Abstract
Heart failure causes significant morbidity and mortality. Distinguishing risk factors for incident heart failure can help identify at-risk individuals. Orthostatic hypotension may be a risk factor for incident heart failure; however, this association has not been fully explored, especially in non-white populations.
The Atherosclerosis Risk in Communities study included 12,363 adults free of prevalent heart failure with baseline orthostatic measurements. Orthostatic hypotension was defined as a decrease of systolic blood pressure ≥20 mm Hg or diastolic blood pressure ≥10 mm Hg with position change from supine to standing. Incident heart failure was identified from hospitalization or death certificate disease codes.
Over 17.5 years of follow up, orthostatic hypotension was associated with incident heart failure with multivariable adjustment (hazard ratio 1.54, 95% CI 1.30-1.82). This association was similar across race and gender groups. A stronger association was identified in younger individuals ≤55 years old (hazard ratio 1.90, 95% CI 1.41-2.55) than in older individuals >55 years old (hazard ratio 1.37, 95% CI 1.12-1.69, interaction p=0.034).
The association between orthostatic hypotension and incident heart failure persisted with exclusion of those with diabetes mellitus, coronary heart disease, and those on anti-hypertensives, psychiatric or Parkinson’s medications. However, exclusion of those with hypertension somewhat attenuated the association (hazard ratio 1.34, 95% CI 1.00-1.80).
We identified orthostatic hypotension as a predictor of incident heart failure among middle-aged individuals, particularly those 45-55 years of age. This association may be partially mediated through hypertension. Orthostatic measures may enhance risk stratification for future heart failure development.
Introduction
In 2007, nearly 1,000,000 hospitalizations and over 277,000 deaths in the United States (US) were related to heart failure (HF).1, 2 Over the past three decades in the US, HF prevalence has increased to affect 5.8 million people.3, 4 Early identification of individuals at risk for HF is critical to aggressively modify known HF risk factors, including diabetes mellitus (DM), hypertension, and coronary heart disease (CHD).5-7 Of interest, orthostatic hypotension (OH), has been associated with an increased risk for hypertension,8 CHD,9, 10 and mortality.9, 11, 12 Orthostasis has recently been implicated in HF development in two subgroups of a cohort study in elderly Dutch: those with DM and those in the oldest age group (78 years (mean), range 71-99 years).9 In addition, a recent cohort study in middle-aged Swedish adults (age range 26-61 years) found an association between OH and incident HF that attenuated somewhat after adjustment for traditional HF risk factors.13
Prior studies of the association between OH and HF have largely been in white populations and older individuals. In addition, medications known to cause OH such as anti-hypertensive, Parkinson’s and psychiatric medications have not frequently been included in prior analyses. Thus, we sought to evaluate whether OH is associated with incident clinical HF in middle-aged white and African-American participants in the Atherosclerosis Risk in Communities (ARIC) study; we also sought to further evaluate the contribution of specific medications and known HF risk factors to this relationship. We hypothesized that OH in middle-aged adults would be associated with incident HF, and that this association would be robust to exclusion of those with HF risk factors at baseline (DM, hypertension, and CHD), individuals taking psychiatric or Parkinson’s disease medications, and individuals taking anti-hypertensives that have been previously associated with OH. We also investigated age as an effect modifier of this association.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing longitudinal population-based study of men and women aged 45-64 at enrollment from 4 U.S. communities: Jackson, Mississippi; Washington County, Maryland; eight northern suburbs of Minneapolis, Minnesota; and Forsyth County, North Carolina. The racial distribution in the Maryland and Minneapolis communities were representative of the area, whereas African-Americans were oversampled in Forsyth County (15%) and exclusively sampled in Jackson. There was a 46% response to participate in the initial examination in Jackson and between 65-67% response for the other sites. The Institutional Review Boards from each site approved the ARIC study and informed consent was obtained from all participants. The ARIC study design and rationale, and a comparison between responders and non-responders, has been previously published.14, 15
From the 15,792 baseline examinees, we excluded race groups other than African-American or white as well as African-Americans in Minneapolis and Washington county (n=89). Postural blood pressure (BP) measurements used to define OH were performed at baseline examinations between 1987-1989. We excluded those with missing data for seated BP (n=3) and those missing OH measures (n=2,376). Most missing OH measures were from participants enrolled in the first 6 months of the study, prior to implementation of postural BP measurement. We also excluded individuals with atrial fibrillation on 2-minute rhythm strip at baseline (n=29) and those with prevalent HF (n=606) and/or missing information that precluded the determination of prevalent HF (n=213) at baseline. Those with prevalent HF were excluded from this analysis by the following criteria: (1) answering “yes” when asked “Were any of the medications you took during the last 2 weeks for heart failure?” or (2) stage 3 or “manifest HF” by Gothenburg criteria.16 The remaining 12,363 participants were included in this study.
