Abstract
Lymphopenia (< 1Giga/L) detected before initiation of chemotherapy is a predictive factor for death in metastatic solid tumors. Combinatorial T cell repertoire (TCR) diversity was investigated and tested either alone or in combination with lymphopenia as a prognostic factor at diagnosis for overall survival (OS) in metastatic breast cancer (MBC) patients. The combinatorial TCR diversity was measured by semi quantitative multi-N-plex PCR on blood samples before the initiation of the first line chemotherapy in a development (n = 66) and validation (n = 67) MBC patient cohorts. A prognostic score, combining lymphocyte count and TCR diversity was evaluated. Univariate and multivariate analyses of prognostic factors for OS were performed in both cohorts. Lymphopenia and severe restriction of TCR diversity called “divpenia” (diversity ≤ 33%) were independently associated with shorter OS. Lympho-divpenia combining lymphopenia and severe divpenia accurately identified patients with poor OS in both cohorts (7.6 and 10.6 vs 24.5 and 22.9 mo). In multivariate analysis including other prognostic clinical factors, lympho-divpenia was found to be an independent prognostic factor in the pooled cohort (p = 0.005) along with lack of HER2 and hormonal receptors expression (p = 0.011) and anemia (p = 0.009). Lympho-divpenia is a novel prognostic factor that will be used to improve quality of MBC patients’ medical care.
Keywords: divpenia, first line chemotherapy, lymphodivpenia, lymphopenia, metatastatic breast cancer, overall survival
Introduction
Numerous studies have shown an impact of immune system status on cancer patients’ outcomes. In a series of studies gathering more than 3,000 patients, our group demonstrated that lymphopenia is observed in 20–25% of patients with advanced cancers, including 20% of untreated metastatic breast cancer (MBC) patients.1-4 All lymphocyte compartments are affected including CD4+ and CD8+ T lymphocytes, NK cells, and B lymphocytes.3 Importantly, in MBC patients lymphopenia in combination with PS > 1, is associated with a 20% risk of early death at 1 mo and 50% risk at 3 mo.3,5,6 In addition, lymphopenia is also associated with an increased risk of disease progression and worse long-term survival in three prospectively collected series of patients with MBC at first line, along with non-Hodgkin lymphoma, and soft tissue sarcomas.6
Several studies have shown the presence and function of immune cells in solid tumors that may promote both humoral and cellular antitumor immune responses, contributing to tumor control. Indeed, high numbers of CD8+ T cells infiltrating breast tumor predict favorable clinical outcome.7 However, tumors develop escape mechanisms including production of immunosuppressive cytokines, alteration of dendritic cell subsets differentiation or function,8-10 and recruitment of immunosuppressive regulatory T-cells (Treg).11,12
The recent success of immunotherapy based on anti CTLA-4 antibodies inducing increased overall survival (OS)13 in advanced melanoma confirms the relevance of T cell based anti tumor immunity (for review, see ref. 14) and suggests that education and activation of immune system could promote tumor control.
The T-cell receptor (TCR) combinatorial diversity results from mechanisms by which exons encoding TCR variable regions are assembled in developing T lymphocytes from germline variable (V), diversity (D), and joining (J) gene segments.15,16 Genomic V(D)J rearrangements leading to combinatorial diversity is of fundamental importance for the generation of diverse antigen receptor repertoires.17-19 Several studies demonstrate the importance of this diversity in infectious diseases and in anti-tumor responses. Indeed, constriction of the immune TCR repertoire has been associated with progression in AIDS.20 In melanoma, a progressive widening of the repertoire diversity under chemo-immunotherapy treatment was accompanied by high avidity and tumor reactivity of Melan-A-specific T-cell clones.21
The objective of this study was to assess the impact of the quality of the immune system in MBC patients’ outcomes. Here we demonstrate that a composite score called NDL (Numeration and Diversity of Lymphocytes), combining lymphocyte count and TCR diversity, allowed the identification of a subgroup of MBC patients with poor outcome.
Results
Patients’ characteristics
Table 1 describes the patients’ characteristics in cohorts A and B. Both cohorts were similar except for higher proportion of triple negative (TN) MBC patients in cohort B (22% vs. 8%). None of the patients of cohort A was treated with bevacizumab, whereas 39% of patients from cohort B received bevacizumab-based chemotherapy. All patients with overexpressing HER2/Neu tumors were treated with trastuzumab-based chemotherapy. Median follow-up was respectively 18.8 and 14.3 mo in the cohort A and B.
