Abstract
Intracellular redox homeostasis is crucial for many cellular functions but accurate measurements of cellular compartment-specific redox states remain technically challenging. To better characterize redox control in the nucleus, we targeted a yellow fluorescent protein-based redox sensor (rxYFP) to the nucleus of the yeast S. cerevisiae. Parallel analyses of the redox state of nucleus-rxYFP and cytosol-rxYFP allow us to monitor distinctively dynamic glutathione (GSH) redox changes within these two compartments in a given condition. We observed that the nuclear GSH redox environment is highly reducing and similar to the cytosol under steady state conditions. Furthermore, these sensors are able to detect redox variations specific for their respective compartments in glutathione reductase (Glr1) and thioredoxin pathway (Trr1, Trx1, Trx2) mutants that have altered subcellular redox environments. Our mutant redox data provide in vivo evidence that glutathione and the thioredoxin redox system play distinct but overlapping functions in controlling subcellular redox environments. We also monitored the dynamic response of nucleus-rxYFP and cytosol-rxYFP to GSH depletion and to exogenous low and high doses of H2O2 bursts. These observations indicate a rapid and almost simultaneous oxidation of both nucleus-rxYFP and cytosol-rxYFP, highlighting the robustness of the rxYFP sensors in measuring real-time compartmental redox changes. Taken together, our data suggest that the highly reduced yeast nuclear and cytosolic redox states are maintained independently to some extent and under distinct but subtle redox regulation. Nucleus- and cytosol- rxYFP register compartment-specific localized redox fluctuations that may involve exchange of reduced and/or oxidized glutathione between these two compartments. Finally, we confirmed that GSH depletion has profound effects on mitochondrial genome stability but little effect on nuclear genome stability, thereby emphasizing that the critical requirement for GSH during growth is linked to a mitochondria-dependent process.
Keywords: redox-sensitive sensor, redox, glutathione, yeast
Introduction
Redox homeostasis by multiple dynamic equilibrium adjustments and regulation mechanisms is crucial for many cellular functions, including the proper folding of proteins and maintenance of their activity. Numerous studies in recent years have established that defective redox homeostasis and reactive oxygen species (ROS) are implicated in ageing, neurodegenerative diseases and cancer [1]. Eukaryotic cells have two principle thiol redox control pathways that have been most extensively characterized in the yeast S. cerevisiae: the glutathione redox system that includes the most abundant non-protein tripeptide glutathione (γ-glutamylcysteinylglycine, GSH), glutaredoxins (Grxs) and glutathione reductase (Glr), and the thioredoxin redox system comprising thioredoxin (Trx) and thioredoxin reductase (Trr) [2,3]. GSH, with its relatively low redox potential (−240 mV) and high intracellular abundance (1–13 mM), in equilibrium with its oxidized disulfide form GSSG [4], is often used as a gauge of the cellular redox environment. In S. cerevisiae, GSH biosynthesis occurs in the cytosol via two ATP-dependent steps catalyzed by γ-glutamylcysteine synthetase encoded by GSH1 [5], and glutathione synthetase encoded by GSH2 [6]. Once synthesized in the cytosol, its distribution, availability and redox states in different organelles, including mitochondria, nucleus, and endoplasmic reticulum, further depend on a poorly understood equilibrium between transport, utilization, relative reduction rates of GSSG by Glr, degradation, and excretion. In addition to the GSH-Grx system. S. cerevisiae contains two cytoplasmic Trxs encoded by TRX1 and TRX2 [3]. Thioredoxin reductase encoded by TRR1 directly reduces oxidized Trxs. Although the Trxs and Grxs are similar in structure and have overlapping functions, they are regulated in a different manner [3]. The oxidized, disulfide form of Trx is reduced directly by Trr using NADPH as the electron donor, whereas Grx is reduced by GSH forming glutathione disulfide (GSSG), which is in turn reduced by Glr using electrons donated by NADPH.
Given the importance of thiol redox homeostasis in cellular function, it is useful to monitor the concentration of oxidized and reduced species from a main redox couple such as cellular GSH/GSSG. However, informative measurements of cellular GSH/GSSG redox states remain technically challenging. While the overall cellular GSH and GSSG concentrations can be determined by several conventional methods, their concentrations in an individual compartment remain difficult to estimate. The redox state of GSH as well as the absolute concentration varies from one compartment to another and conventional methods fail to reliably measure these subcellular variations. GSH/GSSG measurements taken via nuclear fractionation essentially disturb cellular as well as organelle integrity resulting in loss of metabolites and undesired changes in redox state, thereby making estimations prone to artefacts. On the other hand, use of redox-active fluorescent dyes has given conflicting results due to a number of factors, including a lack of specificity for the GSH/GSSG couple, irreversibility that prevents measurement of dynamic redox variations, and a lack of compartment specificity [7]. Nevertheless, given the importance of redox pathways operating in the nucleus it is imperative to define the nuclear redox environment and its regulation.
Genetically encoded biosensors may provide an alternative way to overcome the limitations of conventional GSH/GSSG redox measurements [8]. Østergaard and coworkers have developed redox-sensitive yellow fluorescent protein (rxYFP) by inserting an artificial dithiol-disulfide pair in the YFP structure [9]. Both in yeast cells and in vitro, the cysteines in rxYFP specifically equilibrate with GSH and GSSG over a wide range of pH values via rapid disulfide exchange reactions with the cytosolic Grxs. In contrast, the two cytosolic Trxs are unable to undergo thiol-disulfide exchange with this sensor. The ratio of oxidized to reduced rxYFP, that can be assessed via non-reducing SDS-PAGE in which the two forms have different electrophoretic mobilities, can be used to generate an in vivo readout of the GSH:GSSG redox state. Their measurements indicated that the S. cerevisiae cytosol has a redox potential of −289 mV which is considerably more reducing than whole cell redox measurements (−221 to −236 mV) [10]. More recently, exclusive targeting of rxYFP to the S. cerevisiae mitochondrial intermembrane space (IMS) and mitochondrial matrix, respectively, showed that the IMS steady-state GSH/GSSG redox state is considerably more oxidized than the cytosol or matrix, and that IMS GSH/GSSG redox control is maintained independently from the cytosol and matrix [11]. These recent data highlight the importance of examining subcellular compartments separately.
In an effort to precisely characterize the nuclear GSH/GSSG redox environment and its regulation, we have targeted the rxYFP sensor to the nucleus of the yeast S. cerevisiae and compared the GSH/GSSG redox potential differences with the cytosol. We demonstrate exclusive targeting of nucleus-rxYFP to the nucleus and its dynamic response to an exogenous oxidant and reductant. Redox potential measurements using the nucleus-targeted rxYFP sensor reveal that the nuclear redox environment is highly reducing and similar to the cytosol under steady-state conditions. Furthermore, we tested the specificity and ability of this sensor to register nucleus-specific, localized redox fluctuations using GSH and Trx pathway mutants that have altered subcellular redox environments. Subsequently we evaluated the ability of these probes to sense GSH/GSSG redox environment changes during cellular GSH depletion and in response to mild and acute doses of exogenous H2O2. Finally, we observed and confirmed that GSH depletion has a profound effect on mitochondrial genome stability, while GSH depletion has little effect on nuclear genome stability.
Materials and Methods
Yeast strains, media, and growth conditions
Most strains used in this study are isogenic to the S288c-based parental strain RDKY3615 MATa, ura3–52, leu2Δ1, trp1Δ63, his3Δ200, lys2ΔBgl, hom3–10, ade2Δ1, ade8, hxt13::URA3 [12], except those specified, which are W303 derivatives. Gene replacements were made using standard PCR-based homology-directed methods. Double or triple mutations were usually constructed by crossing haploids of opposite mating types that carry the desired mutations followed by dissection of tetrads. Genotypes of spore clones were determined by replica plating, assaying for the markers used to delete the genes. S. cerevisiae cells were grown in standard media including yeast extract peptone dextrose medium (YPD) or synthetic complete medium (SC) lacking appropriate amino acids as indicated. Canavanine resistant mutants (Canr) created by inactivation of the CAN1 gene were selected on SC–Arginine dropout plates containing 60 mg/l canavanine. The S. cerevisiae strains expressing red fluorescent protein (mRFP) -tagged NAB2 was kindly provided by Dr. Palancade [13].
Plasmids
The plasmid carrying NUP49-mCherryFP was kindly provided by Dr. Palancade [14]. The original plasmid pJH208 expressing cytosol-rxYFP was previously described [11]. The plasmid MEHP97 expressing cytosol-rxYFP used in this study was constructed by digesting the plasmid pJH208 with NotI and SacII. The NotI-SacII-digested fragment containing the PGK1 promoter, the coding sequence of rxYFP, and the TDH3 terminator was then inserted into the NotI-SacII-cut LEU2-CEN vector pRS315 to create MEHP97. The nucleus-rxYFP plasmid MEHP170 was constructed as follows. The rxYFP coding region was amplified from the plasmid pJH208 with the following primers: 5′-CCCGTCATATGTCTAAAGGTGAAGAACTGTTTAC-3′, which contains a NdeI site (underlined), and 5′-CGGTCACGCGTTTAAACCTTTCTCTTCTTCTTTGGAACCTTTCTCTTCTTCTTTGGAACCTTTCTCTTCTTCTTTGGTTTATACAGCTCATGCATGC-3′ which contains a MluI site (in bold), a stop codon (in bold and underlined) and three copies of nucleus targeting sequence of SV40 (underlined). The PCR product, fusing three copies of the NLS in frame to the 3′ end of rxYFP was then digested with NdeI and MluI, and ligated to the backbone of NdeI-MluI-cut MEHP97 plasmid DNA, thereby changing the rxYFP to nucleus targeting rxYFP, designated MEHP170.
