Abstract
Tumor associated macrophages (TAMs) in breast cancers foster several aspects of tumor progression and metastasis, and represent a biomarker associated with an unfavorable clinical outcome. As new therapeutic agents selectively targeting leukocytes enter the clinic whose mechanism of action involves diminishing macrophage infiltration or presence in tumors, it becomes increasingly important to identify those tumors heavily infiltrated by TAMs, as well as monitoring TAM response to therapy. MR imaging with iron oxide nanoparticles enables noninvasive quantification of TAMs in tumors, and thus, provides an easily accessible ex vivo assessment of TAMs for prognosis and related treatment decisions.
Keywords: cellular imaging, contrast agents, magnetic resonance imaging, nanoparticles, tumor associated macrophages
Experimental and clinical studies have revealed that tumor-promoting leukocytes regulate essentially all aspects of solid tumor development by providing a diverse assortment of soluble growth and/or survival factors to neoplastic cells, and contribute to tissue remodeling and angiogenesis via delivery of cytokines, potent mediators and extracellular proteases.1-3 Several studies have recently demonstrated that mammary carcinomas are functionally regulated by leukocytes, most notably both CD4+ T cells and monocytes/macrophages.4-6 Moreover, we have revealed that blocking macrophage infiltration into mammary carcinomas significantly enhances efficacy of standard-of-care chemotherapy and extends overall survival of tumor-prone mice.5 These data, derived from mouse models of mammary carcinogenesis, correlate with human breast cancer (BC) data revealing that leukocyte complexity predicts clinical outcome,7 as well as response to therapy.5 Together, these findings indicate that identifying patients whose BCs are heavily infiltrated with tumor-promoting leukocytes, specifically tumor-associated macrophages (TAMs), would most likely benefit from combining chemotherapy with TAM-antagonist therapeutics. Thus, development of leukocyte-selective imaging reagents will not only facilitate identification of this patient population, but also will enable monitoring patients during therapy.
Clinically Significant Tools for In Vivo Detection of Macrophages in Solid Tumors
Diagnostic imaging of solid tumors has historically focused on imaging malignant cancer cells, proteins overexpressed by malignant cells, tumor microvascular characteristics and angiogenic markers, or the extracellular matrix surrounding tumors, with approaches selectively evaluating the immune component of tumors lagging. While imaging macrophages in vivo in solid tumors has been reported, this has been accomplished with pre-clinical fluorophores, pre-clinical iron oxide nanoparticles for detection by MR imaging, and other macromolecular probes for optical imaging or fluorescence microscopy. A major limitation with these approaches in that they have not utilized clinically-applicable reagents; thus, we sought to develop an immediately clinically-applicable MR imaging technique for detection of TAMs in BC with ultrasmall superparamagnetic iron oxide nanoparticles (USPIO).8 To achieve this, we utilized a clinically-applicable USPIO, and a mouse model of mammary carcinogenesis (MMTV-PyMT) where macrophage infiltration is well documented and functionally significant.9
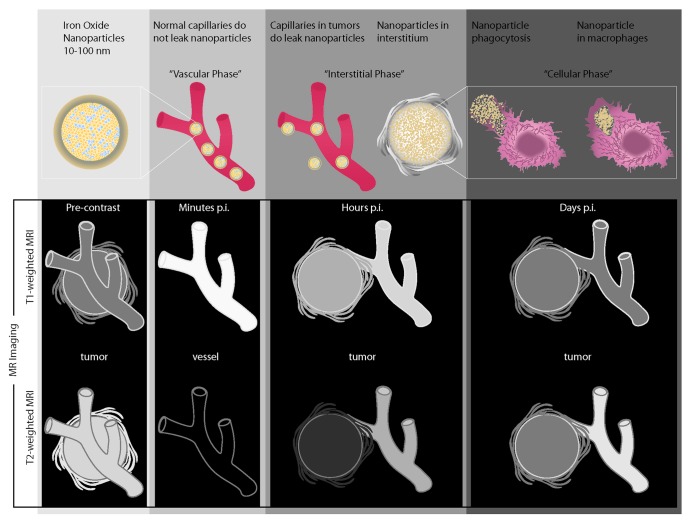
Two classes of clinically applicable iron oxide nanoparticles for MR imaging are currently available for preclinical testing. Superparamagnetic iron oxide nanoparticles (SPIO) have a hydrodynamic diameter of 60–180 nm, whereas ultrasmall SPIO (USPIO) have a diameter of 20–50 nm, both of which have been extensively evaluated in vivo.8-11 Intravenously injected SPIOs are rapidly recognized and phagocytosed by macrophages in the reticuloendothelial system (RES), and are generally too large to traverse the endothelium of tumor microvessels in significant quantities to allow detection with MR imaging, thus smaller USPIOs are superior for in vivo TAM imaging. USPIO transiently escape and delay RES-phagocytosis leading to a prolonged blood half-life of 1–3 h and prolonged tissue perfusion. Clinically-applicable USPIOs, such as Ferumoxytol (Feraheme, AMAG pharmaceuticals) and P904 (Guerbet Group), do not extravasate across intact vascular endothelia in healthy organs. However, in tumors with increased microvascular permeability, USPIOs gradually leak across hyperpermeable vessels into the interstitium, where they exert a combined positive MR signal effect on T1-weighted MR images and a negative MR signal effect on