Table 1. Characteristics of the two independent cohorts.
| |
|
Cohort A (n = 66) |
Cohort B (n = 67) |
||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) |
Median (range) |
59.9 |
(36.5–84.1) |
56.9 |
(34.7–79.9) |
| Menopause |
no |
35 |
53.03 |
19 |
28.78 |
| |
yes |
31 |
46.96 |
47 |
71.21 |
| Adjuvant chemotherapy |
no |
25 |
37.87 |
23 |
34.33 |
| |
yes |
41 |
62.12 |
44 |
65.67 |
| Adjuvant hormonotherapy |
no |
37 |
56.06 |
35 |
52.24 |
| |
yes |
29 |
43.93 |
32 |
47.76 |
| Adjuvant Avastin |
no |
- |
- |
36 |
58.06 |
| |
yes |
- |
- |
26 |
41.94 |
| PS |
0/1 |
51 |
72.27 |
42 |
62.68 |
| |
> 1 |
15 |
22.72 |
25 |
37.32 |
| ER |
No |
15 |
23.07 |
21 |
31.34 |
| |
yes |
50 |
76.92 |
46 |
68.66 |
| PgR |
no |
20 |
30.30 |
33 |
49.25 |
| |
yes |
44 |
66.66 |
34 |
50.75 |
| Her2/neu |
no |
46 |
79.31 |
52 |
81.25 |
| |
yes |
12 |
20.68 |
12 |
18.75 |
| Triple Negative (TNBT) |
no |
60 |
92.30 |
52 |
77.61 |
| yes | 5 | 7.69 | 15 | 22.39 | |
Prognostic value of lymphopenia
In univariate analysis (Table 2), PS > 1 (p = 0.03), liver metastasis (p = 0.02) and hemoglobin < 11.5 g/dL (p = 0·003) were associated with poor prognosis in cohort A, while LDH level was the only prognostic factor in cohort B.
Table 2. Univariate analysis: Cox Model on overall survival on the two cohorts.
| |
|
cohort A |
cohort B |
||||||
|---|---|---|---|---|---|---|---|---|---|
| number | HR | CI (95%) | p value | number | HR | CI (95%) | p value | ||
| Age (years) |
< 60 (R) |
39 |
|
|
0.542 |
40 |
|
|
0.759 |
| |
≥ 60 |
27 |
1.207 |
[0.660–2.206] |
|
27 |
0.907 |
[0.486–1.693] |
|
| Menopause |
no |
35 |
|
|
0.139 |
19 |
|
|
0.030 |
| |
yes |
31 |
1.578 |
[0.862–2.886] |
|
47 |
2.257 |
[1.081–4.716] |
|
| Hemoglobin (g/dl) |
≥ 11.5 (R) |
18 |
|
|
0.002 |
20 |
|
|
0.076 |
| |
< 11.5 |
47 |
0.368 |
[0.198–0.685] |
|
46 |
0.566 |
[0.301–1.562] |
|
| Number of metastatic sites |
1/3 (R) |
35 |
|
|
0.015 |
34 |
|
|
0.202 |
| |
≥ 4 |
31 |
2.134 |
[1.157–3.936] |
|
33 |
1.493 |
[0.807–2.760] |
|
| PNN (Giga/L) |
≥ 7.5 (R) |
58 |
|
|
0.470 |
53 |
|
|
0.001 |
| |
< 7.5 |
7 |
1.470 |
[0.517–4.178] |
|
13 |
0.306 |
[0.151–0.621] |
|
| PS |
0/1 (R) |
51 |
|
|
0.025 |
42 |
|
|
0.105 |
| |
> 1 |
15 |
2.105 |
[1.091–4.060] |
|
25 |
1.662 |
[0.899–3.073] |
|
| Liver metastases |
no (R) |
18 |
|
|
0.038 |
38 |
|
|
0.672 |
| |
yes |
48 |
2.266 |
[1.048–4.898] |
|
29 |
1.140 |
[0.621–2.193] |
|
| Bone metastases |
no (R) |
21 |
|
|
0.626 |
22 |
|
|
0.568 |
| |
yes |
45 |
1.182 |
[0.604–2.310] |
|
45 |
0.831 |
[0.441–1.567] |
|
| Triple negative tumors |
no (R) |
60 |
|
|
0.275 |
52 |
|
|
0.060 |
| |
yes |
5 |
1.960 |
[0.585–6.570] |
|
15 |
1.981 |
[0.970–4.043] |
|
| LDH (Ul/ml) |
< 600 (R) |
41 |
|
|
0.125 |
44 |
|
|
0.015 |
| |
≥ 600 |
13 |
1.779 |
[0.850–3.720] |
|
23 |
3.500 |
[1.272–9.629] |
|
| TCR Diversity (%) |
> 33 (R) |
44 |
|
|
0.042 |
43 |
|
|
0.643 |
| |
≤ 33 |
22 |
1.907 |
[1.023–3.555] |
|
16 |
1.183 |
[0.582–2.405] |
|
| Lymphocytes (Giga/L) |
≥ 1 (R) |
38 |
|
|
0.210 |
35 |
|
|
0.000 |
| |
< 1 |
28 |
1.408 |
[0.802–2.685] |
|
30 |
3.156 |
[1.720–6.547] |
|
| NDL |
1 (R) |
53 |
|
|
0.001 |
54 |
|
|
0.006 |
| > 1 | 13 | 3.108 | [1.573–6.140] | 8 | 3.150 | [1.395–7.110] | |||
