Abstract
Age-appropriate acute stress, such as cold exposure, provokes the secretion of corticotropin releasing factor (CRF) from the hypothalamus, leading to a robust increase of plasma corticosterone in the immature rat. This activation of the hypothalamic-pituitary-adrenal system is accompanied by a stress-induced increase of steady-state CRF-mRNA expression in the hypothalamic paraventricular nucleus (PVN). In the current study, we analysed changes in CRF-mRNA expression in the PVN and the central nucleus of the amygdala (ACe) in the immature rat in response to a single episode of cold stress and three repeated exposures to this same stressor. CRF-mRNA expression in the PVN increased after a single, but not repeated exposures to cold stress, while repeated acute stress increased the content of the CRF peptide in the anterior hypothalamus. In the ACe, repeated episodes of cold stress resulted in increased expression of CRF-mRNA. These findings indicate a differential regulation of CRF gene expression in the PVN and ACe of the immature rat by single and repeated acute stress.
Keywords: corticotropin releasing factor, neonatal rat, hypothalamus, stress, amygdala
The developing rat responds to environmental stressors by activation of the hypothalamic-pituitary-adrenal (HPA) axis (1, 2). However, the mechanisms of this hormonal stress response may differ in the neonatal rat compared with the adult. The developmental period between the third and fifteenth postnatal days has been characterized by attenuated hormonal responses and altered gene regulation in response to stress (2–5).
The neurohormone corticotropin releasing factor (CRF) activates both hormonal and behavioural responses to a variety of stressors. In both the mature and the developing rat, stress leads to CRF release from the hypothalamic paraventricular nucleus (PVN), resulting in increased plasma adrenocorticotropin (ACTH) and corticosterone concentrations. The stress-induced elevation of plasma corticosterone in the immature rat is dependent on CRF secretion as demonstrated using antisera to CRF (2). Subsequent to the stress-induced CRF secretion, a compensatory increase in CRF-mRNA expression in the PVN has been observed in the adult rat (6) as well as in the developing rat starting in the second week of life (2).
CRF-containing cells constitute a significant neuronal population in the central nucleus of the amygdala (ACe). The ACe has been shown to be a key regulator of the stress response mediated by CRF release from the PVN [reviewed in (7)]. For example, ablation or stimulation of the ACe attenuate or mimic, respectively, the effects of stress on CRF release from the PVN (8, 9). However, the modulation of CRF gene expression in the ACe by stress has not been examined in the developing rat.
The spectrum of differential alterations in the neuroendocrine axis of the adult rat by repeated stress compared with a single acute stress has been a topic of intense study. However, there is relatively limited information regarding the modulation of the neuroendocrine axis of an immature rat with repeated stress (10). The goal of this study was to analyse the regulation of CRF in the PVN and the ACe of the immature rat in response to single or repeated episodes of acute cold stress.
Results
Stress-induced alterations of CRF-mRNA in the PVN
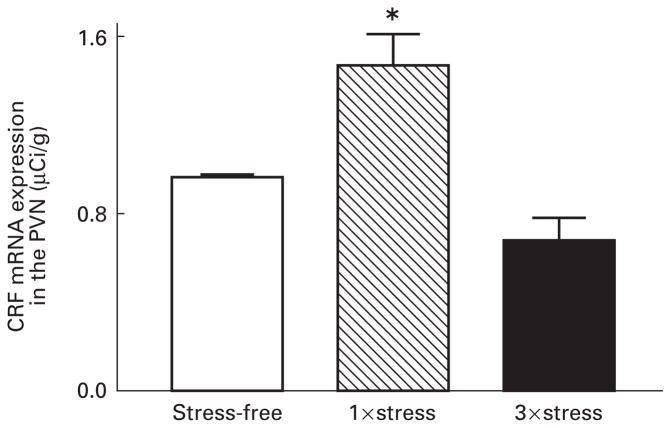
A single exposure to cold stress resulted in a significant elevation of CRF-mRNA expression in the PVN of 9–10-day-old rat pups 4 h after the termination of stress (P<0.01) (Fig. 1). However, when pups sustained this acute stress three times over the course of 24 h, there was no increase in CRF-mRNA in the PVN 4 h after the final exposure (Fig. 1). In fact, there was a trend (P=0.09) towards a reduction of CRF-mRNA in the PVN after repeated acute stress compared with the unstressed controls. Dark-field photomicrographs of the PVN of control rats and those subjected to single or repeated cold stress are shown in Fig. 2, demonstrating that levels of CRF-mRNA in matched sections of the parvocellular dorsomedial PVN are higher following a single exposure to cold stress.
