Abstract
OBJECTIVE:
Antibiotic use rates have declined dramatically since the 1990s. We aimed to determine if, when, and at what level the decline in antibiotic-dispensing rates ended and which diagnoses contributed to the trends.
METHODS:
Antibiotic dispensings and diagnoses were obtained from 2 health insurers for 3- to <72-month-olds in 16 Massachusetts communities from 2000 to 2009. Population-based antibiotic-dispensing rates per person-year (p-y) were determined according to year (September–August) for 3 age groups. Fit statistics were used to identify the most likely year for a change in trend. Rates for the first and last years were compared according to antibiotic category and associated diagnosis.
RESULTS:
From 2000–2001 to 2008–2009, the antibiotic-dispensing rate for 3- to <24-month-olds decreased 24% (2.3–1.8 antibiotic dispensings per p-y); for 24- to <48-month-olds, it decreased 18% (1.6–1.3 antibiotic dispensings per p-y); and for 48- to <72-month-olds, it decreased 20% (1.4–1.1 antibiotic dispensings per p-y). For 3- to <48-month-olds, rates declined until 2004–2005 and remained stable thereafter; the downward trend for 48- to <72-month-olds ended earlier in 2001–2002. Among 3- to <24-month-olds, first-line penicillin use declined 26%. For otitis media, the dispensing rate decreased 14% and the diagnosis rate declined 9%, whereas the treatment fraction was stable at 63%.
CONCLUSIONS:
The downward trend in antibiotic dispensings to young children in these communities ended by 2004–2005. This trend was driven by a declining otitis media diagnosis rate. Continued monitoring of population-based dispensing rates will support efforts to avoid returning to previous levels of antibiotic overuse.
Keywords: antibiotic use, managed care programs, otitis media
What's Known on This Subject:
Overall antibiotic prescribing rates for children declined throughout the 1990s and early 2000s. These declines were concurrent with changes in practice related to acute otitis media, the most common reason for antibiotic treatment in young children.
What This Study Adds:
The downward trend in antibiotic-dispensing rates to young children in 16 Massachusetts communities ended by 2004–2005 and remained stable thereafter. This trend was driven by a declining otitis media diagnosis rate. Antibiotic treatment of diagnosed otitis media remained constant.
Overall antibiotic-prescribing rates for children in the United States declined throughout the 1990s and early 2000s1,2 as a result of awareness by providers3 and parents4 of the growing problem of antibiotic resistance. Specific campaigns to promote judicious antibiotic use likely made important contributions to this dramatic change in pediatric practice.5–7 A recent National Ambulatory Medical Care Survey (NAMCS) study measured a 10% decline in antibiotic-prescribing rates among <15-year-olds between 1993–1994 and 2007–2008, with rates stable after 1999–2000.8 However, uncertainties remain as to whether the overall decline in antibiotic use in young children has ended and, if it has, whether current use appropriately balances benefits and harms for individuals and populations. Furthermore, use of certain antibiotic classes, particularly broad-spectrum agents, increased in the 1990s,9,10 and little is known about the continuing trends in use of these agents.
Overall decreases in antibiotic use have been concurrent with changes in practice related to acute otitis media (AOM), the most common reason for antibiotic treatment in young children.11 National population-based antibiotic prescription rates for AOM declined throughout the 1990s,1 although prescribing of broad-spectrum antibiotics for AOM increased from 1998 to 2004.12 In 2004, the American Academy of Pediatrics and the American Academy of Family Physicians released new clinical practice guidelines for AOM diagnosis and management.11 The guidelines included renewed attention to diagnostic criteria and allowed for an “observation” option, which entailed deferring antibacterial treatment of selected children for 2 to 3 days. However, adoption of this practice has been limited.13,14 When antibiotic treatment is warranted, amoxicillin is generally recommended as the first-line treatment.15
Trends in overall antibiotic use among Massachusetts children have mirrored nationwide trends, and a substantial decreasing trend was previously documented in this population in 1998–2003.7 The objectives of this study were to: (1) determine if, when, and at what level the decline in annual antibiotic-dispensing rates in young children ended between 2000–2001 and 2008–2009; and (2) determine which antibiotic categories and diagnoses substantively contributed to the observed trends.