T2-weighted MR images (Fig. 1). The T1-effect is proportional to the quantity of protons in the interstitium. In tissues/tumors heavily infiltrated by TAMs, nanoparticles are slowly phagocytosed by macrophages,11 and nanoparticles retained in TAMs in turn exert a T2-signal effect on delayed MR scans, several hours to days after nanoparticle administration.11 Intracellular iron oxides exert only minimal or no T1 effect due to lack of interactions with protons.12 This “decoupling” of T1- and T2-signal effects can be used as a non-invasive imaging indicator for TAM phagoctosis of the USPIO. Once within cells, nanoparticles undergo a slow metabolization;11 thus, baseline MR signal intensities of tumors and macrophage infiltrates are regained after several days or weeks.11
Figure 1. In vivo distribution of intravenously injected superparamagnetic iron oxide nanoparticles with resultant characteristic enhancement profiles of vessels and tumor tissue on T1- and T2-weighted MR scans at different time points after injection.
Implications for Patient Management
The ability to observe TAMs in BCs non-invasively and longitudinally in vivo with a clinically applicable imaging reagents would aid understanding of temporal and pathophysiological changes of this cell population in the tumor microenvironment. Moreover, USPIOs that detect TAMs can be applied to study the effect of immune-response modifiers on tumoral inflammatory-type processes by direct in vivo MR imaging. Since macrophage density in BC correlates with clinical outcome, this information could direct clinical decision making by stratifying patients to individualized treatment options. For example, patients with BCs with marked macrophage infiltration could be directed to novel macrophage-antagonist or immune reprogramming therapies, while patients with node-negative tumors without significant leukocyte infiltration could be spared. USPIO-mediated TAM imaging could also facilitate development, monitoring and regulatory approval for new classes of immune-based drugs. Since clinical trials of new therapeutic drugs and combination therapies are expensive and take years to complete, the immediate value and impact of this imaging approach could be immense. The described TAM MR imaging technique is in principle readily clinically applicable, could provide a new diagnostic tool to identify patients who will benefit from intensified and anti-inflammatory therapies, help to improve and tailor individualized therapeutic options, and ultimately, improve long-term outcomes. Since a generalized novel concept for tumor imaging via TAM specific biomarkers is addressed, this imaging test might not only be suitable for TAM imaging in BC patients but also patients with a variety of other tumor types.
Acknowledgments
We acknowledge support from the NIH/NCI R01CA140943, NIH/NCI R21CA156124, the Stanford Center for Cancer Nanotechnology Excellence and Translation NCI CCNE-T U54 U54CA151459 and a Stanford Cancer Institute 2011 Developmental Cancer Research Award Grant.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19456
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–26, 229, discussion 230-2. [PubMed] [Google Scholar]
- 3.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNardo DG, Brennan DJ, Rexhepai E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity in breast cancer predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–75. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–11. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daldrup-Link HE, Rydland J, Helbich TH, Bjørnerud A, Turetschek K, Kvistad KA, et al. Quantification of breast tumor microvascular permeability with feruglose-enhanced MR imaging: initial phase II multicenter trial. Radiology. 2003;229:885–92. doi: 10.1148/radiol.2293021045. [DOI] [PubMed] [Google Scholar]
- 9.Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, et al. MRI of tumor-associated macrophages with clinically-applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17:5695–704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daldrup-Link HE, Simon GH, Brasch RC. Imaging of tumor angiogenesis: current approaches and future prospects. Curr Pharm Des. 2006;12:2661–72. doi: 10.2174/138161206777698774. [DOI] [PubMed] [Google Scholar]
- 11.Corot C, Robert P, Ide´e JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471–504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Simon GH, Bauer J, Saborovski O, Fu Y, Corot C, Wendland MF, et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. Eur Radiol. 2006;16:738–45. doi: 10.1007/s00330-005-0031-2. [DOI] [PubMed] [Google Scholar]