Fig. 1.
Relative abundance of corticotropin releasing factor (CRF) mRNA in the hypothalamic paraventricular nucleus (PVN) of 9–10-day-old rats following single and repeated exposure to cold stress. In situ hybridization (ISH) was performed to detect CRF-mRNA in brain sections from rats sacrificed under stress-free (SF) conditions or rats subjected once (1×) or three times (3×) to cold stress 4 h prior to sacrifice, as described in materials and methods. Single exposure to cold stress resulted in a significant increase in CRF-mRNA in the PVN (*P<0.01) compared with SF controls. Repeated exposure to cold stress resulted in a nonsignificant trend toward decreased expression of CRF-mRNA in the PVN (P=0.09). Each bar represents the average with the standard error of the mean of 16–21 samples from 4–5 brains per group.
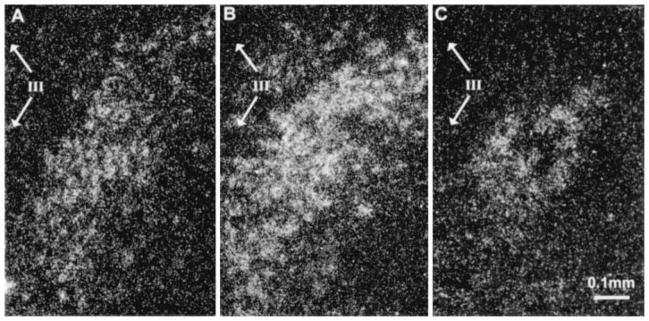
Fig. 2.
Dark-field photomicrographs of CRF-mRNA hybridization signal in the PVN. Nine or 10-day-old rats were either killed under stress-free conditions (A) or subjected once (B) or three times (C) to cold stress 4 h prior to death. A single exposure to stress resulted in elevated levels of CRF-mRNA in the PVN with an apparent increase in the number of cells with detectable signal. CRF-mRNA was detected by ISH in 20 μm coronal brain sections. The third ventricle is represented by III; bar represents 0.1 mm.
Stress-induced differences in CRF peptide content in the hypothalamus
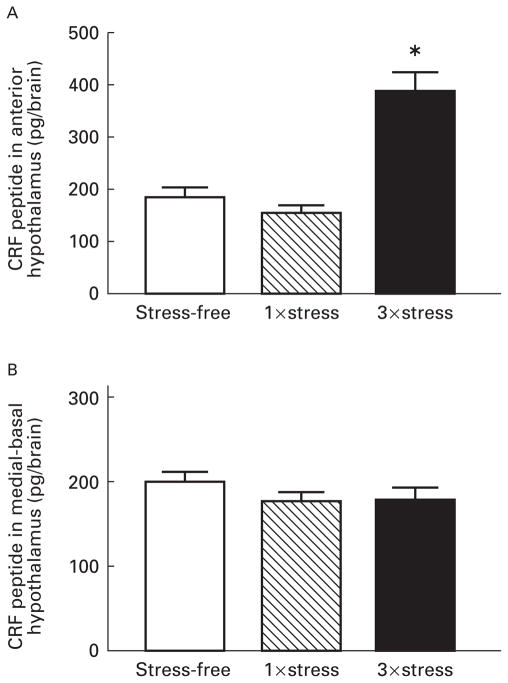
Radioimmunoassay was used to measure the amount of the peptide, CRF, in the anterior hypothalamus (containing the paraventricular, suprachiasmatic and supraoptic nuclei) and in the medial-basal hypothalamus (containing the median eminence and the arcuate nucleus). Although there was no change in CRF peptide content in the anterior hypothalamus following a single exposure to cold stress, repeated stress resulted in a significant (P<0.001) enhancement in CRF levels in this region (Fig. 3A). CRF content 4 h after a single stress was slightly lower than in stress-free controls (Fig. 3A; 157 pg vs 185 pg). By 28 h following a single stress, CRF content was elevated compared with both the 4 h time-point and to the levels in stress-free controls (238 pg vs 157 pg and 185 pg, respectively). CRF content of the medial-basal hypo- thalamus was not significantly altered by a single or repeated stress (Fig. 3B).