Fluorescence microscopy
The strain transformed with MEHP170 (nucleus-rxYFP) was grown to mid-log phase in selecting SC-Leu medium. For live cell imaging, exponentially growing cells were washed with PBS, resuspended in the mounting solution (75% glycerol in PBS) and mounted on a glass slide covered with polylysine (Sigma). When necessary, 4′,6-diamidino-2-phenylindole (DAPI) was added to the mounting solution to a final concentration of 50 μg/ml. Fluorescent and phase contrast images were captured with a Leica microscope (DMRXA) equipped with a cooled CCD camera MicroMAX (Princeton Instruments) under control of the MetaMorph software (Molecular Devices). Images obtained were processed using ImageJ software.
Isolation of nuclei and cytosol from whole yeast cells
The strain transformed with MEHP170 (nucleus-rxYFP) or MEHP97 (cytosol-rxYFP) was grown to mid-log phase in selecting SC-Leu medium. Nuclei containing subcellular fraction was prepared as described in [15] and cytosolic fraction was obtained as described in [16]. Protein concentrations in the fractions were measured by Quant-iT protein assay kit with the Qubit fluorometer (Invitrogen). Each sample in reduced condition was then subjected to electrophoresis on a 12% NuPAGE Novex Bis-Tris gel with MOPS SDS buffer (Invitrogen). After transfer to a nitrocellulose membrane, appropriate portion of membrane was probed with anti-Pgk1 (Invitrogen), anti-Nop1 (Santa Cruz) or anti-GFP (Invitrogen) antibodies.
Redox western blot
Redox Western blot analysis of rxYFP was adapted from previously described methods [11] with slight modifications. In general, 15 ml of cell cultures at mid-logarithmic phase in selecting SC-Leu medium were acid-quenched with 20% trichloroacetic acid (TCA, Sigma) at 4 °C. Cells were harvested and resuspended in 1 ml of 20% TCA. The resuspended cells were pelleted by centrifugation at 4000 g for 10 min at 4°C and the pellet was immediately frozen in liquid nitrogen and then kept at −80°C. For protein extraction, the cell pellet was resuspended in 400 μl of 20% TCA and lysed with glass beads (200 μl) by vortexing for 5 min at 4°C. The lysed cells were centrifuged and the pellet was washed with cold acetone. The dry pellet was resuspended in 90 μl of TES buffer (100 mM Tris Cl pH 8.8, 10 mM EDTA, 1% SDS) containing 50 mM N-ethylmaleimide (Sigma). Following a 60-min incubation at 30 °C with agitation in the dark, insoluble protein was removed by centrifugation at 13000 g for 10 min at room temperature. Each sample containing about 40 μg of protein was prepared with NuPAGE LDS sample buffer and was separated on a 12% NuPAGE Novex Bis-Tris gel with MOPS SDS buffer (Invitrogen). After transfer to a nitrocellulose membrane, reduced and oxidized forms of rxYFP were probed with a rabbit anti-GFP antibody (Invitrogen) and a secondary anti-rabbit IgG (IRDye, LI-COR) and then analyzed by quantitative immunoblot using an Odyssey Infrared Imaging System (LI-COR). The statistical significance was calculated by the Student’s t-test.
GSH/GSSG assays
Total glutathione (GSH + GSSG), GSH and GSSG in yeast extracts were measured by the 5,5′-dithiobis-2-nitrobenzoic acid (DTNB)-GSSG reductase cycling assay and spectrophotometric analysis of thionitrobenzene (TNB) formation as previously described [17]. The lysate from ~2 × 108 cells grown in SC medium was used to determine total glutathione and GSSG. For GSSG measurements, cell lysates were treated with 2-vinylpyridine (Sigma) prior to the assay, which covalently reacts with GSH (but not GSSG). GSH in the sample was calculated by subtraction of total glutathione with GSSG. The glutathione quantity (total glutathione, GSH or GSSG) for each cell (Q) is estimated by dividing the measured quantity by cell number used in the assay. To estimate intracellular glutathione concentration, cell volumes (V) were estimated from cell major (m) and minor (n) axes using the formula: V = πm2n/6 [18,19]. Cell dimensions were determined microscopically at ×100 magnification using ImageJ. For each strain or condition, at least 100 cells were measured and the average cell volume determined. Total glutathione, GSH or GSSG concentration was obtained by Q/V.
Redox potential calculations
The rxYFP sensor equilibrates with GSH:GSSG pools. Measurement of intracellular concentrations of [GSH] and [GSSG], or the ratio of reduced to oxidized rxYFP have been used to estimate the redox potential using the Nernst equation [9,11]: EGSH = E°′GSH − (60.1 mV/2) log[GSH]2/[GSSG] = E°′rxYFP − (60.1 mV/2) log(rxYFPred)/(rxYFP°x) = ErxYFP (Equation 1). Redox potential E values are expressed in mV. E°′GSH is the standard potential for reduced glutathione, which is −240 mV at pH 7. E°′rxYFP is the standard reduction potential of rxYFP which is −265 mV at pH 7. In this equation, [GSH] enters as a squared term, meaning that the reduction potential is dependent on the GSH/GSSG ratio and the absolute concentration of GSH, while ErxYFP is dependent on the ratio of reduced:oxidized rxYFP but not the absolute concentration. The redox potential of GSH and rxYFP at different pH values were calculated according to the equation: EpH = E°′ + [(pH − 7.0) × −60.1] mV (Equation 2)[4]. In this equation, E°′ is the standard redox potential of GSH or rxYFP at 7.0.
Measurement of mitochondrial DNA mutation and nuclear DNA mutation frequency
The gsh1 strains were inoculated into SC medium containing 500 μM GSH for overnight growth. These preculture cells were diluted to an appropriate density in SC without GSH and cultured for 8 h. The cultures were diluted again and further cultured for another 16 h in SC without GSH. After 24 h GSH depletion, the aliquots of the culture were further diluted and set to inoculate respectively into SC medium without GSH or with indicated concentrations of GSH for additional 24 h. In parallel as a control, one aliquot of preculture was maintained in SC medium containing 500 μM GSH. For measurement of mitochondrial DNA mutation, aliquots of culture were removed at times 0, 8, 24 and 48 h after GSH depletion and plated onto YPD plates. The total number of colonies was counted after a 3-day incubation at 30 °C and respiratory incompetency (petite mutations) was revealed by triphenyl tetrazolium chloride coloration [20]. The frequency of petite mutations was calculated as the number of respiratory incompetent white colonies divided by the total number of colony-forming units (white + red) on YPD plates. For measurement of nuclear DNA mutation frequency, cells removed from the defined culture conditions were plated onto YPD or on SC–Arg dropout plates containing canavanine (for Canr mutations events) to determine the total number of viable cells and the number of mutants, respectively. Canr mutation frequency was presented as the number of Canr mutants in 106 viable cells.
Results
Targeting rxYFP to the nucleus
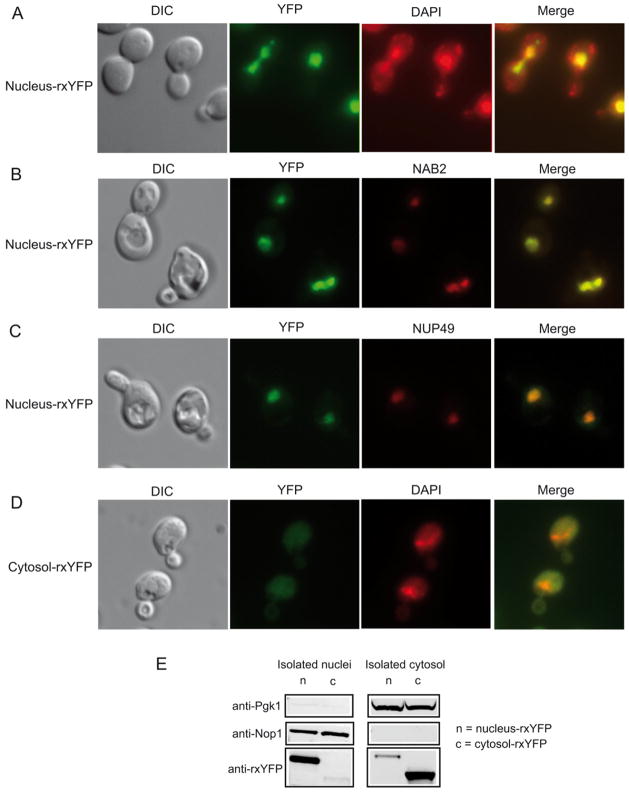
A nucleus-targeted version of rxYFP was created by fusing 3 copies of the nuclear targeting signal of SV40 to the C-terminus of rxYFP in the centromeric vector pRS315. Localization and fluorescence signal of the nucleus-rxYFP sensors were analyzed by fluorescence microscopy. Live yeast cells expressing nucleus-rxYFP were stained with DAPI that reveals both mitochondria (string-like structures) and the nucleus (large and round structure). In all the cells inspected, nucleus-rxYFP co-localized with DAPI staining of the nuclear DNA (Figure 1A). To further confirm the nuclear localization of nucleus-YFP, we transformed nucleus-rxYFP in a NAB2-mRFP strain in which the gene encoding nuclear abundant poly(A) RNA-binding protein 2 (Nab2) is fused with mRFP [13]. Nab2p localizes to the nucleus at steady state. As shown in Figure 1B, Nab2-mRFP localization was homogeneous throughout the whole nuclear region and nucleus-YFP indeed co-localized with Nab2-RFP. We also transformed a plasmid expressing Nup49-mCherryFP fusion protein into a strain carrying nucleus-rxYFP. Nup49 is part of the nuclear pore complex and Nup49-mCherryFP reveals the nuclear periphery [14], allowing us to monitor the relative position of the nucleus-rxYFP with respect to the nucleus. As shown in Figure 1C, nucleus-rxYFP was exclusively located within the compartment limited by nuclear envelope. These experiments demonstrate that nucleus-YFP sensor is localized in the nucleus and is correctly folded with the expected fluorescence properties. In all the following experiments, the nuclear localisation of nucleus-rxYFP was monitored.