Methods
Setting
The study population consisted of children aged 3 to <72 months residing in any of 16 communities in Massachusetts who were members of either of 2 commercial health insurance plans: BlueCross and BlueShield of Massachusetts or Harvard Pilgrim Health Care. We included patients enrolled for at least 90 days during 9 years (September 2000–August 2009). Data from September 2003 to August 2004 were excluded from 1 health plan because of incomplete data availability. This study was approved by the institutional review board of Harvard Pilgrim Health Care, which determined that informed consent was not required.
The 16 geographically distinct communities were part of a cluster-randomized trial from 1998 to 2003 of a community-level intervention to promote judicious antibiotic prescribing for children.7 The selection and population size of these communities have been reported previously.16 All communities from the prior study were included because dispensing rates were not significantly lower in intervention communities than control communities across the current study period.
Data Collection
The total number of days each child was enrolled within each age group (3–<24-month-olds, 24–<48-month-olds, and 48–<72-month-olds) in 1 of the health plans each year was determined by using enrollment files, then divided by 365; the denominator of all population-based rates was aggregate person-years (p-y) within each age group and study year. As children aged during the study period, they were allowed to cross age group boundaries and therefore could contribute to multiple study years and up to 2 age categories in a given study year. Claims data were used to identify oral antibiotic dispensings (primary prescriptions and refills) and diagnoses assigned during clinic and emergency department visits according to International Classification of Diseases, Ninth Revision codes. Each dispensing was linked to the diagnosis assigned during the most recent outpatient visit in the previous 3 days, if one occurred. If no visit occurred in this time frame, the dispensing was classified as “unlinked.”
Oral antibiotics were identified by using a list of National Drug Codes based on the 2008 Healthcare Effectiveness Data and Information Set17 and the American Hospital Formulary Service Drug Information18 via First DataBank.19 These drugs were then classified into 7 categories (Table 1).
TABLE 1.
Categories of Oral Antibiotics Commonly Used in the Pediatric Outpatient Setting
| Antibiotic Category | Generic Name |
|---|---|
| First-line penicillin | Amoxicillin, ampicillin, dicloxacillin, oxacillin, penicillin V potassium |
| Second-line penicillin | Amoxicillin-clavulanate |
| First-generation macrolide | Erythromycin, erythromycin-sulfisoxazole |
| Second-generation macrolide | Azithromycin, clarithromycin |
| Cephalosporin | Cefaclor, cefadroxil, cefdinir, cefditoren, cefixime, cefpodoxime, cefprozil, ceftibuten, cefuroxime, cephalexin, cephradine |
| TMP/SMX | Trimethoprim-sulfamethoxazole |
| Other | Ciprofloxacin, clindamycin, doxycycline, gatifloxacin, gemifloxacin, levofloxacin, linezolid, lomefloxacin, loracarbef, metronidazole, minocycline, moxifloxacin, nitrofurantoin, norfloxacin, ofloxacin, sulfisoxazole, telithromycin, tetracycline, trimethoprim, vancomycin |
International Classification of Diseases, Ninth Revision diagnosis codes were classified into 8 groups: pneumonia (033.0, 033.9, 041.81, 480–486, and 487.0); otitis media ([OM] 381–382, and 384.0–384.2); pharyngitis, including tonsillitis, scarlet fever, and Streptococcus infection (462, 463, 034, and 041.0); sinusitis (461 and 473); bronchitis (466.0 and 490); a composite group of other bacterial diseases, including urinary tract infections, meningitis, and sepsis; viral diseases, including upper respiratory tract infection, common cold, viral pneumonia, and bronchiolitis; and “other,” which included all remaining diagnoses. If >1 diagnosis was recorded for a visit, a primary diagnosis was assigned, giving priority to diagnoses for a potential bacterial source.20
Data Analysis
All analyses were stratified according to age group (3–<24-month-olds, 24–<48-month-olds, and 48–<72-month-olds). Annual population-based antibiotic-dispensing rates (number of antibiotic dispensings divided by number of p-y) with 95% confidence intervals were determined for each age group by year. Years were defined as September 1 to August 31 of the following calendar year, to correspond with the new school year and respiratory illness season. By analyzing annual rates, we controlled for seasonal variability in antibiotic use. To account for clustering of data within communities, generalized estimating equations21,22 were used, assuming a negative binomial distribution for antibiotic dispensings.