Orthostatic Hypotension Definition
At baseline examination, a Dinamap 1846 SX oscillometric device was utilized to ascertain supine and standing BP measurements with a standardized protocol. Supine BP measurement followed an approximately 20-minute supine ultrasound examination; measurements were taken at 30 second intervals for 2 minutes (range of 2 to 5 measurements). Participants then stood upright and a standing BP measurement was taken as their feet touched the ground. Standing BP measurements continued at 30 second intervals for 2 minutes (range of 2 to 5 measurements). OH was defined as a decrease in systolic BP ≥ 20 mmHg or a decrease in diastolic BP ≥ 10 mmHg when the average supine BP was compared to the average standing BP after exclusion of the first standing BP measurement.12, 17 OH was identified in 612 individuals at the baseline examination.
Ascertainment of incident heart failure
Incident HF cases were accrued through 2008 (mean 17.5 years of follow-up) and were ascertained through annual contacts and review of ICD codes from hospitalizations and death certificates. Incident HF (N=1,720) was defined as the first occurrence of either: 1) Hospital ICD-9 ‘428.x’ (in any position in the diagnosis codes), or 2) ICD-9 ‘428’or ICD-10 ‘I50’ listed on a death certificate (in any position in the diagnosis codes). In HF cases defined by hospitalization with a HF diagnosis, the date of admission was used to define the date of incident HF.
Covariate definitions
All covariate measurements were obtained at baseline. Age, gender, race, educational level, alcohol use, medication use, and smoking status were obtained by self-report from the baseline questionnaire. Height and weight were measured by technicians; body mass index was calculated as weight (kilograms) divided by height squared (meters2). A history of CHD included prior myocardial infarction (defined by either self-report of physician-diagnosed myocardial infarction or by silent myocardial infarction as identified by electrocardiography) or a prior coronary revascularization procedure or coronary artery bypass surgery. Left ventricular hypertrophy was identified by electrocardiography using Cornell criteria.18 DM was defined as any of the following: self-reported history of physician-diagnosed DM, recent use of medication for DM, fasting blood glucose concentration ≥ 126 mg/dL, or non-fasting blood glucose concentration ≥ 200 mg/dL. Methods for the measurement of blood levels of glucose have been previously described.19 Standardized methodology was used to measure BP and resting heart rate, as previously described.15 Hypertension was defined as either resting seated systolic BP ≥140 mm Hg or diastolic BP ≥ 90 mm Hg or recent use of antihypertensive medications. Seated BP was measured at the beginning of the baseline examination following a five minute resting period; BP values were the average of the second and third measurements.15
Statistical analysis