The incidence of lymphopenia (< 1 Giga/L) was 39% and 45% respectively for cohorts A and B higher than in previous series.1-4 In both cohorts, lymphopenia was associated with OS, with a median OS of 11.2 and 10.4 mo, vs. 25.1 and 24.5 mo for patients with lymphocyte count ≥ 1Giga/L, in cohort A and B respectively (Fig. 1A and B). The difference was statistically significant in cohort B (p = 0.0002) but not in cohort A (p = 0.210).
Figure 1. Overall survival of patients according to their baseline lymphocyte counts (< 1 Giga/L or ≥ 1Giga/L) for patients enrolled in cohort A (A) and cohort B (B). Comparison of TCR diversity in healthy subjects and patients from cohorts A and B (C). Overall survival according to their baseline TCR diversity (≤ 33% or > 33%) for patients enrolled in cohort A (D) and cohort B (E).
Prognostic value of low TCR diversity (divpenia)
Analysis of TCR diversity on 26 healthy subjects showed a highly homogenous diversity (median = 67%; range [59–77]) (Fig. 1C). By contrast, TCR diversity in both MBC patients cohorts demonstrated reduced and highly dispersed diversity (for cohort A: median = 46.4%, range [1.1–83.7]; for cohort B: median = 47.46%, range [6.2–70.3]). Regardless the lymphocyte count, TCR diversity varied substantially between patients.
In cohort A, divpenia (≤ 33%) was correlated with low hemoglobin level (55% of divpenic patients had also anemia, p = 0.022, khi-deux Pearson), lymphopenia (50% of divpenic patients were also lymphopenic, p = 0.026). Conversely, in cohort B, divpenia was found associated with age (50% of divpenic patients were ≥ 60 y old, p = 0.007), number of metastatic sites (p < 10−4) and liver metastasis (44% of divpenic patients had liver metastasis, p = 0.027).
In cohort A, divpenia was associated with poor prognosis (p = 0.038) (Fig. 1D) with a median OS of 9.7 mo for divpenic patients vs. 21.7 mo for non-divpenic patients. The difference was not statistically different in cohort B (Fig. 1E).
Prognostic value of lympho-divpenia
As shown in Figure 2A, within lymphopenic patients, divpenia can identify patients with a reduced median OS (7.6 mo for divpenic patients compared with 24.4 mo for non divpenic patients p = 0.018). However, divpenia had no impact for non-lymphopenic patients (Fig. 2B).
Figure 2. Overall survival according to baseline TCR diversity (≤ 33% or > 33%) in cohort A within lymphopenic patients (A) or non lymphopenic patients (B).
An interaction between these two variables was demonstrated using a Cox model and the variable (lymphopenia × divpenia) has a stronger impact on OS than the two independently [p = 0.001, HR = 3.108, CI95% (1.573–6.014)].
NDL analysis on healthy subjects showed only non-lymphopenic and non-divpenic subjects (Fig. 3A). In contrast, MBC patients from cohorts A (Fig. 3B) and B (Fig. 3C) were distributed in all four NDL compartments: a similar low lymphocyte count, was either associated with a low TCR diversity or a diverse TCR repertoire. Conversely, normal lymphocyte count was associated with low TCR diversity or normal diversity. Interestingly, lympho-divpenic patients (NDL score 1) had poor OS in cohort A [median OS was 7.6 mo compared with 24.4 for the non-lympho-divpenic patients (p = 0.0006)] (Fig. 3D) and cohort B [median OS of 10.6 mo vs 22.9 for the non-lympho-divpenic patients (p = 0.0035)] (Fig. 3E).