Fig. 3.
Corticotropin releasing factor (CRF) peptide levels in the anterior hypothalamus increase with repeated exposure to cold stress. CRF peptide extracted from the anterior hypothalamus (A) or the medial-basal hypothalamus (B) was measured by radioimmunoassay. Rats were sacrificed under stress-free conditions (SF), 4 h after a single exposure (1×), or repeated exposures (3×) to cold stress. The values are expressed as the mean total CRF peptide content of each brain region, obtained from at least five rats per group. Error bars represent the standard error of the mean, and the star indicates a significant difference from SF controls (P<0.001).
Stress-induced differences in CRF-mRNA in the ACe
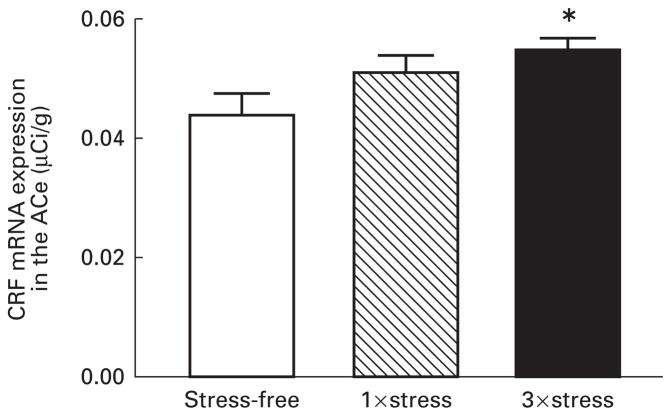
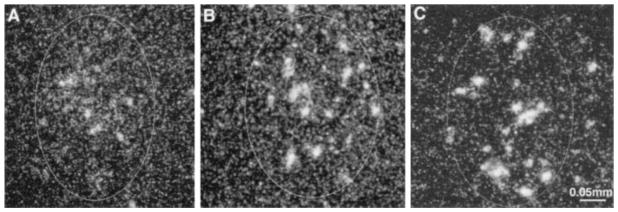
Analysis of CRF-mRNA expression following single and repeated cold stress in the immature rat revealed that repeated (three times) stress caused an increase in CRF-mRNA in the ACe (P<0.01) (Fig. 4). A single exposure to cold stress resulted in a trend (P=0.107) toward increased expression of CRF-mRNA in this nucleus. Dark-field photomicrographs of the ACe of controls and of rats subjected to a single or repeated cold stress are shown in Fig. 5.
Fig. 4.
Relative abundance of CRF-mRNA in the central nucleus of the amygdala (ACe) of 9–10-day-old rats following a single or repeated exposure to cold stress. Hybridization signal for CRF-mRNA was analysed in the ACe of rats sacrificed under stress-free (SF) conditions or rats subjected once (1×) or three times (3×) to cold stress 4 h prior to sacrifice (see materials and methods). Repeated stress resulted in a significant increase in CRF-mRNA expression in the ACe (*P<0.01) compared with SF controls. Each bar represents the mean (standard error of the mean of 17–30 samples from 4–6 brains per group.
Fig. 5.
Dark-field photomicrographs of CRF-mRNA hybridization signal in the central nucleus of the amygdala of 9–10 day-ld rats. Animals were either sacrificed under stress-free conditions (A), or exposed once (B) or three times (C) to cold stress 4 h prior to sacrifice. CRF-mRNA expression was elevated in the ACe after repeated exposure to cold stress. Bar represents 0.05 mm.
Stress-induced plasma corticosterone
Plasma corticosterone of stressed pups was higher than that of stress-free controls. Levels were obtained at the time of sacrifice of the animals, i.e. 4 h after the last stress episode. Therefore, these plasma corticosterone values do not reflect peak levels, which occur 40–60 min after the termination of cold stress at this age (2). Plasma corticosterone concentrations averaged 1.12 ± 0.09 μg/dl in controls, 1.73 ± 0.13 μg/dl 4 h after a single stress, and 0.93±0.12 μg/dl 28 h after a single stress. Three stress episodes resulted in plasma corticosterone concentration of 1.50±0.98 μg/dl 4 h after termination of the last stress episode.