The annual overall dispensing rate for each age group during the study period was inspected for a change in the linear trend. We used “hockey stick” regression23 within each age group to fit 2 distinct linear trends over time. Briefly, this method allows detection of a deviation from a simple linear trend, by dividing the overall study period into 2 subperiods with different linear slopes. We identified the most likely year, if any, for a change in trend by using the quasi-likelihood information criterion.24 We then tested the null hypothesis of a single linear trend in the whole period against the alternative of a change in linear trend in this “most likely” year.
Our goal was to understand if the observed trend in overall antibiotic dispensings was the result of changes among frequent users (eg, children who would have previously received ≥5 dispensings per year now receiving only 4), changes among occasional users, or changes among users equally distributed across the frequency distribution. To discriminate among these possibilities, the predicted probability of the average person in each age group receiving 0, 1, 2, 3, 4, or ≥5 dispensings per year was determined for the first and last study years by fitting an ordinal logistic regression model across the 6 levels of dispensing frequency. To account for individuals enrolled for partial years, this model included seasonality adjustments with the following independent variables: age and age-squared at enrollment start, categorical study year, sine and cosine of the enrollment day of the study year (eg, being enrolled on September 1, 2001, would result in sine [1] and cosine [1]), and the interactions of total days by sine and cosine of the enrollment day of the study year.
We also estimated rates stratified according to antibiotic category and associated diagnosis. Rates for the first and last study years were compared by fitting a negative binomial generalized estimating equation model. The models accounted for clustering by community and included a categorical variable for study year and an offset variable of the log of total days enrolled. Finally, to distinguish trends due to changing diagnosis rates from changing disease management, the annual treatment fraction for each diagnosis was calculated as the fraction of total visits that were associated with an antibiotic dispensing. All analyses were conducted by using SAS 9.2 (SAS Institute, Inc, Cary, NC).
Results
The study included a total of 172 044 p-y of observation contributed by 5918 to 9913 children per year from 1 insurance plan and 1947 to 4173 children per year from the other plan. The average contribution of individual children in each of the 3 age groups for each of the 9 study years ranged from 0.55 to 0.64 p-y; <1 p-y was contributed on average as children aged into and out of 1 age category within 1 study year and joined (and left) the insurance plan. The percentage of female study participants across age groups ranged from 48.5% to 49.6%.
Trends in Overall Antibiotic-Dispensing Rates
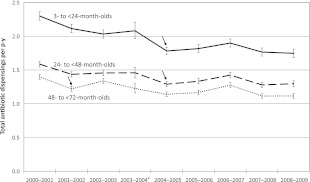
From 2000–2001 to 2008–2009, the overall annual antibiotic-dispensing rate for 3- to <24-month-olds decreased 24% from 2.3 to 1.8 antibiotic dispensings per p-y; for 24- to <48-month-olds decreased 18% from 1.6 to 1.3 antibiotic dispensings per p-y; and for 48- to <72-month-olds decreased 20% from 1.4 to 1.1 antibiotic dispensings per p-y (Fig 1). Analyses identified 2004–2005 as the most likely year for a change in the linear trend of dispensing rates for 3- to <24-month-olds and for 24- to <48-month-olds. For both age groups, the model with 1 slope was rejected in favor of the model with 2 slopes (P < .001); rates declined until 2004–2005 and remained stable thereafter. For 48- to <72-month-olds, 2001–2002 was the most likely year for a change in the linear trend, and the model with 1 slope was again rejected in favor of the model with 2 slopes (P < .001).
FIGURE 1.
Total antibiotic dispensings per p-y for members of 2 large health insurers in 16 Massachusetts communities, 2000–2001 to 2008–2009, according to 3 pediatric age groups. Dispensing rates with 95% confidence intervals are shown. Arrows indicate the most likely year for a change in linear trend for each age group. a The confidence intervals for 2003–2004 are wider than those for other years because data from only 1 insurer were available.