Figure 3. NDL representation on healthy subjects (n = 26) (A), cohort A (n = 66) (B) and cohort B (n = 67) (C). Overall survival according to their lympho-divpenic status (NDL score 1) in the cohort A (n = 66) (D) and cohort B (n = 62) (E).
Univariate and multivariate analysis for the pooled cohorts
We pooled patients of cohorts A and B. In univariate analysis, lympho-divpenia appears as the most significant predictive factor of poor prognosis (p < 10−4) (Table S1) together with PS > 1 (p = 0.0054), anemia (p = 0.0005) and lymphopenia (p = 0.0011). Divpenia tended toward significance (p = 0.073) (Table S1). Median OS of patients with lympho-divpenia was 8.1 mo compared with 23.6 mo for non-lympho-divpenic patients (Fig. 4A).
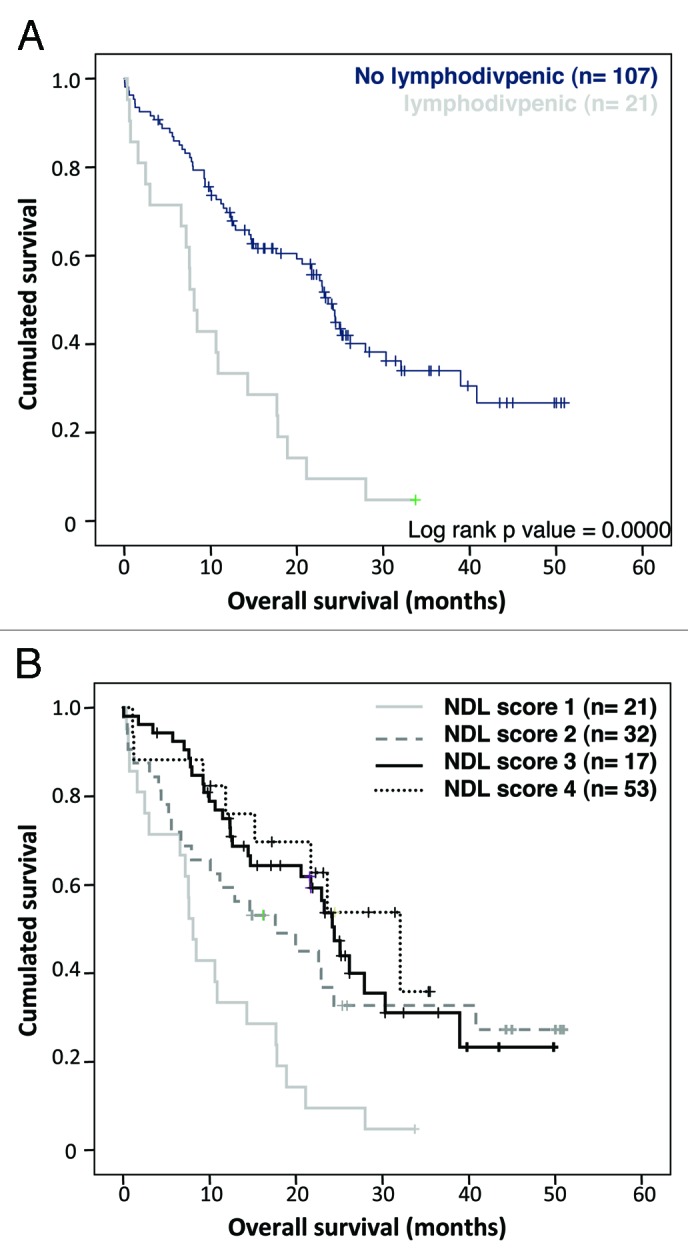
Figure 4. (A) Overall survival according to their lympho-divpenic status (NDL score 1) on the pooled cohort (n = 123) (B) Overall survival according to the different NDL scores i.e: lympho-divpenia (NDL score 1), lymphopenia alone (NDL score 2), divpenia alone (NDL score 3) and neither lymphopenia nor divpenia (NDL score 4).
Interestingly, segregation of patients according to each NDL score separately beside the small size of each part (Fig. 4B) demonstrated that lympho-divpenia (n = 21) as well as lymphopenia only (n = 32) seems to be of poor prognosis in contrast to divpenia alone (n = 17) or non lymphopenic nor divpenic patients (n = 53).