We modeled the relationship between OH and time to incident HF using multivariable Cox proportional hazards regression. Log negative log survival curves and time interaction tests were used to evaluate the proportional hazard assumption for OH and all covariates. Models were adjusted for all above covariates. Additional models were stratified by age, race, and gender. In secondary analyses, we excluded those with DM (n=1,236), hypertension (n=3,807), CHD (n=414), those taking psychiatric or Parkinson’s disease medications (n=726), those taking anti-hypertensives including Angiotensin-Converting Enzyme (ACE) inhibitors, diuretics, and beta-blockers (n=2,758) and those who were censored or developed HF (n=175) in the first two years of the study. Calcium channel blockers were not included in the medication analysis due to less frequent association with OH; clonidine was also not included in this analysis as no participants were taking this medication at baseline. All analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
Those who developed HF during follow up (n=1,720) were more likely to be over 55 years old, male, African-American, obese, have less than a high school education, and were less likely to be current alcohol users (Table 1). Known HF risk factors, including DM, hypertension, and CHD were more common among individuals who developed incident HF. OH at baseline was 7% more common among those who developed incident HF (11%) than those who did not (4%). When ACE inhibitor, beta-blocker, and diuretic use were evaluated among participants with hypertension at baseline, ACE inhibitor use was more common among those who developed HF than those that did not (13% versus 10%); beta-blocker and diuretic use did not significantly differ among hypertensives for development of incident HF. We additionally found that the average supine systolic BP at baseline was approximately 22 mm Hg higher in those with OH (n = 612, SBP 146 mm Hg, SE 1.0) than in those without OH (n = 11,751, SBP 124 mm Hg, SE 0.2).
Table 1.
Characteristics of the Atherosclerosis Risk in Communities (ARIC) Population (N = 12,363) at Baseline (1987-1989) by Development of Incident HF
| Characteristics | Incident HF (N = 1,720 |
No Incident HF (N = 10,643) |
|---|---|---|
| Age > 55 years, %* | 60 | 38 |
| Male, %* | 53 | 44 |
| African American, %* | 35 | 25 |
| BMI >30, %* | 40 | 24 |
| Study Center, %* | ||
| Jackson, MS | 31 | 22 |
| Forsyth County, NC | 24 | 27 |
| Minneapolis, MN | 17 | 27 |
| Washington County, MD | 28 | 24 |
| Less than high school education, %* | 38 | 20 |
| Current smoker, %* | 36 | 24 |
| Current alcohol use, %* | 46 | 59 |
| Parkinson’s or Psychiatric Medications, %* | 8 | 6 |
| Diabetes Mellitus, %* | 29 | 8 |
| Mean Supine Systolic BP, mm Hg (SE)* | 136 (0.6) | 123 (0.2) |
| Mean Heart Rate, beats per minute (SE)* | 69 (0.3) | 66 (0.1) |
| Left Ventricular Hypertrophy, %* | 6 | 2 |
| Coronary Heart Disease, %* | 13 | 3 |
| Orthostatic Hypotension, %* | 11 | 4 |
| Hypertension, %* | 53 | 29 |
| ACE Inhibitors †,‡ | 13 | 10 |
| Beta-blockers† | 30 | 28 |
| Diuretics† | 41 | 41 |
p value <0.0001
Percents among participants with hypertension
p value = 0.006 among participants with hypertension
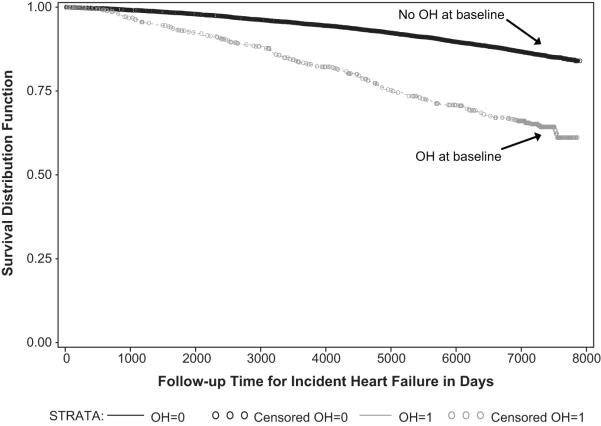
We identified a strong unadjusted association between baseline OH and incident HF (hazard ratio (HR) 3.02 (95% CI 2.59-3.52, Figure 1). We then adjusted for several covariates collected at baseline that had the potential to be confounders; we found the association was somewhat attenuated but still robust (HR 1.54, 95% CI 1.30-1.82, Table 2).
