Abstract
Peripheral blood stem cell (PBSC) infusions are associated with complications such as elevated blood pressure and decreased creatinine clearance. Patients with sickle cell disease experience similar manifestations, and some have postulated release of plasma-free hemoglobin with subsequent nitric oxide consumption as causative. We sought to evaluate whether the infusion of PBSC grafts containing lysed red blood cells (RBCs) leads to the toxicity observed in transplant subjects. We report a prospective cohort study of 60 subjects divided into 4 groups based on whether their infusions contained dimethyl sulfoxide (DMSO) and lysed RBCs, no DMSO and fresh RBCs, DMSO and no RBCs, or saline. Our primary end point, change in maximum blood pressure compared with baseline, was not significantly different among groups. Tricuspid regurgitant velocity and creatinine levels also did not differ significantly among groups. Our data do not support free hemoglobin as a significant contributor to toxicity associated with PBSC infusions. This study was registered at clinicaltrials.gov (NCT00631787).
Introduction
Infusion of peripheral blood stem cells (PBSCs) can be associated with systemic toxicities, such as elevated blood pressure (BP) and serum creatinine, and cardiac arrhythmias.1–7 Multiple hypotheses have been generated to account for such toxicity, including acute volume expansion or a direct effect of the cryopreservative dimethyl sulfoxide (DMSO),3,4,6 but supporting data remain controversial.3,7–13
The volume of infused red blood cells (RBCs) contained within PBSC grafts has been positively correlated with a greater number of side effects.1 RBC lysis, which occurs during the freeze-thaw process, leads to the release of free hemoglobin (Hb), which could hypothetically result in impaired vasomotor tone because of nitric oxide (NO) consumption. Indeed, infusion of Hb-based blood substitutes leads to similar toxicities.14–16 Further, some of the complications of sickle cell disease (SCD) have been theorized to result largely from hemolysis and the liberation of free Hb.17,18 Our hypothesis was that the release of free Hb from thawed peripheral blood allografts explains the similar toxicity observed during PBSC infusions. To test this hypothesis in vivo, we conducted a prospective cohort study comparing 3 groups of subjects undergoing PBSC transplants that received grafts containing RBCs and/or DMSO and a group of healthy volunteers that received normal saline.
Methods
The protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDKK). All of the subjects gave written informed consent in accordance with Declaration of Helsinki. The study was registered at clinicaltrials.gov (NCT00631787). Group 1 included subjects who received thawed whole grafts containing DMSO and lysed RBCs (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Group 2 included subjects who received fresh grafts containing mostly intact RBCs and no DMSO. Group 3 included subjects who received thawed purified PBSCs. Group 4 included age and sex-matched healthy control subjects who received normal saline in equal volume as the first group.
The primary outcome was the difference between the baseline and maximum systolic and diastolic BP across groups. Secondary outcomes included acute changes in tricuspid regurgitant velocity (TRV) as determined by transthoracic echocardiography, organ function (serum creatinine and brain natriuretic peptide, NT-proBNP; Siemens Vista Analyzer, Siemens Healthcare Diagnostics), and markers of hemolysis (serum lactate dehydrogenase [LDH]), haptoglobin (Siemens Vista Analyzer, Siemens Healthcare Diagnostics) and cell-free Hb (plasma Hb ELISA kit; Bethyl Laboratories) before, during, and after the infusion. Further, the reactive hyperemia index (RHI) as determined by EndoPAT was compared among groups (see supplemental Methods).
Sample size was determined by evaluating retrospective BP data collected from subjects who had previously undergone PBSC transplants at the National Institutes of Health (supplemental Table 1). We expected a mean increase in systolic BP of 30 mmHg in the group receiving DMSO and lysed RBCs and 15 mmHg in the group receiving DMSO without RBCs. Based on these data, we initially enrolled 9 subjects into each group giving us an 83% power to detect a difference of 15 mmHg between the group that received lysed RBCs and the other treatment groups, assuming a standard deviation of 10 and a 2-sided significance level of 0.017. A planned analysis performed after 36 subjects were enrolled revealed no differences among groups.