Discussion
The major findings of this study are that in the developing rat, a single acute stress increases CRF-mRNA expression in the PVN, but not in the ACe. Conversely, recurrent acute stress up-regulates CRF-mRNA levels in the ACe, while CRF-mRNA expression in the PVN is not enhanced.
These findings extend earlier studies of the effects of acute stress on CRF-mRNA expression in the PVN of the developing rat. The original report demonstrated that a single age-appropriate cold stress resulted in an increase of CRF-mRNA in the PVN of 9-day-old rats (2), consistent with compensatory up-regulation of CRF gene transcription after enhanced secretion of hypothalamic CRF (6). It should be emphasized that the stressor employed here consists mainly of exposure to a cold environment. The pups are separated from their mother during the cold exposure and for up to 6 h later. However, this manipulation differs substantially from the maternal separation paradigm in regards to the effects of the latter on the activation of the HPA of the infant rat: Rosenfeld et al. (11) demonstrated that an 8 h separation from the mother (even if repeated three times) was insufficient to enhance either basal plasma corticosterone or the subsequent stress response in the 12-day-old rat. Thus, the relatively short separation period accompanying the cold exposure in the current paradigm (≤6 h) is unlikely to contribute significantly to the changes observed in CRF-mRNA and CRF peptide expression in the hypothalamus and ACe.
In the adult rat, repeated stress can lead to an increase (6, 12, 13), decrease (14, 15) or no change (16) in CRF-mRNA expression in the PVN. It is not clear why stress paradigms differ in their effects on CRF-mRNA expression in this nucleus. One hypothesis is that the different physical and psychological elements of each stressor influence gene expression. For example, repeated injection of hypertonic saline results in a persistent increase in CRF-mRNA (6). This manipulation is likely to be predominantly an exogenous physiological stress. In contrast, the endogenous physiological stress of adjuvant-induced arthritis (chronic inflammation) results in decreased expression of CRF-mRNA (14). In addition, the animal may habituate to repeated exposures to a psychological stressor, such as restraint, leading to the observed absence of alteration in CRF-mRNA expression in this paradigm (16). In the present study, we used the age-appropriate stressor of cold, considered a composite of physiological and of psychological stress (1), and found that repeated exposure to this stress did not increase CRF-mRNA expression in PVN neurons. This finding is in line with the results observed with psychological stress in the adult rat (17). Teleologically, habituation to chronic disease or recur- rent stress by alteration of the enhanced CRF expression would be advantageous for the survival of the animal. However, in the case of acute physiological stressors, adaptation to the stressor may be detrimental.
A potential mechanism for the lack of CRF-mRNA increase in the PVN with repeated acute stress is negative feedback via glucocorticoids (18). Glucocorticoids have been shown to depress hypothalamic CRF-mRNA expression (19, 20). The present study measured plasma corticosterone only at the time of sacrifice, 4 h after the stress exposure (or the last in a series of exposures). Cold stress-induced plasma corticosterone concentration peaks at 40–60 min after the stress with a variable decay profile (2). Therefore, with the exception of demonstrating that stress-free controls and pups stressed once 28 h earlier have low plasma corticosterone concentrations (approximately 1 μg/dl), we cannot make any quantitative statements regarding the magnitude of the corticosterone response after repeated stress, compared with a single stress.
It has been shown that persistently elevated plasma cortico- sterone concentrations may be maintained in rats subjected to repeated stress, leading to increased glucocorticoidreceptor binding in the hippocampus and PVN and down-regulation of CRF-mRNA expression in the PVN (20–24). This notion is supported by decreased CRF-mRNA expression in the PVN of infant rats subjected to the chronic stress of altered maternal rearing (25). In these pups, both basal and stress-induced plasma corticosterone concentrations are elevated compared with controls (26). In addition, a recent report documented decreased CRF-mRNA in the PVN of maternally-deprived infant rats with elevated plasma corticosterone concentrations (15). However, other investigators have not found significant alteration in CRF-mRNA expression with maternal deprivation (27).