Figure 1.
Unadjusted Kaplan-Meier Survival Plot: Time to Incident HF, Stratified by Presence or Absence of Orthostatic Hypotension at Baseline Examination, the ARIC study (1987-2008)
Table 2.
Association of Baseline Orthostatic Hypotension with Incident HF, Stratified by Age, Race/Gender and Secondary Analyses with Exclusions; the ARIC study (1987-2008)
| Models | N | Incident HF Hazard Ratios |
95% Confidence Intervals |
|---|---|---|---|
| Unadjusted | 12,363 | 3.02 | (2.59-3.52) |
| Overall adjusted* | 11,743 | 1.54 | (1.30-1.82) |
| Age-stratified analysis † | |||
| Age ≤55 years | 6,868 | 1.90 | (1.41-2.55) |
| Age >55 years | 4,875 | 1.37 | (1.12-1.69) |
| Race and gender stratified analysis † | |||
| White Women | 4,528 | 1.59 | (1.13-2.23) |
| African-American Women | 1,885 | 1.60 | (1.14-2.24) |
| White Men | 4,155 | 1.31 | (1.00-1.72) |
| African-American Men | 1,175 | 1.71 | (0.98-2.99) |
| Secondary Analyses with Exclusions | |||
| Exclusion of diabetes at baseline† | 10,507 | 1.50 | (1.22-1.84) |
| Exclusion of hypertension at baseline† | 7,936 | 1.34 | (1.00-1.80) |
| Exclusion of individuals on anti- hypertensives (ACE inhibitors, beta- blockers or diuretics) at baseline† |
9,111 | 1.47 | (1.17-1.85) |
| Exclusion of CHD at baseline† | 11,329 | 1.52 | (1.27-1.82) |
| Exclusion of psychiatric or Parkinson’s medications at baseline† |
11,017 | 1.43 | (1.19-1.72) |
| Exclusion of HF cases from first 2 years of follow-up† |
11,568 | 1.60 | (1.35, 1.90) |
Models adjusted for baseline variables: age, gender, race by center, body mass index, educational level, smoking, alcohol use, diabetes mellitus, mean supine systolic blood pressure, resting heart rate, left ventricular hypertrophy, coronary heart disease, and hypertension
For secondary analyses, all of the above covariates were adjusted for except the stratified covariates
In a secondary age-stratified analysis, we noted a higher hazard ratio for participants <55 years old (HR 1.90, 95% CI 1.41-2.55) compared to those >55 years old (HR 1.37, 95% CI 1.12-1.69; p value = 0.034 for age interaction). In analyses stratified by race and gender, associations persisted in all groups with no significant variation in magnitude between the various race and gender groups (Table 2).
In additional secondary analyses excluding participants with baseline DM and CHD, we found little change in the association between baseline OH and incident HF (Table 2). However, the association between baseline OH and incident HF was attenuated with exclusion of those with hypertension at baseline (HR 1.34, 95% CI 1.00-1.80). Yet, the HR for incident HF was not markedly higher in those with baseline OH and hypertension (HR 1.63, 95% CI 1.33-2.01). We additionally found that excluding individuals taking ACE inhibitors, beta-blockers, or diuretics at baseline yielded very little change in the association between OH and HF (HR 1.47, 95% CI 1.17-1.85). Neither exclusion of individuals on psychiatric or Parkinson’s medications at baseline nor exclusion of HF cases occurring during the first two years of follow up yielded a substantial change in the association between OH and incident HF (Table 2).