Figure 1.
Localization of nucleus-rxYFP. (A) Wild-type yeast cells expressing nucleus-rxYFP and stained by DAPI were analyzed by fluorescence microscopy. Differential interference contrast (DIC) image and overlay image of YFP and DAPI signal (merge) are also shown. (B) NAB2-mRFP strain transformed with a nucleus-rxYFP expressing plasmid. (C) Wild-type yeast cells expressing nucleus-rxYFP and Nup49-mCherry were also analyzed for localization of YFP relative to the nuclear marker. (D) Wild-type yeast cells expressing cytosol-rxYFP and stained by DAPI were analyzed by DIC and fluorescence microscopy. (E) Nuclei or cytosol from intact cells were prepared (Materials and Methods) and subjected to Western blot analysis using antibodies directed against Pgk1 (cytosol marker), Nop1 (nucleus marker) and rxYFP. In the right panel, isolated nuclei (40 μg of protein) from cells expressing nucleus-rxYFP (n) or cytosol-rxYFP (c) was prepared and analyzed. The same blot was cut and blotted for indicated antibody. In the right panel, cytosol portion (40 μg of protein) from cells expressing nucleus-rxYFP (n) or cytosol-rxYFP (c) was prepared and analyzed. The same blot was cut and blotted for indicated antibody.
Localization and fluorescence signal of cytosol-rxYFP, expressed from a centromeric vector, were also verified by fluorescence microscopy. This sensor was found to be homogenously localized in the cytosol as previously reported (Figure 1D) [11], in contrast to the nuclear localization of nucleus-rxYFP (Figure 1A–C).
To confirm the correct localization of the nucleus-rxYFP and compare to cytosol-rxYFP, we conducted a Western blot analysis of isolated nucleus and cytosolic fractions from WT strains transformed with nucleus- or cytosol-rxYFP. As shown in Figure 1E, distribution of cytoplasmic protein Pgk1 and nucleolar protein Nop1 demonstrated the equal loading and high purity of nuclear fractions from two strains expressing respectively nucleus-rxYFP and cytosol-rxYFP (Figure 1E, left panel), and the equal loading and purity of cytosol fractions from these two strains (Figure 1E, right panel). Western blotting using anti-GFP revealed that the nucleus-rxYFP was found predominantly in the nuclei preparation, in contrast to cytosol-rxYFP that was found predominantly in cytosol preparation.
Nucleus-rxYFP senses the nuclear glutathione redox status and responds to oxidant and reductant treatment
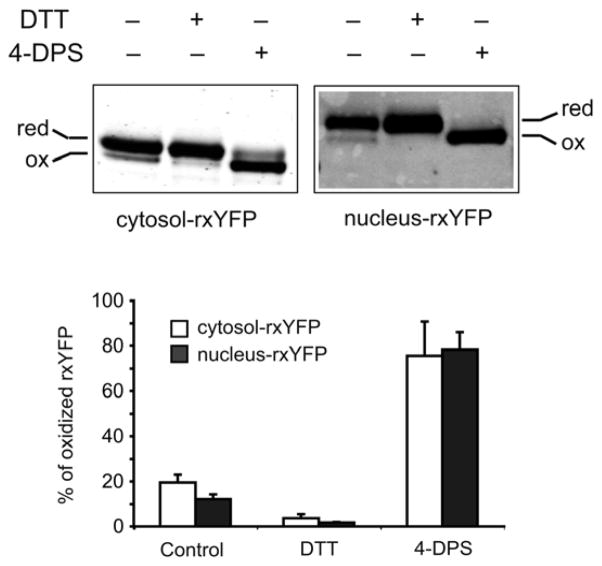
In order to separately measure the nuclear and cytosolic GSH/GSSG redox state, rxYFP expressed from nucleus- or cytosol-rxYFP constructs was monitored by redox Western blots. As shown in Figure 2 and Table 1, cytosol-rxYFP was ~19% oxidized under steady-state conditions in wild-type cells (S288c), which is similar to published results [9,11], whereas nucleus-rxYFP was ~12% oxidized. Nucleus-rxYFP is, at most, slightly more reduced than cytosol-rxYFP, but without statistical significance (P > 0.05). We tested the reactivity and response of targeted nucleus-rxYFP in comparison to cytosol-rxYFP using the strong thiol-oxidant 4,4′-dithiodipyridine (4-DPS, Sigma) or the disulfide reductant dithiothreitol (DTT, Sigma). Treatment of the cells with 4-DPS (180 μM final concentration) or DTT (50 mM final concentration) for 20 min shifted the majority of nucleus- or cytosol-rxYFP toward the oxidized or reduced state, respectively (Figure 2). Therefore, nucleus-rxYFP constructs are responsive to redox changes generated by exogenous reagents, similar to cytosol-rxYFP [11].
Figure 2.
Nucleus-rxYFP senses the nuclear GSH/GSSG redox status and responds to oxidant and reductant treatment. Wild-type yeast cells expressing cytosol- or nucleus-rxYFP were treated with 180 μM 4-DPS or 50 mM DTT for 20 min and collected for redox Western blot analysis. Reduced (red) and oxidized (ox) forms of rxYFP were quantified using an Odyssey Infrared Imaging System. Two representative blots are shown. The reported values are the mean ± SD of at least three independent experiments.
Table 1.
Glutathione concentrations and redox potential measurements in the whole cell, nucleus and cytosol.
| Strain | Whole cell measurement | Nucleus | Cytosol | ||||
|---|---|---|---|---|---|---|---|
| GSH (mM)a | GSSG (mM)a | EGSH (mV) | oxidized rxYFPb | ErxYFP (mV) | oxidized rxYFPb | ErxYFP (mV) | |
| Wild-type (S288c) | 4.1 ± 0.3 | 0.052 ± 0.004 | −225 | 12 ± 4 | −291 | 19 ± 7 | −284 |
| Wild-type (W303) | 2.8 ± 0.1 | 0.022 ± 0.004 | −226 | 6 ± 1 | −301 | 8 ± 3 | −297 |
| glr1 (W303) | 4.7 ± 1.3 | 0.6 ± 0.2 | −196 | 53 ± 8 | −263 | 57 ± 5 | −261 |
| trr1 (W303) | 9.3 ± 0.6 | 1.2 ± 0.2 | −206 | 29 ± 5 | −277 | 28 ± 8 | −277 |
| trx1 trx2 (W303) | 3.7 ± 0.3 | 0.57 ± 0.03 | −191 | 19 ± 5 | −284 | 29 ± 3 | −277 |
| trr1 trx1 trx2 (W303) | 6.6 ± 2.6 | 0.45 ± 0.05 | −209 | 17 ± 2 | −284 | 31 ± 7 | −275 |
The reported values are the mean ± SD of three independent experiments.
The reported values are the mean ± SD for 3–7 independent experiments.