In multivariate analysis, three factors were independently associated with worse prognosis (2-fold increased hazard ratio of death): anemia (HR = 2.08, p = 0.009), triple negative (TN) tumors (HR = 2.56, p = 0.011) and lympho-divpenia (HR = 2.52, p = 0.005) (Table 3).
Table 3. Multivariate analysis in pooled cohort.
| HR | CI 95% | significance (p) | |
|---|---|---|---|
| Hemoglobin (≥ 11.5 vs < 11.5)(g/L) |
0.481 |
[0.277–0.834] |
0.009 |
| Triple negative tumors (Yes vs No) |
2.563 |
[1.239–5.304] |
0.011 |
| NDL (> 1 vs 1) | 2.521 | [1.332–4.771] | 0.005 |
Discussion
In this study, we showed that lympho-divpenia identified a subgroup of patients particularly at risk of early death (≤ 8 mo) that appears refractory to standard chemotherapy.
Immune status is now emerging as an essential biomarker of the biology of the tumor and its microenvironment with a global impact on patient outcome. On top of the well-documented negative impact of lymphopenia on OS for MBC patients and other advanced solid tumors,6,22,23 these results further demonstrate that TCR diversity is frequently reduced in a subset of MBC patients and that divpenia may be assessed in clinic to better define the treatment strategy.
Added value of the NDL to segregate patients
Lymphocyte count and diversity are the two dimensions required for an efficient immune response.6,24 While a minimum number of lymphocytes is required, TCR diversity could reflect the capacity to recognize an antigen.
Lympho-divpenic patients cumulating a low lymphocyte count and a low combinatorial TCR diversity highlighting a profound immunodeficiency present a shorter OS.
Analysis of each NDL score separately suggests that lymphopenia alone is of poorer prognosis than divpenia alone. This observation will be confirmed in a larger cohort of MBC patients under accrual. This will also be evaluated on metastatic lung carcinoma currently enrolled in a prospective clinical trial (LYMPHOS1: NCT01306188) to confirm the prognostic value of lympho-divpenia in diverse clinical situations since we demonstrated that lymphopenia has a negative impact in different solid tumors.6,23
Studying immune system will help to propose personalized treatments that would limit toxic effects and improve life expectancy. Indeed, NDL score will allow identification of lympho-divpenic patients that will represent candidate for the development of new strategies aiming at (1) reconstituting a functional immune system prior to chemotherapy treatment or (2) privileging targeted therapies over chemotherapies.
Different molecules have been developed and evaluated for their ability to favor immune T-cells reconstitution. Whereas, IL-2 was known to favor CD4+ T-cell expansion, two clinical trials (SILCAAT and ESPRIT) performed in HIV individuals failed to demonstrate any clinical benefit, perhaps because of a concomitant expansion of Treg endowed with immunosuppressive functions.25
In contrast, accumulated evidences in clinical trials, indicate the potent lymphopoietic effect of rhIL-7 (CYT107, Cytheris), on both CD4+ and CD8+ T-cells subsets excluding Treg expressing low levels of IL-7Rα receptor.26 Interestingly, in HAART resistant HIV individuals presenting with low CD4+ T cell counts (0,25 Giga/L), a short period of rhIL-7 treatment increased and maintained their CD4+ T cell pool above the > 0,5 Giga/L threshold for one year.27 Such long lasting effects were also observed in lymphopenic cancer patients where T cell counts increased few weeks after the IL-7 cycle and remaind stable.6,24,28
IL-7 therapy could also favor TCR diversity as recently demonstrated in HIV patients.29 The impact of CYT-107 in the restoring lymphocyte counts and TCR repertoire diversity patients is currently under investigation in MBC in our institution in a randomized Phase II trial (ELYPSE 7 clinical trial, NCT01368107).
Origin of lymphopenia and divpenia
It remains unclear whether lymphopenia and divpenia are the cause or the consequence of tumor aggressiveness. Both parameters could result from the tumor burden and/or dissemination status as well as on host characteristics (patient age or genetic polymorphisms).
As the study enrolled only previously untreated patients, lymphopenia or divpenia do not result from previous exposure to cytotoxic agents as described by others.30,31
In the pooled series, lymphopenia correlated with patients’ age analyzed as continuous variable (p = 0.04) and PS status (p = 0.001) and an inverse correlation was observed between TCR diversity and age when tested as continuous variables (p = 0.001). Thus reduced thymic function in elderly32 may contribute to the reduced number and TCR diversity of circulating lymphocytes in tumor bearing patients. In this context, Mackall and colleagues31 demonstrated the rapid CD4+ T cell pool reconstitution in young cancer patients treated with intensive chemotherapy. In contrast, adults exhibit deficiencies in this pathway suggesting that rapid T cell regeneration requires residual thymic function.