Discussion
In a population-based cohort of white and black middle-aged adults, we found a significant association between OH and incident HF that was robust to adjustment for multiple HF risk factors. This association did not differ substantially between groups stratified by sex and race. Interestingly, we found that the association between OH and HF was stronger for those ages 45-55 years than for those ages 56-64 years. Prior studies have been limited to largely white populations, the elderly, and in some cases have lacked information on medications known to cause OH. Our findings were robust to exclusion of participants with DM, CHD, those taking psychiatric or Parkinson’s medications, and those taking specific anti-hypertensives (ACE inhibitors, diuretics, and beta-blockers). However, exclusion of those with hypertension modestly attenuated the association between OH and HF.
Numerous compensatory mechanisms work to maintain BP immediately following positional change from supine to upright. The carotid baroreceptor response plays a major role in this process through increasing sympathetic activity and inhibiting parasympathetic activity, in turn leading to catecholamine release, vasoconstriction, and increased heart rate.20-22 Abnormalities in any of these processes can lead to OH.
OH has been observed in patients with DM,23 in whom autonomic neuropathy, a subtype of diabetic peripheral neuropathy, is the main cause of OH.24 Diabetic autonomic neuropathy can cause dysfunction of autonomic nerves that regulate cardiac function and vascular response to positional changes to ultimately result in OH. In addition, insulin has inherent vasodilatory effects that may contribute to OH in diabetics.25 However, we found that OH was associated with HF even among individuals without DM at baseline, suggesting that diabetic autonomic neuropathy was not a substantial contributor to this relationship.
In hypertensive individuals with OH, proposed pathophysiological mechanisms for OH include impaired baroreceptor responsiveness, increased vascular stiffness related to arteriosclerosis, presence of left ventricular hypertrophy, and medication-related side effects.26-29 Anti-hypertensive medications, including diuretics and ACE inhibitors, have been associated with OH.30 However, certain beta-blockers have been theorized to have potential pressor effects in elderly, mildly hypertensive patients.31 In our study, hypertension was defined to include those with elevated BP or reported antihypertensive medications use at baseline. When participants with hypertension were excluded, the association between OH and HF attenuated somewhat, which may have been partly related to loss of statistical power as a large number of participants were excluded from the analysis. This attenuation was not likely due to the effect of anti-hypertensive medications, as exclusion of individuals taking ACE inhibitors, diuretics, or beta-blockers at baseline had little effect on the association between OH and HF. The attenuation may suggest that hypertension and OH could contribute to incident HF through a similar pathway, such as through recumbent hypertension, as supine BP was approximately 22 mm Hg higher in those with OH than in those without OH at baseline. However, it is important to note that adjusting for supine BP in our overall adjusted model did not eliminate the association between OH and HF.
While the exact mechanism for OH that precedes HF development is uncertain, we speculate that early atherosclerotic disease may affect one or more of the previously described compensatory responses to positional change and manifest as OH prior to HF development. Furthermore, since many conditions, including hypertension, DM and CHD, are associated with both OH and HF, such competing factors may facilitate the association between OH and incident HF (Figure 2).
Figure 2.