After the planned analysis, sample size was increased to determine whether subjects with SCD were more susceptible to cell-free Hb infusions. Group 1 recipients of lysed RBCs were thus subdivided into 2 groups based on whether or not the subjects had SCD (group 1a with SCD, group 1b without SCD). The standard deviation of the increase in systolic BP was assumed to be 12 in group 1a and 10 in the other 3 transplanted groups. A sample size of 15 was selected for group 1a and 11 for each of groups 1b, 2, and 3 (total 48) giving a power to detect a difference of 15 mmHg in the increase in systolic BP of 0.81 for group 1a versus 1b and 0.82 for group 1b versus 2 and group 1b versus 3. Twelve control subjects were matched to the first 12 subjects enrolled in group 1. A 2-sided significance level of 0.017 (Bonferroni rule, 0.05/3) was selected to control for multiple comparisons.
Results and discussion
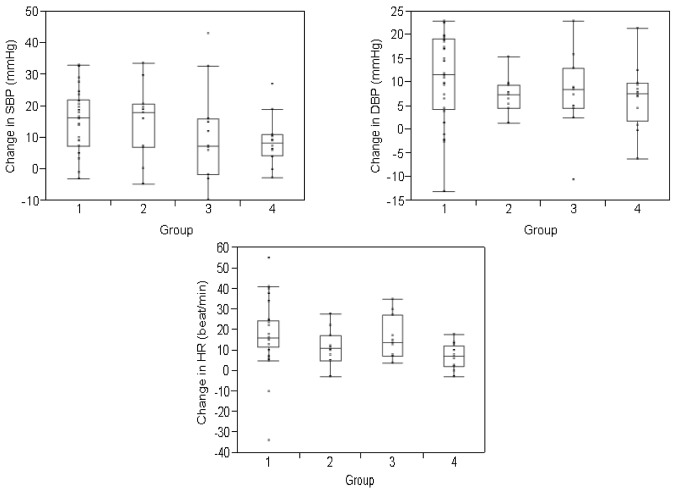
A total of 60 subjects completed the study (supplemental Table 2). There was no statistically significant difference in our primary end point, change in maximum systolic and diastolic BP compared with baseline, among groups (Figure 1). However, systolic and diastolic BP increased significantly within each group during the infusion, including in the healthy volunteers that received saline (supplemental Table 3), providing direct evidence for volume leading to corresponding BP changes. Further, unlike previous studies that suggest that PBSC-associated systemic toxicity is related to DMSO infusion,3,4,6–9 there was no significant difference in BP or organ function changes in subjects that did or did not receive DMSO, challenging DMSO as the etiology for PBSC-associated toxicity. Details regarding the PBSC grafts and/or normal saline infused are included in supplemental Results and supplemental Table 4.
Figure 1.
Change in blood pressure and heart rate during the infusion. Change represents the mean difference between the measured variables (systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) at baseline and the maximum value attained during the study period. BP and heart rate were measured every 15 minutes for the first hour and then once hourly for a total of 4 hours in healthy volunteers and for a total of 8 hours or until the infusion was complete, whichever longer, in transplanted subjects. Values are divided according to study group. The change in blood pressure and heart rate was not significantly different among groups. The boxes represent the 25th and 75th percentiles and border the median horizontal lines. The top and bottom horizontal lines represent the maximum and minimum values, excluding outliers.
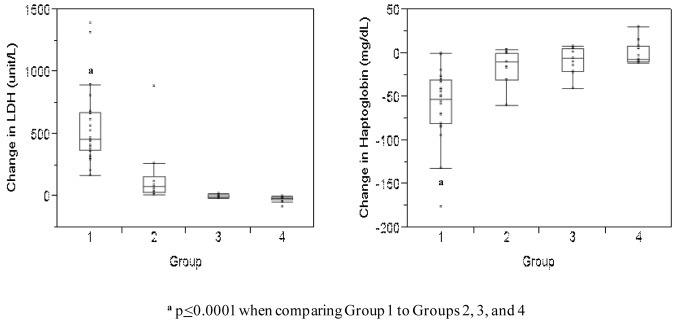
As expected, the median increase in lactate dehydrogenase (LDH) levels was greatest in subjects that received lysed RBCs (median +462.5 unit/L in group 1) compared with the groups that did not receive RBCs (median −5 unit/L in group 3 and median −14 unit/L in group 4, P < .0001) and the group that received fresh RBCs (median +79 unit/L in group 2, P < .0001, Figure 2). Haptoglobin levels decreased significantly more in subjects that received lysed RBCs (median −52.5 mg/dL) compared with subjects that did not receive lysed RBCs (median −10 mg/dL in group 2, −6 mg/dL in group 3, and −7 mg/dL in group 4 subjects, P < .0001; Figure 2). These data demonstrate that significant amounts of free Hb were infused in group 1 subjects.