Increased plasma glucocorticoid concentrations and consequent down-regulation of CRF-mRNA expression with repeated stress may also depend on AVP release from the PVN and activation of ACTH release. We have shown that elevation of plasma corticosterone in infant rats after a single acute stress is dependent on CRF. However, a role for AVP has also been demonstrated (5). In paradigms of chronic or repeated stress, an increase in AVP signalling has been demonstrated in the adult (28–31). This switch from CRF-to AVP-mediated ACTH release is thought to be controlled both by corticosterone-dependent and independent mechanisms (13). Thus, AVP-induced increase in plasma corticosterone after recurrent stress in the infant rat may further contribute (via negative feedback) to the reduction of CRF-mRNA in the PVN.
In the current study, levels of CRF in the anterior hypothalamus were elevated after repeated cold stress but not after a single exposure (Fig. 3A). This result is consistent with repeated and chronic stress paradigms in adult rats which demonstrate increased CRF content in the PVN (32, 33). The causes of the discrepancy between the effects of single and multiple acute stress episodes on peptide and mRNA levels in the PVN are not fully resolved. One factor that may contribute to this discrepancy is the time-point after stress at which CRF expression was measured. We assayed CRF-mRNA and peptide 4 h after the termination of the stressful episode. At that time, it is conceivable that the mRNA had already been up-regulated but that insufficient peptide had been transcribed to alter immunoreactive peptide content. Following repeated stress, CRF peptide content may be elevated as a result of the increased transcription induced by previous stress, while glucocorticoid feedback has already resulted in down-regulation of further CRF gene expression (measured by CRF-mRNA). Indeed, our finding of increased peptide 28 h after a single stress supports this notion. In addition, regulation of intracellular stores of the peptide and axonal transport to the median eminence may be important in determining relative peptide and mRNA concentrations.
Despite the elevation of CRF in the anterior hypothalamus, there was no change in CRF content in the medial-basal hypothalamus, suggesting that the stores of CRF peptide in the median eminence are not depleted with the repeated stress (Fig. 3B). Because the content of CRF in the medial-basal hypothalamus was only measured at a single time-point, changes in content earlier or later than 4 h after stress cannot be excluded. However, the observed pattern suggests enhanced CRF availability for release in the repeated stress paradigm; this is consistent with facilitation of the stress response documented in both the adult (34) and the developing rat (10). In concordance with Akana et al. (34), we also found no increase of CRF-mRNA in the PVN after repeated stress. Our findings are consistent with a model of facilitation in which input to the PVN results in increased peptide content and secretion without significant alterations in steady-state mRNA expression.
A strong candidate source of CRF-enhancement in the PVN is input from the ACe. Recent experiments in the adult rat have shown that expression of CRF-mRNA in the ACe is responsive to stress (35), and that corticosterone increases expression of CRF-mRNA in the ACe, but decreases expression in the PVN (20, 36). The present study in the immature rat demonstrates that repeated stress results in decreased CRF-mRNA expression in the PVN and increased expression in the ACe, consistent with the data in the adult (36). The 25% increase in CRF-mRNA expression in the ACe demonstrated in the current study (Fig. 4) is comparable with the changes observed following corticosterone treatment in the adult (20–40%) (36). In addition, we found a 59% increase in CRF-mRNA in the PVN after a single exposure to cold stress. These changes in total steady state CRF-mRNA are quite remarkable given that even complete elimination of negative feedback, via adrenalectomy, resulted only in a 52% increase of CRF-mRNA expression in the PVN (19).
The mechanism for the findings reported here (Fig. 6) may derive from the effects of repeated stress on plasma glucocorticoid levels, and the differential effects of glucocorticoids on the ACe and PVN: repeated stress is likely to result in cumulatively more corticosterone production, causing decreased CRF-mRNA in the PVN but increased expression in the ACe (36). Thus, the current study provides a mechanism by which increased CRF-mRNA expression in the ACe may maintain an organism’s response to stress even in the presence of elevated corticosterone. Furthermore, these results indicate a differential regulation of CRF in the PVN and in the ACe and, within each region, in response to a single or repeated acute stress. Changes in the expression of CRF-mRNA in the ACe of the immature rat may play a role in the long-term alterations of the neuroendocrine system observed in response to early-life stress.