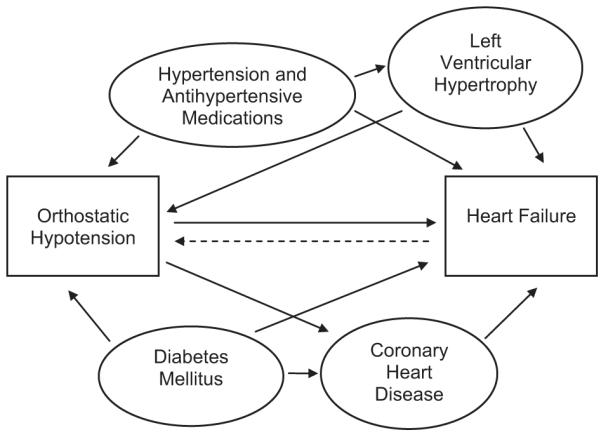
Demonstrating Theory of Competing Factors for Association Between Orthostatic Hypotension and Incident Heart Failure
To address possible under-ascertainment of prevalent HF at baseline, we performed an analysis excluding those with a HF hospitalization during the first two years of follow-up, which yielded very little change in the association between OH and HF. A two-year time period was selected for this analysis as most prevalent HF cases would likely require hospital admission over the course of two years.32
Of interest, we found that age was a significant effect modifier of the association between OH and incident HF; the association was stronger in adults 45-55 years old (HR 2.42, 95% CI 1.82-3.23) compared to those 56-64 years old (HR 1.70, 95% CI 1.38-2.10). In a Swedish population-based cohort study, younger adults 26-44 years old with OH also had higher risk of incident HF [HR 2.43 (95% CI 1.48-3.97)] than older adults 45-61 years old [HR 1.16 (95% CI 0.90-1.48)].13 However, the opposite association with age was found in a population-based cohort study of elderly Dutch from Rotterdam, in which the strongest association between OH and incident HF was in the oldest participants (mean 78 years, range 71-99; HR 1.32, 95% CI 1.04-1.67).9 In this same study, when age was divided into tertiles, no significant association between OH and incident HF was found in the two younger age groups (age ranges 55-63 and 63-71 years). In sum, the differential associations between OH and HF in various age groups are difficult to compare across studies, given that the Malmo and ARIC studies are in younger cohorts than the Rotterdam study. Yet, these divergent findings among age groups suggest that separate mechanisms may account for OH in the elderly compared to younger age groups. Another possibility is that the risk conferred by OH for incident HF may assume a U shaped age distribution with younger and older adults at highest risk. In the elderly, OH has been associated with the presence of multiple comorbidities,9, 25, vascular stiffness, 33 and with baroreflex dysfunction.34, 35 We speculate that OH in younger, apparently healthy individuals may indicate sub-clinical cardiac dysfunction and/or vascular stiffness related to atherosclerotic disease. In both elderly and young, OH has been associated with increased mortality risk.12, 36, 37
Both the Malmo and Rotterdam studies are in predominantly white populations, whereas our study has both white and African-American participants. As such, we could evaluate effect modification by race in our study. When stratified by race and gender, we observed that the hazard ratio associating OH and incident HF was lowest in white men and highest in African-American men, although the differences noted between race and gender groups were not statistically significant and confidence intervals overlapped substantially. In addition, the association between OH and incident HF was not statistically significant in African-American men, which can be partly attributed to a smaller subgroup sample size.
Our main study limitations were the definitions of prevalent and incident HF. At baseline examination, no specific question regarding a previous HF diagnosis was asked of participants. As a result, criteria of self-reported treatment for HF and the Gothenburg HF criteria were used as a proxy.16 To address potential prevalent HF cases not identified at enrollment, we performed a secondary analysis as previously discussed. With regard to the definition of incident HF, because HF was not a formalized outcome when the ARIC study was initiated, HF diagnostic codes were utilized to define the outcome without physician review for validation. This may have resulted in under-ascertainment of less severe cases of HF as only hospital and death certificate codes were used to define this outcome, though ICD-9 code 428 and ICD-10 code I50 are specific and are the most frequently documented HF codes.38, 39 Another limitation is the lack of echocardiographic study results to define HF as systolic or diastolic. However, similar readmission and mortality rates have been found in HF patients with ejection fractions of >40% compared to ejection fractions of <40%.40 Another limitation was that we were unable to evaluate OH at follow up visits as OH measures were only available from the baseline examination. Finally, hypertension was defined either through multiple measurements taken at the baseline visit or by report of taking antihypertensive medications, which may have over-estimated the number of participants with prevalent hypertension and affected our secondary analysis of this population.
The strengths of this study include its prospective design and long-term follow-up of a large, well-characterized, white and African-American cohort. Furthermore, this is the first cohort study to evaluate the relationship between OH and incident HF in both white and African-American participants. Compared to prior evaluations of OH and incident HF, we performed secondary analyses to evaluate known HF risk factors and medications that are known to cause OH.
Perspectives.