Total intracellular GSH and GSSG were measured at 4.07 mM and 0.052 mM, respectively (Table 1). Assuming an average intracellular pH value of 7.0 [21], the whole cell redox potential EGSH was calculated from the Nernst equation (Equation 1, see Materials and Methods) by inserting the standard reduction potential of GSH at pH 7.0 (E°′GSH = −240 mV) along with the absolute concentrations [GSH] and [GSSG]. This yields the value −225 mV, which is similar to a previous report [10]. The ratio of reduced-to-oxidized rxYFP was also used to generate redox potentials of the nucleus and cytosol. The yeast cytosolic pH measurements for glucose-grown cells range from 6.8 to 7.2 [21–24], and to the best of our knowledge, the precise pH of the nucleus is not known. For both S. cerevisiae and human cells, most authors assume that the nuclear pH equals that of the cytosol [21,25], so pH 7.0 was used to calculate the redox potential values for both nucleus and cytosol. With the standard reduction potential of rxYFP (E°′rxYFP) which is −265 mV at pH 7, and ratio of reduced-to-oxidized rxYFP, the nuclear redox potential calculated from the Equation 1 was −291 mV, whereas the cytosolic redox potential corresponded to −284 mV. Therefore, we conclude that the nuclear GSH redox environment is highly reduced and comparable to the cytosol. It is important to note that thiol-disulfide redox reactions are pH-dependent. If the pH were higher or lower than 7.0, the calculated redox potentials of GSH or rxYFP would accordingly decrease or increase by 6.0 mV for every 0.1 unit change in pH (Equation 2, see Materials and Methods).
Nucleus-rxYFP senses the subcellular redox status in mutants with deficient GSH or Trx redox systems
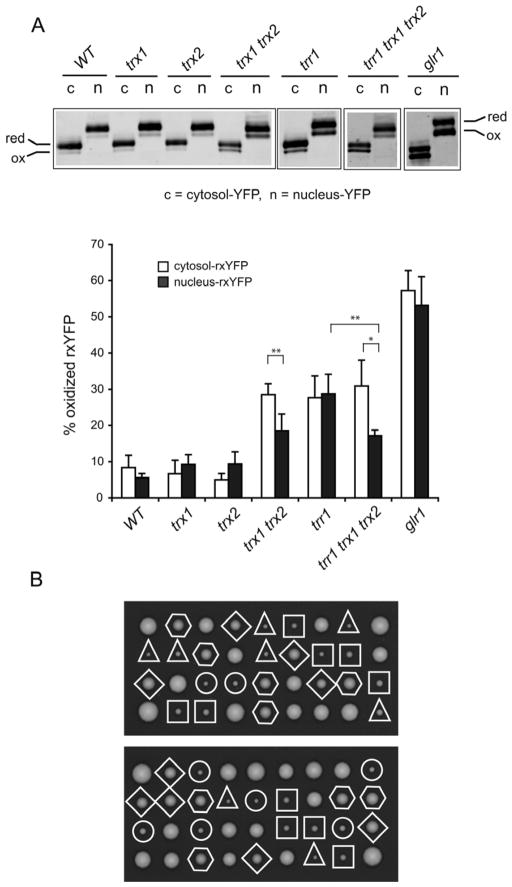
In order to determine whether nucleus-rxYFP is responsive to in vivo redox changes and how the nuclear redox state is affected by deficient GSH or Trx redox systems, we compared the redox state of nucleus- and cytosol-rxYFP in glr1, trx1, trx2, trx1 trx2, trr1 and trr1 trx1 trx2 mutants. Because a trr1 deletion is lethal in the S228c background but viable in the W303 background, we chose to perform this set of analyses in W303-based isogenic strains. In the wild-type cells, cytosol-rxYFP was ~8.4% oxidized under steady-state conditions, whereas nucleus-rxYFP was ~5.6% oxidized (Figure 3A, Table 1). Again as in strain S288c, these differences between the cytosol and nucleus redox states in W303 wild type background were also found to be statistically insignificant upon replicate analysis (P > 0.05). The redox state varied slightly between the S288c- and W303-based wild-type strains, with W303-based cells exhibiting a slightly more reducing state in both the cytosol and nucleus. Variation of GSH/GSSG redox potentials for several wild-type yeast strains has been previously reported [26].
Figure 3.
rxYFP redox response in various mutant cells (W303 background) affecting GSH or TRX redox systems and effects of deletions of thioredoxin genes on the growth of trr1 mutants. (A) Representative redox Western blots of the samples are shown. Percentage of oxidized rxYFP was quantified using an Odyssey Infrared Imaging System. The reported values are the mean ± SD for 3–7 independent experiments. *P < 0.05, **P < 0.01. (B) Tetrads from diploids heterozygous for trr1, trx1 and trx2 were dissected and analyzed for the presence of selection or auxotrophic markers (trr1::kanMX4, trx1::HIS3, and trx2::URA3). Circles indicate trr1 single mutants; triangles indicate trr1 trx1 mutants; squares indicate trr1 trx2 mutants; diamonds indicate trr1 trx1 trx2 mutants; hexagons indicate trx1 trx2 mutants.
In glr1 mutant cells, as previously demonstrated [11], cytosol-rxYFP underwent dramatic redox changes (~57% oxidized) compared with isogenic wild-type cells (~8% oxidized) (Figure 3A, Table 1). There was also a similar shift (~53%) in the percent of oxidized nucleus-rxYFP upon deletion of the GLR1 gene. Glr1, which is responsible for regenerating GSH from GSSG, is localized to the cytosol and mitochondrial matrix, therefore deletion of this gene would have direct consequences for both of these compartments, while the effects on the nucleus are unclear. However, the difference in the oxidation state for nucleus-rxYFP and cytosol-rxYFP in glr1 cells was not significant (P > 0.05). Loss of Trr1 also shifted GSH/GSSG redox balance to a more oxidized state with both cytosol- and nucleus-rxYFP ~30% oxidized (Figure 3A, Table 1). Loss of Trx1 or Trx2 did not remarkably affect the cytosolic and nuclear redox state. Interestingly, a synergistic effect was observed in the double mutant trx1 trx2 compared with each single mutant, demonstrating that the Trxs have overlapping roles in redox homeostasis (Figure 3A), consistent with previous findings [26]. Furthermore, cytosol-rxYFP is significantly more oxidized in the trx1 trx2 mutant (~29%) than nucleus-rxYFP (~19%) (P < 0.01). Intriguingly, while cytosol-rxYFP oxidation was similar in trr1 and trx1 trx2 (~29%) mutants, the nucleus-rxYFP was significantly less oxidized in the latter mutant (~30% versus ~19% respectively, P < 0.01). We reasoned that this could be attributed to the presence of oxidized thioredoxins that would accumulate as a result of TRR1 inactivation. In this regard we measured the rxYFP redox state in a trr1 trx1 trx2 triple mutant. Expectedly, the trr1 trx1 trx2 triple mutant displayed a similar redox profile as the trx1 trx2 double mutant (Figure 3A), suggesting that indeed the more oxidized rxYFP state observed in the nucleus of trr1 cells depends on the presence of oxidized Trx1 and Trx2. Further, the differences in the nuclear and cytosolic redox states in trx1 trx2 and trr1 trx1 trx2 mutants indicate that the nuclear redox may be regulated independently of the cytoplasm to some extent.
Since the trr1 single mutant exhibited a severe growth defect, we tested if mutations in TRX1 and TRX2 genes could also suppress this growth defect phenotype similar to the restoration of redox state. For this purpose, we analyzed the meiotic products obtained from diploids that were heterozygous for trr1, trx1 and trx2 deletions. As shown in Figure 3B, additional deletion of TRX1 or TRX2 could not improve the growth of trr1 mutants, as judged by colony size. In contrast, simultaneous deletion of TRX1 and TRX2 suppressed the growth defects of trr1 mutants. Colonies formed by trr1 trx1 trx2 mutants were of the same size as those formed by the trx1 trx2 mutants. These observations indicated that the severe growth defect of trr1 mutants is caused by the presence of oxidized Trx1 and Trx2 that probably leads to the strong redox deregulation in the nucleus as revealed by rxYFP redox analysis.
To compare the targeted rxYFP redox potential measurements with whole cell GSH and GSSG levels, total intracellular GSH and GSSG were measured in isogenic W303-based wild-type, glr1, trr1, trx1 trx2 and trr1 trx1 trx2 mutants and GSH redox potentials (EGSH) were determined (Table 1). In glr1, trr1, trx1 trx2 and trr1 trx1 trx2 mutants, whole cell GSSG levels were dramatically elevated (20–55 fold), while GSH levels were similar or only slightly increased compared to the wild-type strain. Whole cell redox potentials (EGSH) were calculated from the Nernst equation (see Materials and Methods) using the absolute concentrations [GSH] and [GSSG] and the standard reduction potential of GSH (−240 mV) [4], giving an EGSH value of −226 mV for W303-based wild-type cells and from −209 mV to −191 mV for the four mutants. The EGSH values were higher in all these mutants, indicating a more oxidizing redox environment compared to wild-type cells. TRR1 deletion had the most pronounced effect on elevation of both GSH and GSSG and this effect is dependent on the presence of Trx1 and Trx2 (Table 1). Both GSH/GSSG quantification and the rxYFP data (above) suggest that the significantly oxidized nuclear GSH redox environment in the trr1 mutant is partially due to the presence of the Trx1 and Trx2. The localized cytosolic and nuclear redox potentials (ErxYFP) of wild-type and mutant strains were also calculated by inserting the ratio of reduced-to-oxidized nucleus- and cytosol-rxYFP into the Nernst equation using the standard reduction potential of rxYFP (E°′rxYFP = −265 mV at pH 7) (Table 1). Globally, the nuclear and cytosolic GSH/GSSG redox environments in these mutants are substantially more oxidized compared to wild-type. Additionally, we note that the nuclear and cytosolic GSH/GSSG redox potential estimated from nucleus- and cytosol-rxYFP (ErxYFP) is considerably more reducing (lower) than the estimated GSH/GSSG redox potential (EGSH) obtained by overall cellular GSH and GSSG measurements (Table 1), demonstrating the utility of targeted, genetically-encoded redox sensors in providing more accurate, compartmentalized redox measurements.