Figure 2.
Change in LDH and haptoglobin levels in each study group. These data represent the difference between the median baseline levels and median levels measured at the end of each infusion. Levels are subdivided according to study group. The boxes represent the 25th and 75th percentiles and border the median horizontal lines. The top and bottom horizontal lines represent the maximum and minimum values, excluding outliers.
There was also no significant difference in the change in TRV or NT-proBNP during the infusion comparing the 4 groups of subjects. Further, group 1 recipients experienced no significant change in TRV (mean from 1.9 m/s to 2.1 m/s, P = .063) or NT-proBNP (mean from 1650 pg/mL to 1624 pg/mL, P = .82) during the PBSC infusion. Changes in creatinine levels also were not significantly different comparing groups. In addition, if a significant amount of NO was consumed during the infusion, the RHI would be expected to decrease significantly after infusion in subjects that received lysed RBCs. RHI change in group 1 subjects was not significantly different compared with the other groups (supplemental Table 5). Cell-free Hb concentrations drawn from the subjects after infusion were unreliable (supplemental Figure 2), probably because of technical challenges including ex vivo hemolysis.19
Given our hypothesis that RBC lysis and release of free Hb with NO scavenging explains the toxicity of thawed PBSC infusion, we sought to determine whether subjects with SCD might be more susceptible. There was no significant difference in the primary end point, change in systolic and diastolic BP during the infusion compared with baseline, comparing group 1a (with SCD) to group 1b (without SCD) subjects (supplemental Table 5). Further, the change in TRV, NT-proBNP, and creatinine was not significantly different comparing the subjects with SCD to the other groups. These results are important because the removal of RBCs from the graft, a process that could result in cell loss, is not necessary. It is possible that our trial failed to detect a small effect of infused cell-free Hb because we powered our study based on known BP effects of infused products. In summary, our data do not support that the infusion of lysed RBCs and consumption of NO explains the systemic toxicity associated with PBSC infusions in this acute setting and suggest a limitation of the role of lysed RBCs in the pathophysiology of SCD.
Supplementary Material
Acknowledgments
The authors thank the transplant principal investigators Drs John Barrett, Michael Bishop, Richard Childs, Dan Fowler, Dennis Hickstein, Elizabeth Kang, Steven Pavletic, and their research nurses for helping to recruit and enroll their patients. They also thank the clinical nurses for helping to execute the study, Ms Cyndy Brenneman and Mr Wen Li for performing transthoracic echocardiography, Ms Laurel Mendelsohn for running research studies, Ms Cassie Seamon and Ms Eleni Footman for help with EndoPAT, Ms Becky Rothwell for assistance with statistical analysis, Ms Quyen Chau, Mr Michael McGann, and Ms Alyce McKelvy from the Cell Processing Lab for help with analysis of graft contents, and Dr Hanh Khuu and other staff in the Department of Transfusion Medicine and the Holter monitor technicians for helping to ensure that the protocol was completed. Lastly, they thank Dr Alan Schechter for reviewing the paper.
This work is supported by the Intramural Research Program of NIDDK and NHLBI at the NIH.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.D.F., D.S., M.M.H., G.J.K., and J.F.T. designed the protocol, and reviewed and edited all data and wrote the paper; C.D.F., W.A.C., and M.E.L. recruited subjects, conducted the study, and reviewed the paper; X.Z. and E.W. designed the statistical analyses, and reviewed all data and the paper; and H.U., V.H., and R.P.W. performed data analysis and reviewed all data and the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John F. Tisdale, 9000 Rockville Pike, Bldg 10, Rm 9N-112, Bethesda, MD 20892; e-mail: johntis@nhlbi.nih.gov.