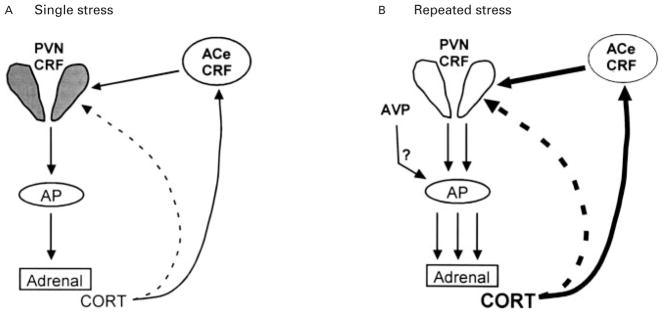
Fig. 6.
Schematic representation of the modulation of neuroendocrine components of the stress response in the immature rat with single or repeated stress. (A) A single stress is associated with CRF release from the hypothalamus, leading to elevated plasma corticosterone (CORT) levels. CORT negatively regulates CRF expression in the hypothalamic paraventricular nucleus (PVN, dashed line) but tends to up-regulate CRF-mRNA production in the central nucleus of the amygdala (ACe, solid line). (B) With repeated stress, cumulative plasma CORT [augmented perhaps also by vasopressin (AVP) secretion and activation of pituitary corticotrophs] further inhibits CRF-mRNA levels in the PVN. However, a significant augmentation of CRF-mRNA levels in the ACe (darkly shaded ACe-CRF) is evident. AP, anterior pituitary.
Materials and methods
Experimental design
In the morning (10.00 h) of postnatal day 9, two groups of rat pups were subjected to cold stress. Four hours after the termination of the stress, one group of rats was sacrificed (1×stress group). The other group was again subjected to cold stress and then returned to the mother (total separation time from the mother was 5–6 h). The next morning (10.00 h), this group was subjected to cold stress for a third time and sacrificed 4 h after the termination of this final exposure (3×stress group). The time-point of 4 h after cold exposure was chosen to coincide with peak compensatory CRF-mRNA expression in the PVN (2). The control group of rats was sacrificed under stress-free conditions (within 2 min of disturbance). For analysis of CRF peptide content, additional rat litters were divided into groups consisting of: control (sacrificed under stress-free conditions), single cold exposure, with sacrifice time of 4 h or 28 h later, and multiple (3×) stress group which was sacrificed 4 h after the termination of the third stress episode. All rats were sacrificed at the same time of day (14.00–15.00 h). For technical reasons, experiments were performed in batches to allow for 5–6 rats in each experimental group. However, each experiment was performed with two mixed litters of the same age and included all experimental groups.
Animals and tissue preparation
All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care Committee. Timed-pregnancy Sprague Dawley-derived rats (Zivic-Miller, Zelienople, PA, USA) were maintained in an NIH-approved animal facility on a 12 h light/dark cycle. Cages were monitored every 12 h for the presence of pups and the date of birth was considered day 0; litters were culled within 24 h of delivery to 12 pups. At the appropriate time-points, rats were decapitated and the brains were rapidly removed and either microdissected on ice and frozen for quantification of CRF or frozen on dry ice for subsequent in situ hybridization. Trunk blood was collected at time of sacrifice for analysis of plasma corticosterone levels by radioimmunoassay (ICN, Irvine, CA, USA) as previously described (37). Assay sensitivity was 0.5 μg/dl and interassay variability was less than 5%.