The quantification of total tumor burden at first relapse remains yet difficult to assess but could be evaluated by the number of metastatic sites. Whereas in the pooled cohort, no correlation was observed between lymphopenia and number of metastatic spreading, divpenia strongly correlated with the number of metastatic sites involved (≤ 3 vs > 3, p < 10−4) suggesting association between divpenia and tumor aggressiveness and underlining the independence of the two dimensions of both NDL parameters.
The influence of patients’ genetic polymorphisms on lymphocyte counts and diversity in cancer patients remains unknown. Data reported in HIV patients strongly suggest the relevance of genes products involved in T cell proliferation (IL-2, IL-2Rβ, IL-2Rγ, IL-15, IL-15Rα) or apoptotis (TRAIL, Bim, TNFα and IFNγ) in regulating the peripheral T-cell pool size during anti-retroviral therapy. Indeed, number of polymorphisms in these genes, alone or in combination, and several haplotypes were found associated with the magnitude of CD4+ T-cell recovery.33 Analyses of polymorphisms may help to elucidate mechanisms underlying lymphopenia in our study.
Lymphopenia and divpenia may result not only from reduced thymic function but also from the destruction of lymphocytes elicited by breast tumor cells expressing pro-apoptotic ligands,34 a blockade of lymphocyte proliferation via inhibitory molecules (PD-L1) expressed by tumor cells,35 or a reduced capacity of lymphocytes to respond to TCR stimulation due to an alteration of ζ chain expression.36 Soluble factors secreted or induced by tumor cells may also impact systemic lymphopoiesis. Moreover, this lymphopenia could also result from a chronic stimulation of peripheral T cells favored by tumor cells leading to their depletion by activation induced cell death (AICD).37 In this context, in HIV infection, CD4+ lymphopenia resulted from AICD due to chronic activation of the immune system by HIV infection, endotoxinemia or other cues rather than a direct destruction of infected CD4+ T cells (for review see ref. 38).
To a lower extent, the mechanism of AICD may also culminate in the loss of expanded specific T cell clones, favoring the appearance of holes in the TCR diversity. In line with this assumption, 36% of lymphopenic patients presented also an altered TCR diversity (lympho-divpenia).
Materials and Methods
Patients
Sixty-six patients with MBC who received first-line chemotherapy at Léon Bérard Cancer Center between 2004 and 2007 were enrolled in the development cohort (cohort A). A validation cohort (cohort B) of 67 patients with MBC was enrolled in a prospective clinical trial between 2006 and 2010. Patients with a known HIV disease were excluded from these two studies.
Control healthy subjects used for TCR diversity analyses were recruited on the basis of absence of auto-immune or infectious disease and cancer. Pregnant women, subjects recently vaccinated or on steroids over the last 3 mo were also excluded.
Previously validated prognostic factors were collected to characterize both cohorts (age, menopausal status, performance status (PS), hemoglobin, LDH, neutrophils (PNN), hormonal receptor status (ER, PgR), Her2/neu amplification, metastatic sites and number of metastatic sites).
Peripheral blood samples were collected for full blood count evaluation before initiation of chemotherapy and cryo-preserved (day 0).
Written informed consent was obtained from each patient. The institutional ethics committee approved both study protocols before implementation.
Combinatorial diversity analysis (Multiplexe PCR assay)
Human TCR diversity was measured using Human ImmunTraCkeRβ® test (ImmunID Technologies; Grenoble, France) on genomic DNA extracted using standard techniques from total peripheral mononuclear cells. Multi-N-plex PCR was performed using an upstream primer specific of all functional members of a given TRBV family and a downstream primer specific of a given TRBJ segment allowing the simultaneous detection of several V–J rearrangements in the same reaction. Twenty-three different reactions allow covering of all (276) the possible rearrangements.
PCR products were generated using iProof enzyme (Bio-Rad) with cycling condition as followed (98°C for 3 min, 98°C for 20 sec, 72°C for 20 sec and 72°C for 3 min 30 sec and reducing the annealing temperature by one degree every cycle until reached 68°C which was then repeated 23 times. Finally one cycle of 10 min at 72°C was performed). PCR reactions were stopped at the exponential step of the PCR. Normalization was performed on the actin gene amplified in the same PCR run. All V–J products, with a maximum amplicon size of ~5 kb, were separated according to their size on a 0.8% agarose gel, directly stained with SyberGreen and quantified using a CCD camera (Vilbert Lourmart; France). PCR signals were detected and analyzed using the Constel’ID software (ImmunID Technologies).