Distribution of Number of Dispensings per Year
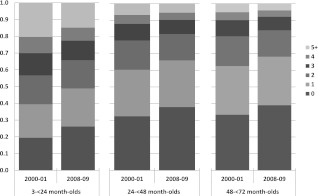
From the first to the last study year for the average 3- to 24-month-old, the predicted probability of receiving 0 antibiotics per year increased from 0.19 to 0.26 and of receiving ≥5 antibiotics decreased from 0.20 to 0.15, with minimal changes in other categories (Fig 2). Similar patterns were seen in the older age groups.
FIGURE 2.
Predicted probabilities for the first (2000–2001) and last (2008–2009) study years of the total number of antibiotic dispensings per p-y for the average person in each age group.
Trends in Dispensing Rates According to Antibiotic Category
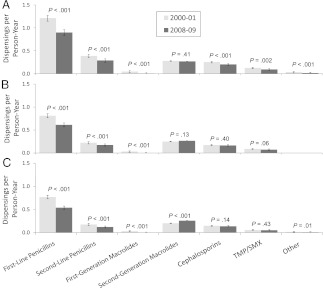
Four antibiotic categories accounted for the great majority of all antibiotic use (Fig 3): first- and second-line penicillins, second-generation macrolides, and cephalosporins. As expected, trimethoprim/sulfamethoxazole, first-generation macrolides, and other agents together accounted for a small minority of antibiotic use.
FIGURE 3.
Values for antibiotic dispensings/p-y for the first (2000–2001) and last (2008–2009) study years for 7 antibiotic categories. Dispensing rates with 95% confidence intervals are shown, along with P values for the difference in rates within each antibiotic category. A, 3- to <24-month-olds; B, 24 to <48-month-olds; C, 48- to <72-month-olds. TMP/SMX, trimethoprim-sulfamethoxazole.
Although first-line penicillins remained the most commonly prescribed antibiotic category, a decline in their use contributed most to the overall decline in antibiotic use (Fig 3). In 3- to <24-month-olds, first-line penicillin use declined 26% from 1.21 antibiotic dispensings per p-y in 2000–2001 to 0.90 antibiotic dispensings per p-y in 2008–2009. Concurrently, second-line penicillin (amoxicillin-clavulanate) use similarly declined 25% from 0.39 antibiotic dispensings per p-y in 2000–2001 to 0.29 antibiotic dispensings per p-y in 2008–2009.
Of all evaluated antibiotic categories and age groups, only dispensings of second-generation macrolides, which are both costly and possibly less effective than other antibiotics for some common infections such as AOM,11,25 significantly increased among 48- to <72-month-olds, from 0.20 to 0.26 antibiotic dispensings per p-y (P < .001).
Trends in Dispensing Rates According to Diagnosis
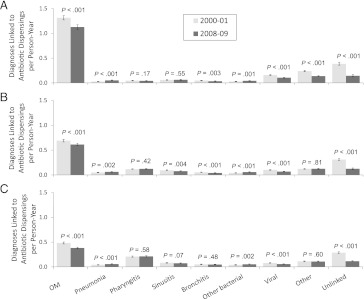
By far, the largest fraction of antibiotic use continues to be for treatment of OM, although the dispensing rate for this condition decreased from 2000–2001 to 2008–2009 in each age group (P < .001) (Fig 4). Among 3- to <24-month-olds, the antibiotic-dispensing rate for OM decreased 14% from 1.31 antibiotic dispensings per p-y in 2000–2001 to 1.13 antibiotic dispensings per p-y in 2008–2009. Similar declines of 12% and 21% were observed for 24- to <48-month-olds and for 48- to <72-month-olds, respectively. In general, a decrease in diagnosis-specific dispensing rates could be attributable to decreases in the diagnosis rate or the treatment fraction of diagnosed cases, but for OM, the treatment fraction remained constant. In 3- to <24-month-olds, for example, the OM diagnosis rate declined 9% from 2.03 visits per p-y in 2000–2001 to 1.84 visits per p-y in 2008–2009, but the treatment fraction only slightly declined over the study period, remaining stable at ∼63% (Supplemental Table 2), suggesting that the decline in antibiotic use was driven by a decline in the OM diagnosis rate, with minimal change in management practices.