Dynamic and concurrent oxidation of nucleus- and cytosol- rxYFP during GSH depletion
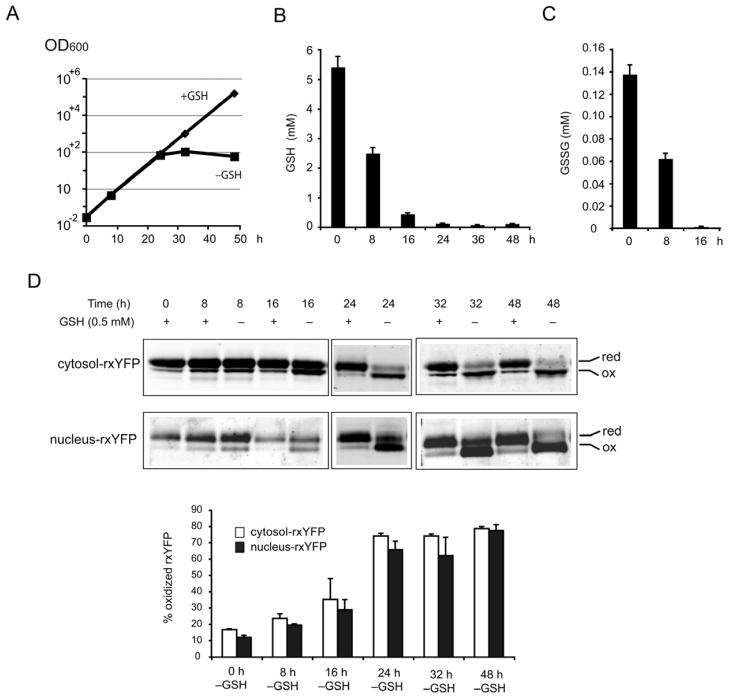
Given that GSH plays key roles in subcellular thiol redox control, ROS detoxification, and iron homeostasis [4,27,28], it is important to determine the compartment-specific redox changes during GSH depletion. The effects of GSH depletion in the gsh1 mutant, which is defective in GSH synthesis, on the cytoplasmic, mitochondrial matrix and IMS redox state were recently described using the redox-sensitive green fluorescent protein (roGFP) [29]. However, it is not known whether GSH depletion influences the nuclear redox state. Moreover, a recent study using chemical GSH-depleting agents buthionine sulfoximine (BSO) or diethyl maleate (DEM) on mammalian cells indicated that nuclear GSH pools resist depletion as compared to cytosolic pools, thereby suggesting that nuclear GSH pools might be critical for growth [30]. We decided to address these issues using rxYFP probes and an alternative genetic strategy to deplete GSH. Growth and survival of gsh1 strains are dependent on availability of exogenous GSH [31], therefore depletion of GSH in gsh1 strains leads to a growth arrest after a substantial number of cell divisions. We concurrently analyzed cell growth, intracellular GSH and GSSG concentrations, and cytosol- and nucleus-rxYFP redox changes at defined time points (0, 8, 16, 24, 32 and 48 h) during GSH depletion in gsh1 cells. As shown in Figure 4A, 4B and 4C, in the first 24 h after onset of depletion, gsh1 cells in medium lacking GSH grow at a similar rate as the culture in medium supplemented with GSH, although intracellular GSH and GSSG decreased drastically. After about ~ 8–10 divisions in the first 24 h, the growth stalled. At this point, GSH was estimated to be 0.136 mM and GSSG was not detectable in our assay. These limited divisions probably reflect the consumption of GSH accumulated during preculture in GSH-containing medium. As expected, at time 0, the redox state of cytosolic- and nucleus-rxYFP in gsh1 cells was similar to wild-type cells and remained reduced over the 48h experimental period when grown in medium supplemented with GSH. A modest oxidation of cytosolic- and nucleus-rxYFP was detectable 8 h after onset of depletion (Figure 4D). The oxidized form reached the maximum (60% – 70%) at 24 h, a time point corresponding to the cessation of growth of gsh1 cells (Figure 4A). Cytosolic- and nucleus-rxYFP remained highly oxidized during the subsequent time points of analysis. The rxYFP oxidation at the 24-h time point likely reflects the detection limit of the probe, hence it is quite possible that both nuclear and cytosol GSH/GSSG redox environments are even more oxidizing than what is being measured in gsh1 cells at later time points (24 h and after) during GSH depletion. It is also to note that the redox measurements at later time points (24 h and after) might be less reliable due to the fact that the redox probes may be influenced by other redox systems or protein thiols themselves that provide the cellular redox buffering capacity under these conditions [32], and the remaining little GSH may not be in equilibrium with the redox probes. Nevertheless, the fact that the time course of cytosolic and nuclear rxYFP oxidation during the first 16 h of GSH depletion was indistinguishable, suggests dynamic and concomitant changes in cytosolic and nuclear redox environment during GSH depletion. It appears that once the intracellular GSH concentration decreases below a critical threshold and/or the intracellular redox potential increases to a critical level by 24 h after depletion, gsh1 cells stop growing.
Figure 4.
Dynamic subcellular redox changes of gsh1 cells during GSH depletion. The gsh1 cells expressing the rxYFP sensors were inoculated into SC–Leu medium containing 500 μM GSH for overnight growth. These preculture cells were diluted to an appropriate density in SC–Leu with or without 500 μM GSH. During the 48 h time course, cultures were diluted when necessary to maintain exponential growth. (A) Cell density (OD600) of gsh1 cells was monitored at 0, 8, 24, 32 or 48 h after onset of GSH depletion. (B) and (C) Time course of intracellular GSH (B) and GSSG (C) concentrations during GSH depletion. Samples were removed 0, 8, 24, 32 and 48 h after onset of GSH depletion and prepared for GSH and GSSG quantification. (D) Redox Western blot of the samples removed from two parallel cultures, with or without GSH, at 0, 8, 24, 32 and 48 h after onset of GSH depletion. Percentage of oxidized forms of rxYFP was quantified using an Odyssey Infrared Imaging System. In A–D, the reported values are the means ± SD for two independent experiments.
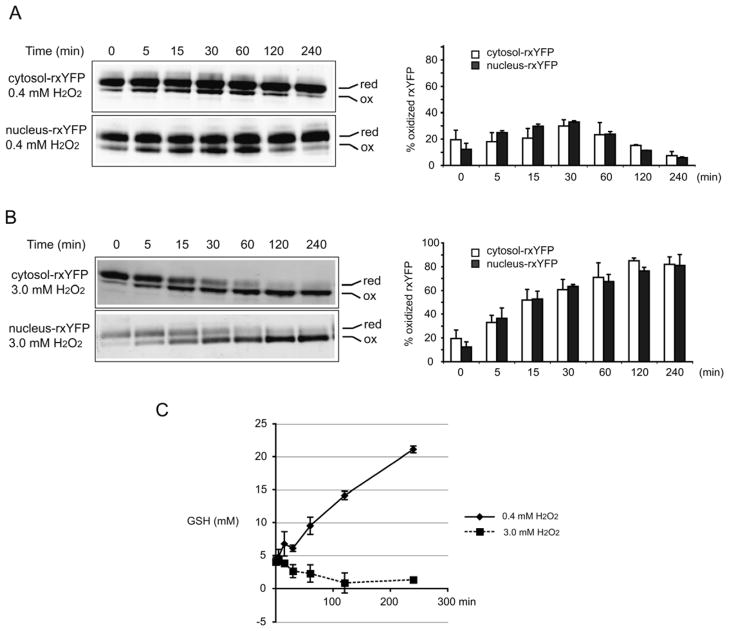
The rxYFP sensors register real-time dynamic subcellular redox changes in response to hydrogen peroxide (H2O2) bursts
Exogenous H2O2 is largely used as an oxidant to induce intracellular oxidative stress and cell death. To understand the dynamics of cytosolic and nuclear redox changes during an exogenous H2O2 burst, a time-course analysis of cytosol- and nucleus-rxYFP, GSH and GSSG concentrations, and corresponding EGSH was performed in wild-type cells expressing cytosol- or nucleus-rxYFP. Cells at mid-exponential phase were treated with a moderate dose (0.4 mM) or high dose (3 mM) of H2O2, which resulted in 70% or <5% viability, respectively, after 4 h treatment (data not shown). In the presence of 0.4 mM H2O2, both cytosol- and nucleus-rxYFP shifted to increased oxidation and reached the maximum value (~30.0%) by 30 min. Subsequently, the proportion of oxidized form decreased and reached a level more reduced than that observed before adding H2O2 (Figure 5A). In the presence of 3 mM H2O2, both cytosol- and nucleus-rxYFP became rapidly oxidized, shifting to ~30% oxidized after 5 min and steadily increasing to ~80% oxidized over the 240-min experimental period (Figure 5B). Globally, cytosol- and nucleus-rxYFP responded in a similar manner to H2O2 bursts, suggesting dynamic and concomitant changes in the cytosol and nuclear redox environment in response to two different doses of H2O2. Interestingly, treatment with 0.4 mM H2O2 resulted in higher intracellular GSH concentrations (Figure 5C), consistent with the previous observations that the H2O2 stress induces activation of the general environmental stress response cluster genes and the specific H2O2 stimulon [33,34]. In contrast, a lethal dose of H2O2 resulted in a significant decrease of intracellular GSH (Figure 5C), which is an early hallmark in the progression of cell death in response to a variety of apoptotic stimuli. The rxYFP response and GSH quantification indicate that cells are capable of restoring the reduced cytosolic and nuclear redox environments in response to moderate dose of H2O2 and even adapting to the stress situation by establishing a more reducing GSH/GSSG potential compared to non-stressed cells. In contrast, the cytosolic and nuclear redox environments are overwhelmed and irreversibly affected by the lethal dose of H2O2.