Statistical analysis
In order to analyze TCR diversity impact on clinical outcome, the study population was divided into tertiles on the basis of the diversity. The threshold for the first tertile corresponding to ≤ 33% combinatorial diversity was called severe “divpenia.”
Furthermore, we built a NDL score assessing the lymphocyte count as a function of the combinatorial diversity. Four NDL scores were defined using < 1 vs. ≥ 1 Giga/L for lymphocyte count and ≤ 33% vs. > 33% for diversity (Fig. 4). Patients with lymphopenia and divpenia were called “lympho-divpenia.”
Survival analysis. OS was defined as the time from patient’s inclusion to the date of death or the date of the last follow-up for alive patients. Survival distributions were estimated by the Kaplan-Meier method.39
Univariate analysis. To assess the relationship between OS and all relevant biological and/or clinical data, reliable prognostic factors were included in univariate Cox proportional hazard regression model. Prognostic factors commonly used in previous studies (e.g., PS, age, hemoglobin, LDH levels and PNN count) were dichotomized using previously published cutoff. Lymphopenia (< 1 Giga/L vs. ≥ 1 Giga/L) and TCR divpenia (≤ 33% vs. > 33%) were also used as dichotomous variables. Candidate prognostic factors with a 0.10 level of significance in univariate analysis were then included in the multivariate analysis.
Multivariate model analysis. Independent prognostic variables of OS were identified by a Cox regression analysis using a backward selection procedure.40 The added value of NDL in the models was evaluated using a likelihood ratio test (LRT); likelihood scores of the model evaluated with and without the biomarker were compared, considering that lower likelihood scores will indicate better fitting models.41
All statistical analyses were performed independently by the Léon Bérard Center biostatistic department using SAS v.9.2 package (Cary) and ImmunID Technologies using R (GNU Public).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was financially supported by CLARA (Lymphos’1), FUI projects (PLATINE, DIVRESCUE) and INCa translational program (Breast Immune). M.M. is a grant holder of the ANRT with financial support of ImmunID. For his last PhD year, he has been financed by an ARC students’ fellowship. The authors would like to thank the institutional Biological Research Center from the Centre Léon Bérard for providing us with patients PBMC.
Glossary
Abbreviations:
- OS
overall survival
- MBC
metastatic breast cancer
- TCR
T-cell receptor
- TN
triple negative
- PS
performance status
- PNN
poly nuclear neutrophils
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19545
References
- 1.Blay JY, Chauvin F, Le Cesne A, Anglaret B, Bouhour D, Lasset C, et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J Clin Oncol. 1996;14:636–43. doi: 10.1200/JCO.1996.14.2.636. [DOI] [PubMed] [Google Scholar]
- 2.Blay JY, Le Cesne A, Mermet C, Maugard C, Ravaud A, Chevreau C, et al. A risk model for thrombocytopenia requiring platelet transfusion after cytotoxic chemotherapy. Blood. 1998;92:405–10. [PubMed] [Google Scholar]
- 3.Borg C, Ray-Coquard I, Philip I, Clapisson G, Bendriss-Vermare N, Menetrier-Caux C, et al. CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer. 2004;101:2675–80. doi: 10.1002/cncr.20688. [DOI] [PubMed] [Google Scholar]
- 4.Ray-Coquard I, Borg C, Bachelot T, Sebban C, Philip I, Clapisson G, et al. ELYPSE study group Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003;88:181–6. doi: 10.1038/sj.bjc.6600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray-Coquard I, Ghesquière H, Bachelot T, Borg C, Biron P, Sebban C, et al. ELYPSE Study Group Identification of patients at risk for early death after conventional chemotherapy in solid tumours and lymphomas. Br J Cancer. 2001;85:816–22. doi: 10.1054/bjoc.2001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–91. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 8.Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–34. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 9.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- 10.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–74. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 11.Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, et al. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res. 2011;71:6143–52. doi: 10.1158/0008-5472.CAN-11-0573. [DOI] [PubMed] [Google Scholar]
- 12.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–9. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–84. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232. doi: 10.1016/S0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 16.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–81. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 17.Marodon G, Desjardins D, Mercey L, Baillou C, Parent P, Manuel M, et al. High diversity of the immune repertoire in humanized NOD.SCID.gamma c-/- mice. Eur J Immunol. 2009;39:2136–45. doi: 10.1002/eji.200939480. [DOI] [PubMed] [Google Scholar]
