon in the immune-based treatment of melanoma. As most research has now focused on developing tumor-antigen specific T cells (e.g., vaccination) and activating lymphocytes (e.g., anti-CTLA-4), more research is needed to efficiently recruit these cells to an inflammation-prone tumoral environment.
Glossary
Abbreviations:
- CDKN2A
cyclin-dependent kinase inhibitor 2A
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- gp100
glycoprotein 100
- IDO
indoleamine 2,3-dioxygenase
- PD-L1
programmed cell death 1-ligand 1
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19403
References
- 1.Quaglino P, Marenco F, Osella-Abate S, Cappello N, Ortoncelli M, Salomone B, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409–14. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 2.Speeckaert R, Vermaelen K, van Geel N, Autier P, Lambert J, Haspeslagh M, et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.09.007. In press. [DOI] [PubMed] [Google Scholar]
- 3.Speeckaert R, van Geel N, Luiten RM, van Gele M, Speeckaert M, Lambert J, et al. Melanocyte-specific immune response in a patient with multiple regressing nevi and a history of melanoma. Anticancer Res. 2011;31:3697–703. [PubMed] [Google Scholar]
- 4.Cassarino DS, Miller WJ, Auerbach A, Yang A, Sherry R, Duray PH. The effects of gp100 and tyrosinase peptide vaccinations on nevi in melanoma patients. J Cutan Pathol. 2006;33:335–42. doi: 10.1111/j.0303-6987.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Dorval T, Chaput N, Andre´ F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerhof W, Manini P, Napolitano A, d’Ischia M. The haptenation theory of vitiligo and melanoma rejection: a close-up. Exp Dermatol. 2011;20:92–6. doi: 10.1111/j.1600-0625.2010.01200.x. [DOI] [PubMed] [Google Scholar]
- 7.van den Boorn JG, Melief CJ, Luiten RM. Monobenzone-induced depigmentation: from enzymatic blockade to autoimmunity. Pigment Cell Melanoma Res. 2011;24:673–9. doi: 10.1111/j.1755-148X.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 8.van Geel N, Speeckaert R, Mollet I, Brochez L, De Schepper S, De Wolf J, et al. New in vivo vitiligo induction and therapy model: double blind, randomised clinical trial of efficacy (proof of concept) Pigm Cell Melanoma Res. 2012;25:57–65. doi: 10.1111/j.1755-148X.2011.00922.x. [DOI] [PubMed] [Google Scholar]
- 9.Narayan R, Nguyen H, Bentow JJ, Moy L, Lee DK, Greger S, et al. Immunomodulation by imiquimod in patients with high-risk primary melanoma. J Invest Dermatol. 2012;132:163–9. doi: 10.1038/jid.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]