Figure 5.
Subcellular redox changes in response to H2O2 bursts. Wild-type strains expressing the rxYFP sensors at mid-exponential phase were treated with 0.4 mM H2O2 (A) or 3.0 mM H2O2 (B). Redox Western blots were performed on samples removed before and 5, 15, 30, 60, 120, 240 min after addition of H2O2 (left). Percentage of oxidized forms of rxYFP was quantified using an Odyssey Infrared Imaging System (right). (C) Time course of total intracellular GSH concentration in response to 0.4 mM H2O2 and 3.0 mM H2O2 bursts. Samples were removed before and 5, 15, 30, 60, 120, 240 min after addition of H2O2 and prepared for GSH quantification. The reported values are the mean ± SD of at least two independent experiments.
Effect of GSH depletion on mitochondrial and nuclear genome stability
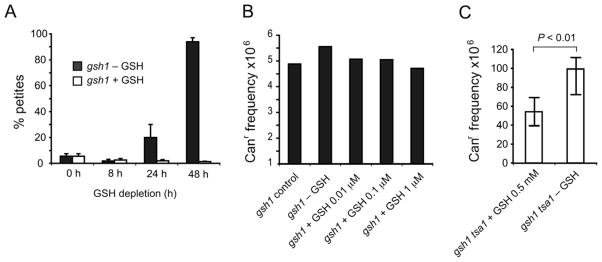
In the absence of GSH, gsh1 cells undergo limited divisions before arrest, probably reflecting the consumption of GSH accumulated during preculture in medium with GSH. In the GSH depletion experiment, a steady increase in the proportion of respiratory incompetent cells was observed, measuring 20% by 24 h and 90% by 48 h after onset of depletion (Figure 6A). This dramatic increase between 24 and 48 h suggests that once intracellular GSH concentrations decrease below a critical threshold, irreversible loss of respiratory competency ensues. This high prevalence of respiratory incompetency in the gsh1 mutant could be completely prevented by supplementation with 5 μM GSH (data not shown). These data indicate a specific requirement for GSH for cell growth and maintenance of respiratory competence and confirm the recent finding on the critical role of GSH in maintaining mitochondrial genome stability [29].
Figure 6.
Effect of GSH depletion on mitochondrial and nuclear genome stability. The strains (S288c background) were inoculated into SC medium containing 500 μM GSH for overnight growth before dilution to an appropriate density in SC without GSH. (A) Effect of GSH depletion on respiratory incompetency. The gsh1 cells were removed at 0, 8, 24, and 48 h after onset of GSH depletion and plated onto YPD plates to form colonies. The frequency of petite mutations was calculated as described in Materials and Methods. The reported values are the means ± SD for 2–5 independent experiments. (B) Effect of GSH depletion on nuclear DNA mutation. The gsh1 cells that underwent 24 h GSH depletion were either inoculated in SC without GSH or supplemented with indicated concentrations of GSH for another 24 h. Cells removed from these defined culture conditions were plated onto YPD and selection plates containing canavanine to determine the total number of viable cells and the number of mutants, respectively. The reported mutation frequency is the median frequency of at least five independent cultures. (C) Effect of GSH depletion on nuclear DNA mutation in tsa1 gsh1 mutants. The tsa1 gsh1 mutants that underwent 24 h GSH depletion were either inoculated into SC medium containing 500 μM GSH or without GSH for another 24 h. Canr mutation frequencies were calculated as above and is the median frequency of 21 independent cultures. The error bars indicate 95% interval confidence based on order statistics with the formula available at http//www.math.unb.ca/~knight/utility/Medlnt95.htm. Statistical significance was evaluated by the Mann-Whitney test using programs available at http://faculty.vassar.edu/lowry/vshome.htm.
Since GSH depletion results in a profound instability of mitochondrial DNA (Figure 6A) and oxidation of the nuclear GSH/GSSG redox state (Figure 4), we studied whether GSH depletion also causes nuclear genome instability. The Canr mutation assay that detects mutations inactivating the CAN1 gene was performed as described in Materials and Methods. We expected that the potential DNA damage associated with 24 or 48 h GSH depletion would be revealed in this experiment. However, as shown in Figure 6B, GSH depletion did not affect nuclear genome stability, in striking contrast with the profound effect of GSH depletion on the mitochondrial genome.
We reasoned that the redox deregulation- or oxidative stress- induced nuclear DNA damage during GSH depletion could be processed by other antioxidants or by different DNA damage repair pathways, thus masking the effect on GSH depletion in gsh1 single mutant. We therefore assessed the nuclear genome stability by combining gsh1 deletion with a peroxiredoxin tsa1 mutant. Tsa1 plays a key role in suppression of a broad spectrum of mutation phenotypes [35,36]. As shown in Figure 6C, the Canr frequency of the gsh1 tsa1 double mutant under GSH depletion conditions was about 2-fold higher than gsh1 tsa1 in medium supplemented with GSH (P < 0.01). This observation suggests that, in the absence of peroxiredoxin Tsa1, GSH depletion and the consequent redox changes have significant effects on nuclear genome stability detectable by the Canr assay.
Discussion
The lack of tools for measuring the status of defined intracellular redox couples in a given compartment has been a limitation for basic research. Genetically-encoded biosensors promise to overcome the limitations of conventional redox measurements [8]. The cytosolic rxYFP, initially developed by Østergaard and coworkers, specifically registers the intracellular GSH:GSSG redox state via disulfide exchange reactions with cytosolic Grxs in yeast [9]. This reaction has a similar equilibrium constant between pH 6.7 and 7.9 and occurs rapidly in the cytosol. The rxYFP sensor has also been exclusively targeted to the yeast mitochondrial matrix and IMS and shows distinct steady-state redox environments and independent GSH metabolism in these two subcellular compartments [11]. Similar GFP-based sensors have been targeted to other subcellular compartments in eukaryotic cells, such as endoplasmic reticulum, lysosome, plastids, and peroxisomes in order to examine dynamic redox changes in these compartments [37–40]. However, no studies have used nucleus-targeted versions of these sensors to specifically monitor fluctuations in the nuclear GSH:GSSG redox potential in response to genetic and environmental factors. Nevertheless, numerous studies have established that the nuclear redox state and the GSH/GSSG pool in particular play important roles in the physiology of the cell [41,42]. To better reflect the nuclear GSH/GSSG redox environment, we targeted rxYFP to the nucleus in yeast. Correct localization of nucleus-rxYFP was unequivocally confirmed by fluorescence microscopy and by fractionation analysis. Nucleus-rxYFP reacted in vivo to addition of an oxidant (4-DPS) or reductant (DTT) and to the elimination of Glr1, Trr1, or simultaneous loss of Trx1 and Trx2. Furthermore, nucleus-rxYFP responded differently to trr1 deletion, trx1 and trx2 double deletion, or trr1, trx1 and trx2 triple deletion, compared to cytosol-rxYFP. These results demonstrate that nucleus-rxYFP selectively registers redox variations within the nucleus, and together with cytosolic rxYFP and other compartment-targeted rxYFP sensors, it is possible to monitor dynamic redox changes within different subcellular compartments.
