Abstract
The impact of CD27 costimulation on human T lymphocyte effector function and memory formation has been limited to evaluations using in vitro cell culture systems and observations from the clinic. We therefore investigated whether CD27 signaling augments antigen redirected human T-cell survival, expansion and function in vitro and in vivo.
Keywords: CD27, T cell, antigen, cancer, chimeric, costimulation, lentivirus, memory, receptor, survival
CD27 (TNFRSF7) is a transmembrane receptor expressed on subsets of human CD8+ and CD4+ T-cells, NKT cells, NK cell subsets and hematopoietic progenitors and induced in FOXP3+ CD4 T-cells and B cell subsets. CD27 commonly serves as a T-cell differentiation antigen but its role in functional T-cell memory formation is still emerging. CD27 is dispensable for immune development in mice; however, T-cell memory responses are impaired in formation, kinetics of response and cell number.1 Normal cell division but low [H3]thymidine incorporation by CD27−/− T cells following CD3 stimulation in vitro has implicated CD27 as an enabler of activated T-cell survival.1
The role of CD27 signaling in human T-cell survival and memory formation is more difficult to assess. CD27 facilitates mitogen or CD3-stimulated human T-cell proliferation in vitro,2 but its role in vivo is unclear. We first observed in a trial of adoptive T-cell therapy that the absolute number of transferred human CD27+ tumor antigen-specific T-cells that persist long-term in patients responding to therapy is remarkably stable.3 Ultimately, the CD27+ subset comprised the entire antigen-specific memory pool as CD27- T-cell numbers contracted, implicating a survival advantage.3 T cell persistence is correlated with clinical responses to adoptive immunotherapy, emphasizing the importance of these observations.4 Clinical response is also associated with the transfer of high numbers of CD27+ T cells.5 Together, these results imply that CD27 identifies a human antigen-experienced T-cell precursor to the emergent memory population. Here, CD27 may either actively provide costimulatory signals that improve human T-cell survival and anti-tumor activity in vivo, or alternatively serve as a functionally dispensable marker of the evolving memory pool.
We recently investigated CD27 costimulatory function in primary human T-cells utilizing chimeric antigen receptor (CAR) technology.6 CARs couple antigen-specific targeting of an extracellular antibody scFv with intracellular T-cell signaling domains for the generation of antigen-redirected T cells for therapy. T cells were engineered to express either second generation CARs, which incorporate a TCR CD3ζ intracellular domain (ICD) in tandem with the CD27 ICD, containing the TRAF-binding domain essential for CD27 function, or a first generation CAR containing the CD3ζ ICD alone. Various costimulatory domains have been tested in CAR constructs including CD28, 41BB, ICOS and OX40. These second generation CARs impart augmented functions to T-cells relative to first generation CARs, making this a suitable model system for evaluating costimulatory function in human T-cells.
CD27 bolstered human CAR T-cell activity in vitro resulting in greater Th-1 cytokine secretion (IFNγ, TNFα and IL-2) and cytotoxicity when co-cultured with antigen-expressing cancer cells in vitro, compared with CAR T-cells with CD3ζ alone. CD27 signaling actively reinforced a Th-1 profile in CD4+ and CD8+ CAR T-cells since levels of secreted Th-2 cytokines, IL-4 and IL-10, were uniformly lower in CAR T-cells with CD27-CD3ζ ICDs, compared with CD3ζ alone. Antigen-stimulated CAR T-cells underwent equal cell division with or without the CD27 ICD, but CD27 costimulation fostered upregulated anti-apoptotic Bcl-XL protein expression and resistance to antigen-induced apoptosis, leading to increased numerical expansion. These effects were antigen-dependent, precluding constitutive CD27 activity. This has an important implication on the interpretation of in vitro functional assay results, particularly that the observed increase in net effector function by CD27-costimulated CAR cells in co-culture may reflect augmented cell survival, and not increased function at cellular level.