FIGURE 4.
Total antibiotic dispensings per p-y for the first and last study years according to diagnosis. Dispensing rates with 95% confidence intervals are shown, along with P values for the difference in rates within each diagnosis category. A, 3- to <24-month-olds; B, 24- to <48-month-olds; C, 48- to <72-month-olds.
In addition to OM, there were decreases in 3- to <24-month-olds from 2000–2001 to 2008–2009 for dispensings linked to viral diseases (0.16–0.11; P < .001), bronchitis (0.05–0.04; P = .003), and other diagnoses (0.24–0.14; P < .001). Notably, there was a substantial decrease in dispensings that were unlinked to a recent visit (0.39–0.15; P < .001). There were small increases for dispensings linked to pneumonia (P < .001) and other bacterial diseases (P = .001). Although the rate of pharyngitis diagnoses per p-y seemed to increase over the study period, the fraction of these visits treated with an antibiotic declined (Supplemental Table 2), so overall antibiotic use for pharyngitis was stable for each age group. Figure 4 displays the contribution of diagnoses of interest to overall antibiotic use.
In this population, antibiotic use for nonbacterial conditions was uncommon. In 2008–2009, although the treatment fraction for diagnosed bronchitis was high (range across age groups: 50%–75%) (Supplemental Table 2), the diagnosis itself was assigned infrequently. The treatment fraction for viral diagnoses was low (range across age groups: 5%–8%), also accounting for a small percentage of antibiotics dispensed.
Discussion
Antibiotic use rates in these particular Massachusetts communities for children aged 3 to <48 months decreased from 2000–2001 to 2004–2005 but then stabilized. The year the decline ended seemed to be somewhat earlier (2001–2002) for children aged 48 to <72 months. Annual dispensing rates for 2004–2005 to 2008–2009 stabilized at ∼1.8 antibiotic dispensings per p-y for 3- to <24-month-olds, 1.3 antibiotic dispensings per p-y for 24- to <48-month-olds, and 1.2 antibiotic dispensings per p-y for 48- to <72-month-olds. The decrease was largely attributable to lower OM diagnosis rates, as well as a substantial decrease in dispensings unlinked to a visit. A recent NAMCS study highlighted the stable treatment fraction for OM,8 and the decline in OM diagnoses is consistent with prior reports.26 However, the decrease in “unlinked” dispensings is a new finding. Although we cannot confirm the clinical circumstances of these dispensings with available claims data, they may include prescriptions written or refilled without an in-person visit, a practice that was, except for prophylaxis, discouraged by efforts to promote more judicious antibiotic prescribing.
Consistent with data from 9 health plans (1996–2000),27 only a small minority of dispensings in this study seemed to be for clearly inappropriate indications, such as diagnosed viral illnesses. The observed treatment fraction for viral diseases (5%–8% in 2008–2009) was lower than that found in other studies,28,29 including an NAMCS analysis that suggested that 23% of <5-year-olds in 2006 with non-OM acute respiratory tract infection for which antibiotics are rarely indicated received an antibiotic prescription.28 The observed decline in the pharyngitis treatment fraction may reflect greater adherence to recommended testing and treatment practices, now widely reported as quality measures.30 The diagnosis of cough illness as “bronchitis” (as a presumed bacterial infection) is generally believed to be unwarranted in young children.31 Although this diagnosis was rarely assigned in these communities, a substantial fraction of patients thus labeled received antibiotic treatment. This highlights the danger of using diagnostic labels that erroneously signify to parents that antibiotics are necessary.
Despite concern about increasing use of second-generation macrolides,9,32,33 this study showed only a small increase in dispensings of these antibiotics only for 48- to <72-month-olds (Fig 3), to 0.26 per p-y in 2008–2009.