In S288c-based wild-type cells, nucleus-rxYFP was ~12% oxidized, which is similar if not slightly more reduced than cytosol-rxYFP, reflecting similar or at most subtle differences in the thiol-disulfide equilibrium between these two compartments. Assuming that the pH of the nucleus equals that of the cytosol that is approximately pH 7.0 [24,25,43], the nuclear redox potential measured with rxYFP is −291 mV. The rxYFP redox state for the cytosol reported here, −284 mV, is similar to that published previously [11]. A previous report on the mitochondrial matrix and IMS rxYFP redox state demonstrates that the redox balance in these mitochondrial compartments is shifted more toward disulfide formation than in the cytosol. Because reduction potential is strongly dependent on pH, the matrix value (−296 ± 5 mV) is actually more reducing than the cytosol despite the higher percentage of oxidized rxYFP in this compartment. Furthermore, the IMS GSH/GSSG redox potential (−255 ± 3 mV) measured by IMS-rxYFP is considerably more oxidizing than the cytosol and mitochondrial matrix [11]. Taken together, the steady-state redox state of rxYFP in several subcellular compartments analyzed in the present study and in Hu et al [11] establishes that the order from most reducing to most oxidizing is mitochondrial matrix > nucleus ≥ cytosol > IMS. A similar trend was observed in mammalian cells measured with direct GSH/GSSG quantification or with the redox sensor roGFP1 [44]. The values of redox potentials of these four compartments are considerably more reducing than the whole cell redox measurements using absolute concentration of [GSH] and [GSSG], reported as −225 mV in this study (Table 1) and −232 mV in a work by Hwang et al [10]. The significant differences between whole cell GSH/GSSG redox measurements and subcellular GSH-specific redox sensor measurements emphasize the importance of examining subcellular compartments separately. It is highly possible that redox compartmentalization is linked to organelle functions and redox signaling. For instance, the nuclear compartment is relatively reducing, perhaps reflecting an environment necessary for normal DNA metabolism, cell growth and deactivation of a number of redox-regulated transcription factors. Additional investigation of redox regulation and cellular response to oxidative stress in the context of redox compartmentalization should improve understanding of these critical mechanisms in diverse physiological and pathological processes.
Our nuclear and cytosolic GSH/GSSG redox measurements in Trx pathway mutants provide independent evidence of functional overlap between the GSH and Trx redox systems and within the Trx system in maintaining redox homeostasis. Previous genetic analyses indicate that the Trx-Trr system shares functional overlap with the glutathione system and that the Trx-Trr system helps prevent overaccumulation of GSSG in cells lacking the glutathione reductase Glr1 [45–48]. Our present study provides new evidence that the Trx-Trr system is specifically involved in controlling nuclear GSH/GSSG redox homeostasis even in the presence of Glr1. Trx1 and Trx2 appear to share overlapping function in this regard. Furthermore, our data suggest that the strong redox deregulation in the nucleus caused by Trr1 mutation is mediated by both oxidized Trx1 and Trx2. Indeed, trr1 trx1 trx2 triple mutants exhibit better fitness than the trr1 single mutant in standard YPD medium (Figure 3B), further pointing to the adverse effect of oxidized thioredoxins.
One interesting question is whether cytosolic and nuclear compartments have different redox characteristics and distinct redox regulation. It is generally presumed that nuclear pores should allow diffusion of low molecular weight GSH and GSSG. However, several studies in animal and plant cells provide evidence for the existence of functionally distinct pools of GSH/GSSG in the nucleus that are dynamically regulated during the cell cycle [42,49,50]. Furthermore, studies on 3T3 fibroblasts have shown that addition of the γ-glutamylcysteine synthetase inhibitor BSO significantly decreases the total cellular GSH pool; however, the nuclear GSH pool is much more resistant than the cytosolic pool to BSO-mediated depletion [30]. Little is known regarding the mechanisms for distinct nuclear and cytosolic redox control, as well as the kinetics of GSH/GSSG redox flux and exchange between these two compartments in yeast. In the present study, we observed only subtle, if any, redox differences between these two compartments in wild-type cells. However, stress conditions generated by trx1 trx2 double deletion or trr1 trx1 trx2 triple deletion resulted in statistically significant redox differences between the two compartments, suggesting that the nuclear GSH/GSSG redox state could be at least partially regulated independently of the cytoplasm. On the other hand, nucleus- and cytosol-rxYFP registered dynamic and concurrent oxidation during GSH depletion and in response to two doses of H2O2 bursts at defined time points, suggesting that these stress conditions have similar effects on the two GSH/GSSG pools. However, one has to exert some caution while analyzing the rxYFP redox measurements in such stress conditions. Transient difference in nuclear and cytosolic GSH/GSSG pools during GSH depletion may not be reflected in the time points analysed in the present study. The redox measurements particularly at later time points (24 h and after) during GSH depletion might be less reliable due to the reasons mentioned above (see Results section). Similarly, exogenous H2O2 may rapidly and homogenously diffuse to the cytosol and nucleus, leading to homogenous oxidation of both cytosolic and nuclear GSH/GSSG pools through the action of glutathione peroxidases. Future studies examining other types of stress conditions will reveal which type of stress specifically influences cytosolic vs. nuclear GSH/GSSG pools. Overall, these data favor the view that the highly reduced yeast nuclear and cytosolic GSH/GSSG redox states are maintained independently and under distinct but subtle redox regulation. Nucleus- and cytosol-rxYFP register compartment-specific localized redox fluctuations that may be largely influenced by exchange of GSH and/or GSSG between these two compartments. Indeed, both active and passive mechanisms of regulation and exchange cannot be excluded.
GSH is involved in numerous processes in cells including redox buffering, detoxification of organic and inorganic hydroperoxides, detoxification of xenobiotics, cellular signaling and iron–sulfur cluster assembly [4,27,51,52]. Here we find that GSH homeostasis plays a vital role in the maintenance of mitochondrial DNA and respiratory competency of cells, consistent with a previous report [29]. Although gsh1 cells are capable of undergoing ~ 8–10 divisions in medium lacking GSH before growth arrest, the remarkable increase of respiratory incompetence that occurs after 8–10 cell divisions suggests that a threshold concentration is essential for maintenance of mitochondrial DNA. Both iron-dependent and iron-independent mechanisms have been proposed [29]. In contrast with the profound effect of GSH depletion on mitochondrial DNA damage and redox state, nuclear genome stability was not significantly affected in the Canr assay. It is possible that the efficient antioxidant systems and DNA repair mechanisms may mask the mutagenic effect induced by the absence of GSH. Indeed, the gsh1 tsa1 double deletion mutant under GSH depletion displayed higher mutation frequency than the gsh1 tsa1 double mutant supplemented with GSH. Another reason for the greater importance of GSH in the maintenance of mitochondrial DNA stability might be because mitochondria are a substantial source of reactive oxygen species, while the nuclear DNA is present in chromatin, which may provide greater protection from oxidative damage. This also raises the question of whether there is an essential role for the nuclear pool of GSH/GSSG, which is not well defined. Madeo et al. reported that accumulation of reactive oxygen species caused by GSH depletion triggered the apoptotic pathway in S. cerevisiae and that hypoxia or radical scavenging prevented apoptosis [53]. All together, our results suggest that nuclear genome instability is not a major consequence caused by GSH depletion, although we cannot exclude the possibility that the mutator assay does not reflect all aspects of genome instability.
Conclusion
Intracellular redox homeostasis is crucial for many cellular functions but accurate measurements of cellular compartment-specific redox states remain technically challenging. We targeted a yellow fluorescent protein-based redox sensor rxYFP to the nucleus of the yeast S. cerevisiae. Parallel analyses of the redox state of nucleus-rxYFP and cytosol-rxYFP allow us to monitor distinctively dynamic glutathione redox changes within these two compartments in a given condition. These data show robustness of the rxYFP sensors in measuring real-time compartmental redox changes and provide in vivo evidence that glutathione and the thioredoxin redox system play distinct but overlapping functions in controlling subcellular redox environments. This is the first report of exclusive targeting of a YFP-based redox sensor to the yeast nucleus to specifically monitor fluctuations in the nuclear glutathione redox potential compared with that in cytosol in response to genetic and environmental factors.
Highlights.
A redox-sensitive yellow fluorescent protein (rxYFP) is targeted to the yeast nucleus.
Nucleus- and cytosol- rxYFP can monitor compartment-specific dynamic redox changes.
Glutathione and thioredoxin system play overlapping roles in subcellular redox control.
Nuclear redox may be regulated independently of the cytosol.
Oxidized nuclear redox environment upon GSH depletion does not affect nuclear genome stability.
Acknowledgments
We thank Benoit Palancade (Institut Jacques Monod, Paris) for providing the plasmid carrying NUP49-mCherry and the S. cerevisiae strain expressing mRFP-tagged NAB2. We thank Jérémie Soeur, Amélie Heneman, Dorothée Baille from Huang’s laboratory for helpful discussions. This work was supported by grants from Action Thématique et Incitative sur Programme (ATIP and ATIP-Plus) of Centre National de la Recherche Scientifique (CNRS) and from Institut Curie (to MEH), and NIH grant GM086619 (to CEO). Michèle Dardalhon and Laurence Vernis are research scientists at Institut National de la Santé et de la Recherche Médicale (INSERM).