Acute cold paradigm
Rat pups were subjected to cold stress as previously described (2). Briefly, pups were separated from the mother and subjected to maximally tolerated cold exposure as defined by development of rigor and little response to tactile stimulus. Maximum exposure required 40–50 min at 4 °C and resulted in an average rectal temperature of 11 °C. Following cold stress, pups were placed on a euthermic pad where they regained normal core temperature of 33–34 °C in 10–15 min
In situ hybridization histochemistry
In situ hybridization histochemistry was performed as described previously (2, 37). Briefly, 20 micrometer coronal sections were collected on gelatincoated slides and stored at −80 °C. These tissue sections were thawed, airdried, fixed in paraformaldehyde, dehydrated and rehydrated through graded ethanols. Sections were exposed to 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8) and dehydrated. Prehybridization and hybridization steps were performed at 40 °C in a humidified chamber in a solution of 50% formamide, 5×SET, 0.2% SDS, 5×Denhardt’s, 0.5 mg/ml salmon sperm DNA, 0.25 mg/ml yeast tRNA, 100 mM dithiothreitol and 10% Dextran sulphate. Following a 1 h prehybridization, sections were hybridized overnight (20 h) with a deoxyoligonucleotide probe complementary to the coding region of CRF-mRNA and 3′ end-labelled with 35S-dATP as previously described (37). Sections were then washed as described (2) and apposed to film (Hyperfilm β–Max, Amersham, IL, USA) for 5–7 days. Selected sections were also dipped in emulsion (NTB-2, Eastman Kodak, Rochester, NY, USA) and exposed for 3–4 weeks.
Acquisition and quantitative analysis of CRF-mRNA in situ hybridization signal
Semiquantitative analysis of CRF-mRNA was performed following in situ hybridization. A digitized image of each brain section was acquired using a StudioStar scanner (AGFA, resolution 1,200×1,200 dots per inch) and analysed using the ImageTool software program ( University of Texas Health Science Center, San Antonio, TX, USA, version 1.25). Densities were calibrated using 14C standards and are expressed in μCi/g after correcting for background by subtracting the density of the hybridization signal over the corpus collosum (for PVN sections) and internal capsule (for ACe sections). For a balanced comparison of the PVN and ACe between the different experimental groups, only the five highest density values were used from each brain. The significance of observed quantitative differences among experimental groups was evaluated using the unpaired Student’s t-test with Welch’s correction for unequal variance as required.
Analysis of CRF peptide
Immediately after decapitation, brains were microdissected to isolate the medial-basal hypothalamus (containing the median eminence and arcuate nucleus) and the anterior hypothalamus (containing paraventricular, suprachiasmatic and supraoptic nuclei, as well as the anterior hypothalamic and medial preoptic areas). Tissue blocks were placed individually in microfuge tubes and frozen in powdered dry ice. CRF peptide was purified by a modification of a previously described procedure (38). Briefly, tissue was thawed in extraction buffer (0.25 N HCl, 0.25 N acetic acid, 4.5 μg/ml pepstatin A (Sigma, St Louis, MO, USA), and 0.5% β-mercaptoethanol) at 48 °C for 3 min followed by homogenization (10 strokes with a micro tissue grinder, Kontes, Vineland, NJ, USA) on ice. The homogenate was sonicated (15 s at 75% power, 0.8 duty cycle, Braun sonicator) a total of four times on ice. The preparation was centrifuged at 15 600 g for 30 min at 4 °C. The supernatant was transferred to a new tube, the centrifugation was repeated, and the supernatant was desiccated to near dryness in a speed-vac. The preparation was resuspended in 400 μl of assay buffer (39) containing 1 μg/ml of phenol red and the pH was adjusted to 7.2–7.6 using NaOH.
Radioimmunoassay (RIA) for CRF peptide was performed as previously described (39) with a slight modification. Assay was performed in polypropylene RIA tubes (Fisher) with rabbit antirat CRF antibody (kindly provided by W. Vale, final dilution of 1:700,000) and 125I-Tyr-rat/human CRF (New England Nuclear, 30,000 cpm/tube). Calibrations to CRF standards were performed using 1:1 serial dilutions of CRF (Bachem, Torrance, CA, USA) ranging from 2000 to 0.25 pg. The final precipitation was achieved using Pansorbin (Calbiochem, La Jolla, CA, USA; final dilution of 1:360).
Acknowledgments
The excellent advice and insightful comments of Dr P. Plotsky are appreciated. We thank Linda Schultz and Mariam Eghbal-Ahmadi for their assistance and Dr W. Vale for providing the CRF antiserum. This work was supported by NIH NS28912.