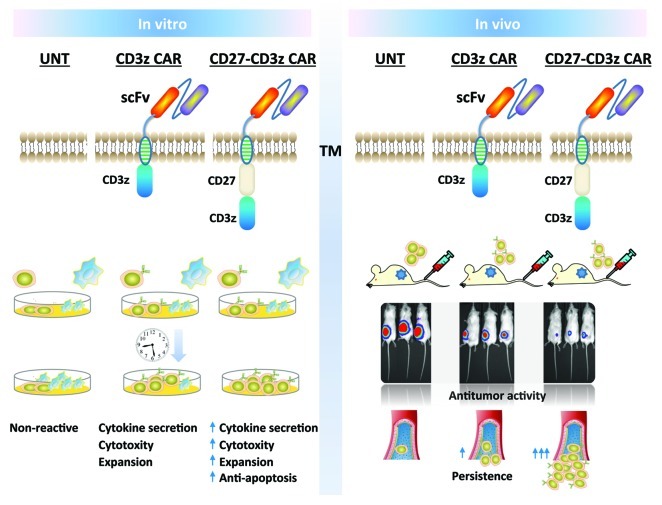
Given the role of CD27 on T-cell survival in mice, the impact of CD27 costimulation on human CAR T-cell survival was evaluated in vivo using a human ovarian cancer xenograft model. CD4+ and CD8+ T cells expressing the CD27-costimulated CAR demonstrated heightened antigen-dependent persistence after infusion. Interestingly, incorporation of a CD28 ICD into CAR T-cells was incapable of achieving such levels of persistence, although incorporation of the ICD from 41BB, another TNF-R family member, did. Persistence under CD28 costimulation was identical to that achieved with CD3z alone, indicating that CD28 does not support human T-cell survival in vivo, consistent with clinical observations.3 Improved T-cell persistence by CD27 containing CARs was associated with improved regression of established cancer. Thus, CD27 costimulation enhances the survival and antitumor activity of human CAR-expressing T cells in vivo. A schematic summary of these results is provided (Fig. 1).
Figure 1. A schematic representation of the impact of CD27 costimulatory function on human T-cell survival, expansion and activity in vitro and in vivo. Primary T cells redirected against a tumor associated antigen via chimeric antigen receptor containing the CD27 intracellular signaling domain mediate improved antitumor activity in vivo upon transfer, which is associated with improved T-cell persistence.
Still, treatment with CAR T cells containing a CD27, CD28 or 41BB ICD yielded similar tumor responses; all superior to a first generation CAR. With discordance in T-cell survival in vivo among these groups, how might this then be explained? In virus-infected mice, CD27 promotes activated CD8+ T-cell survival facilitating increased T-cell accumulation independently of CD28,7 which affects T-cell activity. We hypothesize that increased survival and accumulation of CD27 CAR T cells facilitates a quantitatively increased response, while greater activity but poor survival by CD28 CAR T cells enables similar tumor responses. Formal testing of this hypothesis is under investigation.
Clinical application of CD27 ICD-containing CAR T cells is yet untested. Our data, coupled with prior clinical observations,3,5 suggest that greater T-cell persistence promoted by CD27 costimulation may improve clinical efficacy of adoptive immunotherapy. Mechanisms accounting for CD27-mediated human T-cell survival in vivo may include upregulation of anti-apoptotic molecules, instructed CD4 help8 and autocrine IL-2 production by CD8+ T cells in vivo.9 Identification of CD27 deficiency in individuals with persistent EBV viremia10 suggests that CD27 is dispensable for T-cell development and priming, but required for antigen recall responses, particularly IL-2 production by CD8+ T cells, akin to CD27−/− mouse results.9 With the availability of gene transfer technology, agonistic antibodies and clinical-grade enrichment devices, the impact of CD27 costimulation on T-cell activity and survival is rapidly approaching clinical application.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19458
References
- 1.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–40. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 2.van Lier RA, Borst J, Vroom TM, Klein H, Van Mourik P, Zeijlemaker WP, et al. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987;139:1589–96. [PubMed] [Google Scholar]
- 3.Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–50. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–35. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr CD27 costimulation augments the survival and anti-tumor activity of redirected human T cells in vivo. Blood. 2012;119:696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 7.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–80. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181:1071–82. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 9.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120:168–78. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Montfrans JM, Hoepelman AIM, Otto S, van Gijn M, van de Corput L, de Weger RA, et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol. 2012;129:787–93. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]