- 18.Pasqual N, Gallagher M, Aude-Garcia C, Loiodice M, Thuderoz F, Demongeot J, et al. Quantitative and qualitative changes in V-J alpha rearrangements during mouse thymocytes differentiation: implication for a limited T cell receptor alpha chain repertoire. J Exp Med. 2002;196:1163–73. doi: 10.1084/jem.20021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham HP, Manuel M, Petit N, Klatzmann D, Cohen-Kaminsky S, Six A, et al. Half of the T-cell repertoire combinatorial diversity is genetically determined in humans and humanized mice. Eur J Immunol 2011; Epub ahead of print; PMID:22105329; 10.1002/eji.201141798. [DOI] [PubMed]
- 20.Gorochov G, Neumann AU, Kereveur A, Parizot C, Li T, Katlama C, et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–21. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 21.Palermo B, Del Bello D, Sottini A, Serana F, Ghidini C, Gualtieri N, et al. Dacarbazine treatment before peptide vaccination enlarges T-cell repertoire diversity of melan-a-specific, tumor-reactive CTL in melanoma patients. Cancer Res. 2010;70:7084–92. doi: 10.1158/0008-5472.CAN-10-1326. [DOI] [PubMed] [Google Scholar]
- 22.Capellino AR, Desposorio CR, Hurtado de Mendoza F, et al. Lymphopenia and breast cancer subtypes as prognostic factors in patients with locally advanced breast cancer. ASCO Meeting, 29 2011 e21100(Abstract) [Google Scholar]
- 23.Cézé N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, et al. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305–13. doi: 10.1007/s00280-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 24.Luo W, Liao WJ, Huang YT, Shi M, Zhang Y, Wen Q, et al. Normalization of T cell receptor repertoire diversity in patients with advanced colorectal cancer who responded to chemotherapy. Cancer Sci. 2011;102:706–12. doi: 10.1111/j.1349-7006.2011.01868.x. [DOI] [PubMed] [Google Scholar]
- 25.Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A. 2010;107:10632–7. doi: 10.1073/pnas.1000027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelièvre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morre M, Beq S. Interleukin-7 and immune reconstitution in cancer patients: a new paradigm for dramatically increasing overall survival. Targeted Oncology. 2012;7:55–68. doi: 10.1007/s11523-012-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–14. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakim FT, Cepeda R, Kaimei S, Mackall CL, McAtee N, Zujewski J, et al. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood. 1997;90:3789–98. [PubMed] [Google Scholar]
- 31.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–8. [PubMed] [Google Scholar]
- 32.Ferrando-Marti´nez S, Franco JM, Hernandez A, Ordoñez A, Gutierrez E, Abad A, et al. Thymopoiesis in elderly human is associated with systemic inflammatory status. Age (Dordr) 2009;31:87–97. doi: 10.1007/s11357-008-9084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas DW, Geraghty DE, Andersen J, Mar J, Motsinger AA, D’Aquila RT, et al. AIDS Clinical Trials Group Immunogenetics of CD4 lymphocyte count recovery during antiretroviral therapy: An AIDS Clinical Trials Group study. J Infect Dis. 2006;194:1098–107. doi: 10.1086/507313. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Sun T, Xue L, Han X, Zhang B, Lu N, et al. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067–73. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 35.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding W, Lotze M, et al. Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res. 2001;7(Suppl):947s–57s. [PubMed] [Google Scholar]
- 37.Agrawal S, Marquet J, Delfau-Larue MH, Copie-Bergman C, Jouault H, Reyes F, et al. CD3 hyporesponsiveness and in vitro apoptosis are features of T cells from both malignant and nonmalignant secondary lymphoid organs. J Clin Invest. 1998;102:1715–23. doi: 10.1172/JCI3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossman H. AIDS at 25: a quarter century of medical miracles. MedGenMed. 2006;8:57. [PMC free article] [PubMed] [Google Scholar]
- 39.Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147:62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen CH, George SL. The bootstrap and identification of prognostic factors via Cox’s proportional hazards regression model. Stat Med. 1985;4:39–46. doi: 10.1002/sim.4780040107. [DOI] [PubMed] [Google Scholar]
- 41.Dahm PF, Gail MH, Rosenberg PS, Pee D. Determining the value of additional surrogate exposure data for improving the estimate of an odds ratio. Stat Med. 1995;14:2581–98. doi: 10.1002/sim.4780142307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





