Abstract
We report that cryoablation of primary tumors synergizes with anti-CTLA-4 treatment to mediate rejection of secondary tumors in the TRAMP mouse model of prostate cancer. T cells, in particular CD8+ T cells specific for the TRAMP antigen SPAS-1, were enriched in both secondary tumors and spleens of combination-treated mice.
Keywords: CTLA-4, Ipilumimab, SPAS-1, cryoablation, prostate cancer
The recently FDA approved immunotherapy, Ipilimumab (commercial name Yervoy) is a fully human monoclonal antibody that blocks the function of CTLA-4, an inhibitory receptor expressed by T cells. CTLA-4 is expressed and trafficked to the T-cell surface upon activation, and blockade of this checkpoint pathway, through administration of monoclonal antibodies, induces antitumor immunity by promoting T-cell survival and proliferation.1 In clinical trials, Ipilimumab improved overall survival time of metastatic melanoma patients compared with control groups.2,3 Studies in mouse models, using a monoclonal antibody that blocks the function of mouse CTLA-4, showed that treatment with CTLA-4 blockade as a single agent results in the rejection of immunogenic tumors.4 However, rejection of less immunogenic tumors can be achieved only when CTLA-4 blockade is combined with immunotherapies such as those that induce increased antigen uptake and presentation on MHC Class I molecules (a process called “cross-presentation”) by professional antigen-presenting cells (APCs). These types of therapies include GM-CSF secreting whole tumor cell vaccines,5 radiation6 or a FLT-ligand expressing vaccine.7 It is reasonable to hypothesize that in humans, similar combination therapies administered in conjunction with Ipilimumab may boost their effectiveness and increase survival time in a larger fraction of patients. While this hypothesis is currently being tested in clinical trials combining the use of a GM-CSF-expressing tumor cell vaccine (GVAX) with Ipilimumab,8 we wanted to investigate whether this was also true when anti-CTLA-4 was combined with tumor ablation techniques. Local tumor ablation, such as cryoablation, has occasionally been shown to lead regression of tumor metastases, a phenomenon that could likely be attributed to a systemic anti-tumor immune response resulting from increased, albeit in most cases still insufficient, antigen cross-presentation by professional APC. Starting from the assumption that cryoablation would increase cross-presentation, we hypothesized that administration of anti-CTLA-4 during the destruction of a primary tumor, through cryoablation, would further improve the generation a systemic immune response that would result in the eradication of metastatic disease.9 The possibility of combining anti-CTLA-4 therapy with cryotherapy is appealing because of the low morbidity and cost-effectiveness of such an ablative technique compared with surgical interventions.
Using the transplantable TRAMP model of mouse prostate cancer, we developed a unique system to study the induction of systemic immunity and rejection of a secondary tumor challenge in mice treated with anti-CTLA-4 and cryoablation combination therapy. Our previous work in the TRAMP model led to the discovery of the CD8+ T-cell tumor antigen SPAS-1, which contains a mutation in its antigenic epitope.10 SPAS-1 was identified as the target of a T cell line derived from the spleen and lymph nodes of mice that received a tumor-rejecting immunotherapy: anti-CTLA-4 and GM-CSF-expressing TRAMP cell vaccine. By this method, SPAS-1 was defined due to its highly immunogenic character and was found to be the immunodominant TRAMP antigen. This finding has enabled us to track the specific CD8+ T-cell response in the TRAMP model through the use of MHC Class I tetramers and ELISPOT assays. TRAMP C2 tumors were implanted on the left flank of male mice and cryoablated when they reached about 5–8 mm in diameter. The mice were then treated with anti-CTLA-4 and rechallenged with a second inoculation of TRAMP C2 on the opposite flank. Strikingly, in mice that received both anti-CTLA-4 and cryoablation, the number of SPAS-1-specific CD8+ T cells per mg of tumor was significantly increased compared with control mice. Furthermore, the SPAS-1-specific T cells were enriched in the spleens of the mice receiving the combination treatment, indicating the induction of a systemic tumor-specific immune response. Importantly, the combination of anti-CTLA-4 in vivo blockade and cryoablation also resulted in improved survival and in an increased percentage of secondary tumor rejection. Examination of secondary tumors by histology and flow cytometry revealed that, compared with controls, tumors from combination treated mice exhibited a shift from an immunologically hostile microenvironment with few CD4+ and CD8+ T cells and a low effector to T regulatory cell ratio to an immunologically permissive microenvironment where tumors were highly infiltrated by effector T cells and the ratio of effector to T regulatory cell was high. Furthermore, CD8+ T cells were important for tumor rejection as their depletion resulted in a decreased effect of the combination therapy.