Trends in the use of specific vaccines may have influenced trends in OM diagnosis and overall antibiotics dispensed. During the study period, the Advisory Committee on Immunization Practices expanded the guidelines for influenza vaccination to all children ≥6 months old.34–36 Inactivated and live-attenuated influenza vaccines have been reported to decrease AOM by as much as 30% in some settings.37,38 The percentage of 6- to 23-month-olds in Massachusetts vaccinated for influenza rose from ∼6% in 2002–200339 to ∼46% in 2008–2009.40 We doubt that annual variation in influenza-related illness rates had a substantive impact on the described antibiotic usage trends. According to sentinel sites in Massachusetts participating in the US Outpatient Influenza-Like Illness Surveillance Network, the peak weekly percentage of visits to sentinel providers for influenza-like illness averaged 3.0% during the study period, ranging from 1.7% in 2006–2007 to 5.3% in 2003–2004, and was ∼4.0% for each of the last 2 study years.
Opinions differ on the extent to which heptavalent pneumococcal conjugate vaccine (PCV7) decreased antibiotic use.28,41 PCV7 was introduced in Massachusetts in July 2000, after the downward trend in antibiotic use had already begun,8,27,28 to <2-year-olds universally and to 2- to 5-year-olds at increased risk.42 Coverage of children 19 to 35 months old with ≥3 doses of PCV7 in Massachusetts rose to ∼95% in 2009.43 Initial trials showed PCV7 had only ∼6% efficacy in preventing AOM,44 but subsequent modeling approaches that included the effect of serotype replacement suggested that PCV7 might decrease AOM incidence by up to 12%.45 Although our study provides no direct data on this point, we believe that immunization may have had indirect effects as well; that is, as immunization decreased the risk of serious bacterial infection, physicians became more comfortable not treating febrile infants and young children, allowing physicians to essentially raise their threshold for diagnosing AOM. We suspect that the confluence of PCV7 and increased influenza immunization, and the release of professional guidelines allowing observation in 2004,11 together facilitated a changing culture of practice around AOM.
Reporting rates of antibiotic use from precisely defined populations, rather than by analysis of visit-level data, allows direct analysis of trends in the frequency of office visits as well as treatment practices. Our pharmacy-dispensing rates in 3- to <72-month-olds are not directly comparable with rates recently reported for <15-year-olds by using NAMCS data8; measuring medication-dispensing events rather than prescriptions may better reflect actual use by patients. However, claims data are limited by the lack of: (1) some demographic information, such as race/ethnicity; (2) clinical details; (3) capture of antibiotics for which the health plans did not pay (eg, out-of-pocket payments); and (4) reasons why antibiotics unlinked to a diagnosis were dispensed (eg, incomplete capture of clinical care); this is an important area for further research. Finally, although our data may be precise for the communities studied, they may not represent trends in other parts of the United States.
Conclusions
The downward trend in antibiotic-dispensing rates to young children in these Massachusetts communities, driven by a decline in OM diagnosis rates, seems to have ended by 2004–2005. Possible explanations include true changes in OM incidence (possibly influenced by changes in immunization), parental care-seeking behavior, and/or changes in the diagnostic threshold for OM. A confluence of some or all of these factors is likely responsible. Clinicians recognize that there will always be uncertainty in many of the diagnoses for which antibiotics are commonly prescribed. If clinicians only treated known bacterial disease, antibiotic use would be reduced dramatically, but many patients would likely be undertreated. Whether the right equilibrium that balances the risks of overtreatment (adverse effects for individuals and resistance levels in the population) with the risks of undertreatment of bacterial illnesses has been reached remains unknown. Trends in population-based dispensing rates should continue to be monitored in young children to determine if current rates are sustained, decrease further, or increase in ways that may suggest antibiotic overuse.
Supplementary Material
Acknowledgments
We thank BlueCross and BlueShield of Massachusetts for providing data, Molly Crockett (Influenza Surveillance Coordinator, Massachusetts Department of Public Health) for providing state data on influenza-like illness trends, and Virginia L. Hinrichsen and M. Maya Dutta-Linn for study coordination.
Glossary
- AOM
acute otitis media
- NAMCS
National Ambulatory Medical Care Survey
- OM
otitis media
- PCV7
heptavalent pneumococcal conjugate vaccine
- p-y
person-year
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Research support was provided by the National Institute of Allergy and Infectious Diseases (R01 AI066304). Funded by the National Institutes of Health (NIH).