The abbreviations used are
- ROS
reactive oxygen species
- GSH
reduced glutathione
- Grx
glutaredoxin
- Glr
glutathione reductase
- Trx
thioredoxin
- Trr
thioredoxin reductase
- GSSG
glutathione disulfide
- rxYFP
redox-sensitive yellow fluorescent protein
- IMS
mitochondrial intermembrane space
- YPD
yeast extract peptone dextrose medium
- SC
synthetic complete medium
- Canr
canavanine resistant
- DAPI
4′,6-diamidino-2-phenylindole
- TCA
trichloroacetic acid
- 4-DPS
4,4′-dithiodipyridine
- DTT
dithiothreitol
- H2O2
hydrogen peroxide
- BSO
buthionine sulfoximine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. 4. Oxford: Oxford University Press; 2007. [Google Scholar]
- 2.Herrero E, Ros J, Belli G, Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim Biophys Acta. 2008;1780:1217–1235. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Toledano MB, Kumar C, Le Moan N, Spector D, Tacnet F. The system biology of thiol redox system in Escherichia coli and yeast: differential functions in oxidative stress, iron metabolism and DNA synthesis. FEBS Lett. 2007;581:3598–3607. doi: 10.1016/j.febslet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohtake Y, Yabuuchi S. Molecular cloning of the gamma-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast. 1991;7:953–961. doi: 10.1002/yea.320070907. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Sugiyama K, Izawa S, Kimura A. Molecular identification of glutathione synthetase (GSH2) gene from Saccharomyces cerevisiae. Biochim Biophys Acta. 1998;1395:315–320. doi: 10.1016/s0167-4781(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 7.Bonini MG, Rota C, Tomasi A, Mason PP. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Meyer AJ, Dick TP. Fluorescent protein-based redox probes. Antioxid Redox Signal. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard H, Tachibana C, Winther JR. Monitoring disulfide bond formation in the eukaryotic cytosol. J Cell Biol. 2004;166:337–345. doi: 10.1083/jcb.200402120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ME, Kolodner RD. A biological network in Saccharomyces cerevisiae prevents the deleterious effects of endogenous oxidative DNA damage. Mol Cell. 2005;17:709–720. doi: 10.1016/j.molcel.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Palancade B, Zuccolo M, Loeillet S, Nicolas A, Doye V. Pml39, a novel protein of the nuclear periphery required for nuclear retention of improper messenger ribonucleoparticles. Mol Biol Cell. 2005;16:5258–5268. doi: 10.1091/mbc.E05-06-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chadrin A, Hess B, San Roman M, Gatti X, Lombard B, Loew D, Barral Y, Palancade B, Doye V. Pom33, a novel transmembrane nucleoporin required for proper nuclear pore complex distribution. J Cell Biol. 2010;189:795–811. doi: 10.1083/jcb.200910043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum P, Thorner J. Preparation of isolated nuclei from S. cerevisiae. In: Rose MD, Winston F, Hieter P, editors. Methods in yeast genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1990. pp. 136–139. [Google Scholar]
- 16.Reider SE, Emr SD. Isolation of subcellular fractions from the yeast Saccharomyces cerevisiae. Curr Protoc Cell Biol. 2001:3.8.1–3.8.68. doi: 10.1002/0471143030.cb0308s08. [DOI] [PubMed] [Google Scholar]
- 17.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 18.Wheals AE. Size control models of Saccharomyces cerevisiae cell proliferation. Mol Cell Biol. 1982;2:361–368. doi: 10.1128/mcb.2.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant CM, Collinson LP, Roe JH, Dawes IW. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol Microbiol. 1996;21:171–179. doi: 10.1046/j.1365-2958.1996.6351340.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogur M, StJohn R, Nagai S. Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science. 1957;125:928–929. doi: 10.1126/science.125.3254.928. [DOI] [PubMed] [Google Scholar]
- 21.Orij R, Brul S, Smits GJ. Intracellular pH is a tightly controlled signal in yeast. Biochim Biophys Acta. 2011;1810:933–944. doi: 10.1016/j.bbagen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Breeuwer P, Abee T. Assessment of the intracellular pH of immobilized and continuously perfused yeast cells employing fluorescence ratio imaging analysis. J Microbiol Methods. 2000;39:253–264. doi: 10.1016/s0167-7012(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 23.Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ. In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology. 2009;155:268–278. doi: 10.1099/mic.0.022038-0. [DOI] [PubMed] [Google Scholar]
- 24.Imai T, Ohno T. Measurement of yeast intracellular pH by image processing and the change it undergoes during growth phase. J Biotechnol. 1995;38:165–172. doi: 10.1016/0168-1656(94)00130-5. [DOI] [PubMed] [Google Scholar]
- 25.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 26.Drakulic T, Temple MD, Guido R, Jarolim S, Breitenbach M, Attfield PV, Dawes IW. Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species, and ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2005;5:1215–1228. doi: 10.1016/j.femsyr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Kumar C, Igbaria A, D’Autreaux B, Planson AG, Junot C, Godat E, Bachhawat AK, Delaunay-Moisan A, Toledano MB. Glutathione revisited: a vital function in iron metabolism and ancillary role in thiol-redox control. EMBO J. 2011;30:2044–2056. doi: 10.1038/emboj.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Mirabal HR, Winther JR. Redox characteristics of the eukaryotic cytosol. Biochim Biophys Acta. 2008;1783:629–640. doi: 10.1016/j.bbamcr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Ayer A, Tan SX, Grant CM, Meyer AJ, Dawes IW, Perrone GG. The critical role of glutathione in maintenance of the mitochondrial genome. Free Radic Biol Med. 2010;49:1956–1968. doi: 10.1016/j.freeradbiomed.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Markovic J, Mora NJ, Broseta AM, Gimeno A, de-la-Concepcion N, Vina J, Pallardo FV. The depletion of nuclear glutathione impairs cell proliferation in 3T3 fibroblasts. PLoS One. 2009;4:e6413. doi: 10.1371/journal.pone.0006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JC, Straffon MJ, Jang TY, Higgins VJ, Grant CM, Dawes IW. The essential and ancillary role of glutathione in Saccharomyces cerevisiae analysed using a grande gsh1 disruptant strain. FEMS Yeast Res. 2001;1:57–65. doi: 10.1111/j.1567-1364.2001.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 32.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci USA. 2009;106:422–427. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang X, Tran QT, Goodwin S, Wible RS, Sutter CH, Sutter TR. Yap1 or thiol-reactive chemicals elicits distinct adaptive gene responses. activation by H2O2. Free Radic Biol Med. 2011;50:1–13. doi: 10.1016/j.freeradbiomed.2010.10.697. [DOI] [PubMed] [Google Scholar]
- 35.Huang ME, Rio AG, Nicolas A, Kolodner RD. A genomewide screen in Saccharomyces cerevisiae for genes that suppress the accumulation of mutations. Proc Natl Acad Sci USA. 2003;100:11529–11534. doi: 10.1073/pnas.2035018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iraqui I, Kienda G, Soeur J, Faye G, Baldacci G, Kolodner RD, Huang ME. Peroxiredoxin Tsa1 is the key peroxidase suppressing genome instability and protecting against cell death in Saccharomyces cerevisiae. PLoS Genet. 2009;5:e1000524. doi: 10.1371/journal.pgen.1000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin CD, Wen X, Gazzard L, Nelson C, Scheller RH, Scales SJ. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci USA. 2005;102:17987–17992. doi: 10.1073/pnas.0509035102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenwasser S, Rot I, Sollner E, Meyer AJ, Smith Y, Leviatan N, Fluhr R, Friedman H. Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol. 2011;156:185–201. doi: 10.1104/pp.110.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Lith M, Tiwari S, Pediani J, Milligan G, Bulleid NJ. Real-time monitoring of redox changes in the mammalian endoplasmic reticulum. J Cell Sci. 2011;124:2349–2356. doi: 10.1242/jcs.085530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Go YM, Jones DP. Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal. 2010;13:489–509. doi: 10.1089/ars.2009.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallardo FV, Markovic J, Garcia JL, Vina J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspects Med. 2009;30:77–85. doi: 10.1016/j.mam.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Karagiannis J, Young PG. Intracellular pH homeostasis during cell-cycle progression and growth state transition in Schizosaccharomyces pombe. J Cell Sci. 2001;114:2929–2941. doi: 10.1242/jcs.114.16.2929. [DOI] [PubMed] [Google Scholar]
- 44.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 45.Muller EG. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell. 1996;7:1805–1813. doi: 10.1091/mbc.7.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trotter EW, Grant CM. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2002;46: 869–878. doi: 10.1046/j.1365-2958.2002.03216.x. [DOI] [PubMed] [Google Scholar]
- 47.Tan SX, Greetham D, Raeth S, Grant CM, Dawes IW, Perrone GG. The thioredoxin-thioredoxin reductase system can function in vivo as an alternative system to reduce oxidized glutathione in Saccharomyces cerevisiae. J Biol Chem. 2010;285:6118–6126. doi: 10.1074/jbc.M109.062844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trotter EW, Grant CM. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 2003;4:184–188. doi: 10.1038/sj.embor.embor729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellomo G, Vairetti M, Stivala L, Mirabelli F, Richelmi P, Orrenius S. Demonstration of nuclear compartmentalization of glutathione in hepatocytes. Proc Natl Acad Sci USA. 1992;89:4412–4416. doi: 10.1073/pnas.89.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vivancos PD, Dong Y, Ziegler K, Markovic J, Pallardo FV, Pellny TK, Verrier PJ, Foyer CH. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2009;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- 51.Franco P, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 52.Pocsi I, Prade RA, Penninckx MJ. Glutathione, altruistic metabolite in fungi. Adv Microb Physiol. 2004;49:1–76. doi: 10.1016/S0065-2911(04)49001-8. [DOI] [PubMed] [Google Scholar]
- 53.Madeo F, Frohlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Frohlich KU. Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]