References
- 1.Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 2.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenfeld NM, Leathem JH, Rabii J. Maturation of adrenal stress responsiveness in the rat. Neuroendocrinology. 1980;31:101–105. doi: 10.1159/000123058. [DOI] [PubMed] [Google Scholar]
- 4.Levine S. The pituitary-adrenal system and the developing brain. Prog Brain Res. 1970;32:79–85. doi: 10.1016/S0079-6123(08)61521-6. [DOI] [PubMed] [Google Scholar]
- 5.Muret L, Priou A, Oliver C, Grino M. Stimulation of adrenocorticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 1992;130:2725–2732. doi: 10.1210/endo.130.5.1315256. [DOI] [PubMed] [Google Scholar]
- 6.Lightman SL, Young WS. Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proc Natl Acad Sci USA. 1989;86:4306–4310. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu S, Pelletier G, Vaudry H, Barden N. Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence. Neuroendocrinology. 1989;49:255–261. doi: 10.1159/000125125. [DOI] [PubMed] [Google Scholar]
- 9.Tannahill LA, Sheward WJ, Robinson IC, Fink G. Corticotrophin-releasing factor-41, vasopressin and oxytocin release into hypophysial portal blood in the rat: effects of electrical stimulation of the hypothalamus, amygdala and hippocampus. J Endocrinol. 1991;129:99–107. doi: 10.1677/joe.0.1290099. [DOI] [PubMed] [Google Scholar]
- 10.Walker CD, Dallman MF. Neonatal facilitation of stress-induced adreno-corticotropin secretion by prior stress: evidence for increased central drive to the pituitary. Endocrinology. 1993;132:1101–1107. doi: 10.1210/endo.132.3.8382596. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 13.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 14.Harbuz MS, Rees RG, Eckland D, Jessop DS, Brewerton D, Lightman SL. Paradoxical responses of hypothalamic corticotropin-releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF-41 peptide and adenohypophysial proopiomelanocortin mRNA during chronic inflammatory stress. Endocrinology. 1992;130:1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- 15.Smith MA, Kim S-Y, Van Oers H, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- 16.Lightman SL, Harbuz MS. Expression of corticotropin-releasing factor mRNA in response to stress. Ciba Found Symp. 1993;172:173–189. doi: 10.1002/9780470514368.ch9. [DOI] [PubMed] [Google Scholar]
- 17.Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- 18.Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol. 1992;4:517–526. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 19.Jingami H, Matsukura S, Numa S, Imura H. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985;117:1314–1320. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- 20.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 21.Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky RM, Armanini MP, Packan DR, Sutton SW, Plotsky PM. Glucocorticoid feedback inhibition of adrenocorticotropic hormone secretagogue release. Relationship to corticosteroid receptor occupancy in various limbic sites. Neuroendocrinology. 1990;51:328–336. doi: 10.1159/000125357. [DOI] [PubMed] [Google Scholar]
- 23.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman JP, Wiegand SJ, Watson SJ. Regulation of basal corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid expression in the paraventricular nucleus: effects of selective hypothalamic deafferentations. Endocrinology. 1990;127:2408–2417. doi: 10.1210/endo-127-5-2408. [DOI] [PubMed] [Google Scholar]
- 25.Gilles EE, Guirguis C, Schultz L, Snodgrass SR, Baram TZ. Differential regulation of hypothalamic CRH mRNA by chronic and acute intermit- tent stress in infant rats. Soc Neurosci Abstr. 1996;26:201. [Google Scholar]
- 26.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary- adrenal components in the infant rat. Neurosci Lett. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 29.Scaccianoce S, Muscolo LA, Cigliana G, Navarra D, Nicolai R, Angelucci L. Evidence for a specific role of vasopressin in sustaining pituitary-adrenocortical stress response in the rat. Endocrinology. 1991;128:3138–3143. doi: 10.1210/endo-128-6-3138. [DOI] [PubMed] [Google Scholar]
- 30.de Goeij DC, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992;577:165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- 31.Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: a study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 32.Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas DA, George SR. Single or repeated mild stress increases synthesis and release of hypothalamic corticotropin-releasing factor. Brain Res. 1988;461:230–237. doi: 10.1016/0006-8993(88)90254-5. [DOI] [PubMed] [Google Scholar]
- 34.Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 35.Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 36.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 37.Baram TZ, Lerner SP. Ontogeny of corticotropin releasing hormone gene expression in rat hypothalamus—comparison with somatostatin. Int J Dev Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 38.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 39.Vale W, Vaughan J, Yamamoto G, Bruhn T, Douglas C, Dalton D, Rivier C, et al. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–577. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]