References
- 1.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096–3102 [DOI] [PubMed] [Google Scholar]
- 2.Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315–2322 [DOI] [PubMed] [Google Scholar]
- 3.Stille CJ, Rifas-Shiman SL, Kleinman K, Kotch JB, Finkelstein JA. Physician responses to a community-level trial promoting judicious antibiotic use. Ann Fam Med. 2008;6(3):206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SS, Rifas-Shiman SL, Kleinman K, et al. Parental knowledge about antibiotic use: results of a cluster-randomized, multicommunity intervention. Pediatrics. 2007;119(4):698–706 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Get smart: know when antibiotics work. Available at: www.cdc.gov/getsmart/campaign-materials/about-campaign.html. Accessed April 4, 2012
- 6.Belongia EA, Knobloch MJ, Kieke BA, Davis JP, Janette C, Besser RE. Impact of statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis. 2005;11(6):912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkelstein JA, Huang SS, Kleinman K, et al. Impact of a 16-community trial to promote judicious antibiotic use in Massachusetts. Pediatrics. 2008;121(1). Available at: www.pediatrics.org/cgi/content/full/121/1/e15. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) . Office-related antibiotic prescribing for persons aged ≤ 14 years—United States, 1993-1994 to 2007-2008. MMWR Morb Mortal Wkly Rep. 2011;60(34):1153–1156 [PubMed] [Google Scholar]
- 9.Stille CJ, Andrade SE, Huang SS, et al. Increased use of second-generation macrolide antibiotics for children in nine health plans in the United States. Pediatrics. 2004;114(5):1206–1211 [DOI] [PubMed] [Google Scholar]
- 10.McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992-2000. Emerg Infect Dis. 2003;9(4):432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media . Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–1465 [DOI] [PubMed] [Google Scholar]
- 12.Coco AS, Horst MA, Gambler AS. Trends in broad-spectrum antibiotic prescribing for children with acute otitis media in the United States, 1998-2004. BMC Pediatr. 2009;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernacchio L, Vezina RM, Mitchell AA. Management of acute otitis media by primary care physicians: trends since the release of the 2004 American Academy of Pediatrics/American Academy of Family Physicians clinical practice guideline. Pediatrics. 2007;120(2):281–287 [DOI] [PubMed] [Google Scholar]
- 14.Coco A, Vernacchio L, Horst M, Anderson A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125(2):214–220 [DOI] [PubMed] [Google Scholar]
- 15.Gamboa S, Park MK, Wanserski G, Lo V. Clinical inquiries. Should you use antibiotics to treat acute otitis media in children? J Fam Pract. 2009;58(11):602–604 [PubMed] [Google Scholar]
- 16.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124(1). Available at: www.pediatrics.org/cgi/content/full/124/1/e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Quality Assurance (NCQA). 2008 final NDC list for antibiotic utilization. HEDIS 2008: Healthcare effectiveness data and information set, volume 2. Washington, DC: NCQA; 2007. Available at: www.ncqa.org/tabid/598/Default.aspx. Accessed August 18, 2009
- 18.McEvoy GK, Snow EK, Miller J, Kester L, Welsh OH, eds. American Hospital Formulary Service (AHFS) Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2009 [Google Scholar]
- 19.First DataBank National drug data file plus. Available at: www.firstdatabank.com/Products/national-drug-file.aspx. Accessed August 24, 2009
- 20.Finkelstein JA, Davis RL, Dowell SF, et al. Reducing antibiotic use in children: a randomized trial in 12 practices. Pediatrics. 2001;108(1):1–7 [DOI] [PubMed] [Google Scholar]
- 21.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22 [Google Scholar]
- 22.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130 [PubMed] [Google Scholar]
- 23.Yanagimoto T, Yamamoto E. Estimation of safe doses: critical review of the hockey stick regression method. Environ Health Perspect. 1979;32:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125 [DOI] [PubMed] [Google Scholar]