Implications from our study are that OH appears to be associated with incident HF, which is somewhat attenuated with the exclusion of participants with hypertension. We found a stronger association between OH and HF in younger adults than in older adults. Given our findings, we speculate that OH preceding HF may be a marker of early sub-clinical atherosclerosis that is facilitated by hypertension and potentially by other risk factors to contribute to HF development.
Novelty and Significance.
What Is New?
- The association between OH and incident HF did not vary greatly among white and African-American participants.
- A stronger association between OH and HF was identified in younger (ages 45-55) compared to older adults (ages 56-64).
What Is Relevant?
- The association between OH and HF may be partially explained by hypertension; we speculate that recumbent hypertension may also contribute to this association.
- Medications for hypertension do not appear to play a large role in the OH and HF association.
Summary
- OH appears to be associated with HF development; the strength of this relationship is not as strong when participants with hypertension were excluded from the analysis.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors would like to thank all of the ARIC staff and study participants for their valuable contributions.
Funding Sources Funding: The Atherosclerosis Risk in Communities Study is funded by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022.
Footnotes
Disclosures Conflicts of Interest: Christine DeLong Jones (none), Laura Loehr (none), Nora Franceschini (none), Wayne Rosamond (none), Patricia Chang (none), Eyal Shahar (none), David Couper (none), Kathy Rose (none),
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;(29):1–20. 24. [PubMed] [Google Scholar]
- 3.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970-1974 and 1990-1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 6.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, Shlipak MG. Predictors of heart failure among women with coronary disease. Circulation. 2004;110:1424–1430. doi: 10.1161/01.CIR.0000141726.01302.83. [DOI] [PubMed] [Google Scholar]
- 7.Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–236. doi: 10.1053/euhj.2000.2289. [DOI] [PubMed] [Google Scholar]
- 8.Rose KM, Holme I, Light KC, Sharrett AR, Tyroler HA, Heiss G. Association between the blood pressure response to a change in posture and the 6-year incidence of hypertension: prospective findings from the ARIC study. J Hum Hypertens. 2002;16:771–777. doi: 10.1038/sj.jhh.1001482. [DOI] [PubMed] [Google Scholar]
- 9.Verwoert GC, Mattace-Raso FU, Hofman A, Heeringa J, Stricker BH, Breteler MM, Witteman JC. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. 2008;56:1816–1820. doi: 10.1111/j.1532-5415.2008.01946.x. [DOI] [PubMed] [Google Scholar]
- 10.Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13:571–578. doi: 10.1016/s0895-7061(99)00257-5. [DOI] [PubMed] [Google Scholar]
- 11.Luukinen H, Koski K, Laippala P, Kivela SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–280. doi: 10.1001/archinte.159.3.273. [DOI] [PubMed] [Google Scholar]
- 12.Rose KM, Eigenbrodt ML, Biga RL, Couper DJ, Light KC, Sharrett AR, Heiss G. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk In Communities (ARIC) Study. Circulation. 2006;114:630–636. doi: 10.1161/CIRCULATIONAHA.105.598722. [DOI] [PubMed] [Google Scholar]
- 13.Fedorowski A, Engstrom G, Hedblad B, Melander O. Orthostatic Hypotension Predicts Incidence of Heart Failure: The Malmo Preventive Project. Am J Hypertens. 2010;23:1209–1215. doi: 10.1038/ajh.2010.150. [DOI] [PubMed] [Google Scholar]
- 14.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, Shahar E, Kalsbeek W. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49:1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 17.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 18.Crow RS, Prineas RJ, Rautaharju P, Hannan P, Liebson PR. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension (results from Treatment of Mild Hypertension Study) Am J Cardiol. 1995;75:1233–1238. [PubMed] [Google Scholar]