References
- 1.Kessinger A, Schmit-Pokorny K, Smith D, Armitage J. Cryopreservation and infusion of autologous peripheral blood stem cells. Bone Marrow Transplant. 1990;5(Suppl):25–27. 1. [PubMed] [Google Scholar]
- 2.Alessandrino P, Bernasconi P, Caldera D, et al. Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant. 1999;23(6):533–537. doi: 10.1038/sj.bmt.1701609. [DOI] [PubMed] [Google Scholar]
- 3.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75(3):781–786. [PubMed] [Google Scholar]
- 4.Styler MJ, Topolsky DL, Crilley PA, et al. Transient high grade heart block following autologous bone marrow infusion. Bone Marrow Transplant. 1992;10(5):435–438. [PubMed] [Google Scholar]
- 5.Keung YK, Lau S, Elkayam U, Chen SC, Douer D. Cardiac arrhythmia after infusion of cryopreserved stem cells. Bone Marrow Transplant. 1994;14(3):363–367. [PubMed] [Google Scholar]
- 6.Zenhäusern R, Tobler A, Leoncini L, Hess OM, Ferrari P. Fatal cardiac arrhythmia after infusion of dimethyl sulfoxide-cryopreserved hematopoietic stem cells in a patient with severe primary cardiac amyloidosis and end-stage renal failure. Ann Hematol. 2000;79(9):523–526. doi: 10.1007/s002770000186. [DOI] [PubMed] [Google Scholar]
- 7.Syme R, Bewick M, Stewart D, Porter K, Chadderton T, Gluck S. The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant. 2004;10(2):135–141. doi: 10.1016/j.bbmt.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport AP, Rowe JM, Packman CH, Ginsberg SJ. Cardiac arrest after autologous marrow infusion. Bone Marrow Transplant. 1991;7(5):401–403. [PubMed] [Google Scholar]
- 9.Hameroff SR, Otto CW, Kanel J, Weinstein PR, Blitt CD. Acute cardiovascular effects of dimethyl sulfoxide. Ann N Y Acad Sci. 1983;411:94–99. doi: 10.1111/j.1749-6632.1983.tb47289.x. [DOI] [PubMed] [Google Scholar]
- 10.Rowley SD, Feng Z, Yadock D, Holmberg L, Macleod B, Heimfeld S. Post-thaw removal of DMSO does not completely abrogate infusional toxicity or the need for pre-infusion histamine blockade. Cytotherapy. 1999;1(6):439–446. doi: 10.1080/0032472031000141303. [DOI] [PubMed] [Google Scholar]
- 11.Shlafer M, Matheny JL, Karow AM., Jr Cardiac chronotropic mechanisms of dimethyl sulphoxide: inhibition of acetylcholinesterase and antagonism of negative chronotropy by atropine. Arch Int Pharmacodyn Ther. 1976;221(1):21–31. [PubMed] [Google Scholar]
- 12.Shlafer M. Cardiac pharmacology of dimethyl sulfoxide and its postulated relevance to organ preservation in ischemic or hypoxic states. Ann N Y Acad Sci. 1983;411:170–179. doi: 10.1111/j.1749-6632.1983.tb47299.x. [DOI] [PubMed] [Google Scholar]
- 13.Clifford DH, Lee DC, Lee MO. Effects of dimethyl sulfoxide and acupuncture on the cardiovascular system of dogs. Ann N Y Acad Sci. 1983;411:84–93. doi: 10.1111/j.1749-6632.1983.tb47288.x. [DOI] [PubMed] [Google Scholar]
- 14.Winslow RM. Red cell substitutes. Semin Hematol. 2007;44(1):51–59. doi: 10.1053/j.seminhematol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Amberson WR, Jennings JJ, Rhode CM. Clinical experience with hemoglobin-saline solutions. J Appl Physiol. 1949;1(7):469–489. doi: 10.1152/jappl.1949.1.7.469. [DOI] [PubMed] [Google Scholar]
- 16.Savitsky JP, Doczi J, Black J, Arnold JD. A clinical safety trial of stroma-free hemoglobin. Clin Pharmacol Ther. 1978;23(1):73–80. doi: 10.1002/cpt197823173. [DOI] [PubMed] [Google Scholar]
- 17.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 18.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 19.Waugh W, Daeschner CW, Files BA, Gordon DW. Evidence that L-arginine is a key amino acid in sickle cell anemia: a preliminary report. Nutrition Res. 1999;19(4):501–518. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.