- 25.Courter JD, Baker WL, Nowak KS, et al. Increased clinical failures when treating acute otitis media with macrolides: a meta-analysis. Ann Pharmacother. 2010;44(3):471–478 [DOI] [PubMed] [Google Scholar]
- 26.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997-2004. Pediatrics. 2008;121(2):253–260 [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein JA, Stille C, Nordin J, et al. Reduction in antibiotic use among US children, 1996-2000. Pediatrics. 2003;112(3 pt 1):620–627 [DOI] [PubMed] [Google Scholar]
- 28.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadeem Ahmed M, Muyot MM, Begum S, Smith P, Little C, Windemuller FJ. Antibiotic prescription pattern for viral respiratory illness in emergency room and ambulatory care settings. Clin Pediatr (Phila). 2010;49(6):542–547 [DOI] [PubMed] [Google Scholar]
- 30.National Committee for Quality Assurance (NCQA). Appropriate testing for children with pharyngitis. The State of Health Care Quality: 2004. Washington, DC: NCQA; 2004. Available at: www.ncqa.org/Portals/0/Publications/Resource%20Library/SOHC/SOHC_2004.pdf. Accessed April 4, 2012
- 31.Ramanuja S, Kelkar PS. The approach to pediatric cough. Ann Allergy Asthma Immunol. 2010;105(1):3–8; quiz 9–11, 42 [DOI] [PubMed]
- 32.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994-2007. Pediatrics. 2011;127(3):411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro DJ, Gonzales R, Cabana MD, Hersh AL. National trends in visit rates and antibiotic prescribing for children with acute sinusitis. Pediatrics. 2011;127(1):28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB, Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) . Prevention and control of influenza: recommendations of the ACIP. MMWR Recomm Rep. 2004;53(RR-6):1–40 [PubMed] [Google Scholar]
- 35.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA, Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) . Prevention and control of influenza: recommendations of the ACIP. MMWR Recomm Rep. 2006;55(RR-10):1–42 [PubMed] [Google Scholar]
- 36.Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) . Prevention and control of influenza: recommendations of the ACIP, 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60 [PubMed] [Google Scholar]
- 37.Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995;149(10):1113–1117 [DOI] [PubMed] [Google Scholar]
- 38.Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr Infect Dis J. 2000;19(suppl 5):S66–S71 [DOI] [PubMed] [Google Scholar]
- 39.Santibanez T, Barker L, Santoli J, Bridges C, Euler G, McCauley M, Centers for Disease Control and Prevention (CDC) . Childhood influenza-vaccination coverage—United States, 2002-03 influenza season. MMWR Morb Mortal Wkly Rep. 2004;53(37):863–866 [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among children aged 6-23 months—United States, 2008-09 influenza season. Available at: www.cdc.gov/flu/professionals/vaccination/coverage_6-23months.htm. Accessed March 4, 2011 [PubMed]
- 41.Sox CM, Finkelstein JA, Yin R, Kleinman K, Lieu TA. Trends in otitis media treatment failure and relapse. Pediatrics. 2008;121(4):674–679 [DOI] [PubMed] [Google Scholar]
- 42.Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J, Massachusetts Department of Public Health Epidemiologists . Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24(1):17–23 [DOI] [PubMed] [Google Scholar]
- 43.National Immunization Survey. Estimated vaccination coverage with 3+PCV among children 19-35 months of age by race/ethnicity and by state and local area—US, National Immunization Survey, Q3/2008-Q2/2009. Available at: http://www2a.cdc.gov/nip/coverage/nis/nis_iap.asp?fmt=r&rpt=tab29a_3pcv_race_iap&qtr=Q3/2008-Q2/2009. Accessed October 1, 2010
- 44.Jacobs MR. Prevention of otitis media: role of pneumococcal conjugate vaccines in reducing incidence and antibiotic resistance. J Pediatr. 2002;141(2):287–293 [DOI] [PubMed] [Google Scholar]
- 45.Shea KM, Weycker D, Stevenson AE, Strutton DR, Pelton SI. Modeling the decline in pneumococcal acute otitis media following the introduction of pneumococcal conjugate vaccines in the US. Vaccine. 2011;29(45):8042–8048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.