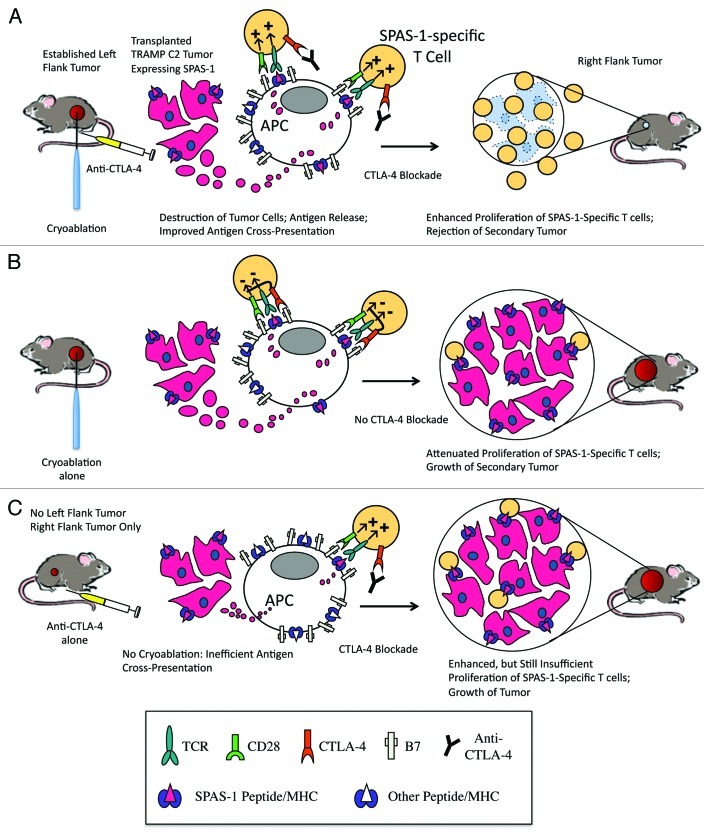
Although the full mechanism of tumor immunity in our model has not been elucidated, it likely involves the exertion of effector function of TRAMP-specific T cells after their activation by activated APCs. Neither cryoablation nor CTLA-4 blockade on their own were sufficient to mediate rejection of secondary tumors (Fig. 1). Therapies such as vaccines, chemotherapies, and radiotherapy that improve antigen cross-presentation may be the most effective treatments to combine with CTLA-4 blockade and we speculate that cryoablation synergizes with CTLA-4 blockade by augmenting antigen cross-presentation. Another possible mechanism is that while a growing intact tumor is likely to have a low level of antigen release in the absence of cryoablation, removal of the primary tumor may result in the removal of a systemic immune suppressive environment. While future studies will delineate the mechanisms by which cryoablation cooperates with CTLA-4 blockade to generate systemic immunity, our current work provides evidence that this combination therapy may improve survival in patients with metastatic disease.
Figure 1. Cryoablation needs to be combined with CTLA-4 blockade to generate systemic immunity of sufficient strength to reject a secondary tumor. (A) A primary TRAMP C2 tumor is inoculated into the left flank of a mouse and allowed to grow until it is large enough to cryoablate. Our model proposes that cryoablation results in the release of antigens, which are taken up by antigen presenting cells (cross-presentation). In the presence of CTLA-4 blockade, SPAS-1 specific T cells maximally proliferate in response to positive signals delivered by the engagement of T cell receptor with SPAS-1 peptide/MHC I complex and CD28 with B7. This enhanced proliferation results in the rejection of a secondary right flank tumor. (B) In the absence of CTLA-4 blockade, CTLA-4 dampens T cell proliferation and a second tumor grows uncontrolled. (C) In absence of cryoablation, diminished antigen cross-presentation results in limited cross-priming of SPAS-1 specific T cells. Even in the presence CTLA-4 blockade, proliferation of SPAS-1 specific T cells is insufficient to mediate rejection of the tumor.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19442
References
- 1.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–13. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 7.Curran MA, Allison JP. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009;69:7747–55. doi: 10.1158/0008-5472.CAN-08-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–15. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waitz R, Solomon SB, Petre EN, Trumble AE, Fassò M, Norton L, et al. Potent Induction of Tumor Immunity by Combining Tumor Cryoablation with Anti-CTLA-4 Therapy. Cancer Res. 2012;72:430–9. doi: 10.1158/0008-5472.CAN-11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fassò M, Waitz R, Hou Y, Rim T, Greenberg NM, Shastri N, et al. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci U S A. 2008;105:3509–14. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]