- 19.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118:496–500. [PubMed] [Google Scholar]
- 20.Mathias CJ. Orthostatic hypotension: causes, mechanisms, and influencing factors. Neurology. 1995;45:S6–11. [PubMed] [Google Scholar]
- 21.Schatz IJ. Orthostatic hypotension. I. Functional and neurogenic causes. Arch Intern Med. 1984;144:773–777. doi: 10.1001/archinte.144.4.773. [DOI] [PubMed] [Google Scholar]
- 22.Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–386. doi: 10.1002/j.1552-4604.1994.tb04977.x. [DOI] [PubMed] [Google Scholar]
- 23.Schneider SM, Robergs RA, Amorim FT, de Serna DG, Duran-Valdez EE, Schade DS. Impaired orthostatic response in patients with type 2 diabetes mellitus after 48 hours of bed rest. Endocr Pract. 2009;15:104–110. doi: 10.4158/EP.15.2.104. [DOI] [PubMed] [Google Scholar]
- 24.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 25.Madden KM, Tedder G, Lockhart C, Meneilly GS. Euglycemic hyperinsulinemia alters the response to orthostatic stress in older adults with type 2 diabetes. Diabetes Care. 2008;31:2203–2208. doi: 10.2337/dc08-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis BR, Langford HG, Blaufox MD, Curb JD, Polk BF, Shulman NB. The association of postural changes in systolic blood pressure and mortality in persons with hypertension: the Hypertension Detection and Follow-up Program experience. Circulation. 1987;75:340–346. doi: 10.1161/01.cir.75.2.340. [DOI] [PubMed] [Google Scholar]
- 27.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing. 2010;39:51–56. doi: 10.1093/ageing/afp192. [DOI] [PubMed] [Google Scholar]
- 28.Fan XH, Wang Y, Sun K, Zhang W, Wang H, Wu H, Zhang H, Zhou X, Hui R. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens. 2010;23:829–837. doi: 10.1038/ajh.2010.76. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DS, Pechnik S, Holmes C, Eldadah B, Sharabi Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2003;42:136–142. doi: 10.1161/01.HYP.0000081216.11623.C3. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Castellote E, Ocon J, Wagner B. Controlled multicenter study with quinapril, hydrochlorothiazide, and combination in patients with moderate to severe hypertension. J Cardiovasc Pharmacol. 1995;26:114–118. doi: 10.1097/00005344-199507000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Cleophas TJ, Grabowsky I, Niemeyer MG, Makel WM, van der Wall EE, Nebivolol Follow-Up Study Group Paradoxical pressor effects of beta-blockers in standing elderly patients with mild hypertension: a beneficial side effect. Circulation. 2002;105:1669–1671. doi: 10.1161/01.cir.0000012745.50229.ac. [DOI] [PubMed] [Google Scholar]
- 32.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 33.Mattace-Raso FU, van der Cammen TJ, Knetsch AM, van den Meiracker AH, Schalekamp MA, Hofman A, Witteman JC. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam Study. J Hypertens. 2006;24:339–344. doi: 10.1097/01.hjh.0000202816.25706.64. [DOI] [PubMed] [Google Scholar]
- 34.Lipsitz LA, Nyquist RP, Jr, Wei JY, Rowe JW. Postprandial reduction in blood pressure in the elderly. N Engl J Med. 1983;309:81–83. doi: 10.1056/NEJM198307143090205. [DOI] [PubMed] [Google Scholar]
- 35.James MA, Potter JF. Orthostatic blood pressure changes and arterial baroreflex sensitivity in elderly subjects. Age Ageing. 1999;28:522–530. doi: 10.1093/ageing/28.6.522. [DOI] [PubMed] [Google Scholar]
- 36.Masaki KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D, Curb JD. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–2295. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 37.Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project) Eur Heart J. 2010;31:85–91. doi: 10.1093/eurheartj/ehp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goff DC, Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160:197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 39.Schellenbaum GD, Rea TD, Heckbert SR, Smith NL, Lumley T, Roger VL, Kitzman DW, Taylor HA, Levy D, Psaty BM. Survival associated with two sets of diagnostic criteria for congestive heart failure. Am J Epidemiol. 2004;160:628–635. doi: 10.1093/aje/kwh268. [DOI] [PubMed] [Google Scholar]
- 40.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]