Abstract
Background
The US has experienced an alarming and unexplained increase in the incidence of esophageal adenocarcinoma (EAC) since the 1970s. A concurrent increase in obesity has led some to suggest a relationship between the two trends. We explore the extent of this relationship.
Methods
Using a previously validated disease simulation model of white males in the US, we estimated esophageal adenocarcinoma incidence 1973–2005 given constant obesity prevalence and low population progression rates consistent with the early 1970s. Introducing only the observed, rising obesity prevalence we calculated the incremental incidence caused by obesity. We compared these to esophageal adenocarcinoma incidence data from the National Cancer Institute’s SEER registry to determine obesity's contribution to the rise therein. Incidences were converted to absolute numbers of cases using US population data.
Results
Using constant obesity prevalence we projected a total of 30,555 EAC cases cumulatively over 1973–2005 and 1,151 in 2005 alone. Incorporating the observed obesity trend resulted in 35,767 cumulative EACs and 1,608 in 2005. Estimates derived from SEER data showed 111,223 cumulative and 7,173 cases in 2005. We conclude that the rise in obesity accounted for 6.5% of the increase in EAC cases that occurred from 1973–2005 and 7.6% in the year 2005.
Conclusion
Using published odds ratios for EAC among obese individuals, we found that only a small percentage of the rise in EAC incidence is attributable to secular trends in obesity.
Impact
Other factors, alone and in combination, should be explored as causes of the EAC epidemic.
Keywords: Esophageal Adenocarcinoma, Obesity, SEER, Disease Model, Simulation Modeling
Introduction
According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry data, the incidence of esophageal adenocarcinoma (EAC) in the US has increased fivefold in the past three decades (1). There is no consensus regarding the cause of this rise in EAC incidence, although increasing gastroesophageal reflux disease (GERD), eradication of Helicobacter pylori infection, use of non-steroidal anti-inflammatory drugs (2–3), and obesity have been suggested (4). Of these risk factors, obesity has received particular attention as a potential causal factor in the rapid rise in esophageal adenocarcinoma incidence (4–5). Two meta-analyses found that the risk of esophageal adenocarcinoma increased approximately two- to threefold in overweight and obese individuals (6–7) and additional studies found a higher risk of esophageal adenocarcinoma in obese individuals than those who are simply overweight (7–8), consistent with an exposure-response effect. Obesity has furthermore been found to be associated with symptoms of gastroesophageal reflux disease and strongly associated with Barrett’s esophagus (BE) (3, 8–9). These findings coupled with the high temporal correlation between obesity prevalence and esophageal adenocarcinoma incidence have led to speculation that the increasing weight trends in the United States may be at least partially responsible for the increase in esophageal adenocarcinoma incidence (7, 10–11).
Previous epidemiological studies, mainly case-control studies, were limited in their ability to estimate the contribution of obesity to the rapid rise of esophageal adenocarcinoma incidence. Conducting a cohort study with sufficiently large sample size and follow-up period is difficult due to the low esophageal adenocarcinoma incidence in the general population. However, mathematical models are able to simulate the natural history of esophageal adenocarcinoma by integrating the best available biologic, epidemiologic, and clinical data. Such a model can be used to estimate the excess cases caused by obesity assuming a causal association. Other applications of such an approach have been demonstrated in breast cancer (12–13). We have previously used an esophageal adenocarcinoma model to estimate the lives that could be saved under a national aspirin chemoprevention program (14–15). The aim of this study was to estimate the excess risk for esophageal adenocarcinoma attributable to obesity using a model constructed and validated with SEER data and the published literature.
Materials and Methods
Model Overview
A previously developed and validated model of esophageal adenocarcinoma (EACMo) (14, 16) was revised to analyze the contribution of obesity to the rise in esophageal adenocarcinoma incidence in the US over the study period of 1973 to 2005. EACMo is a Markov state transition model of esophageal carcinogenesis that tracks the transition of fractions of a population through six health states: Normal, Symptoms of Gastroesophageal Reflux Disease, Barrett’s Esophagus, Undetected Cancer, Detected Cancer, and Death. Extensive details regarding the parameterization, calibration, and validation of the model can be found in a publicly available manuscript (14).
Analysis Overview
The study performed was a multi-phase analysis. To help simplify and convey the process, the analysis is summarized into 5 phases; a more detailed description follows.
Dividing the Population into Obese and Non-Obese Groups
The simulated US population was initially divided by body mass index into Obese and Non-Obese groups and respective models.
Numerous Simulations to Provide Numerous Potential Solutions
The models were run, with each simulation producing a different set of transition probabilities between the health states. The probabilities were randomly selected for each transition from within a wide clinically plausible range to produce ten billion (1010) parameter sets (unique solutions).
Superior Simulation Selection
Two criteria were used: first, target odds ratios; and second, fit to SEER esophageal adenocarcinoma incidence. The top 1,000 simulations were deemed superior and selected.
Model Projections Over 33 Years: Scenarios 1 & 2
The superior parameter sets were used to project a hypothetical US population from 1973–2005. In Scenario 1, the simulation assumed that transition probabilities and all EAC risk factors including obesity prevalences remained constant over the study period. In Scenario 2, the rising prevalence of obesity was incorporated into the model.
Assessing Obesity Contributions by Comparing Projections to SEER
Projected EAC cases in Scenarios 1 and 2 were compared to SEER data and estimates of the number of cases attributable to obesity were calculated.
Analysis Details
In order to isolate the effect of obesity on esophageal adenocarcinoma incidence, we began by dividing the US into two separate populations, Obese and Non-Obese, at a BMI of 30. We then initially simulated each population independently. These two groups were recombined to recreate a whole US population at a later point in the analysis when selecting simulations with superior results.
We ran separate simulations of the esophageal adenocarcinoma model described above for each population. For each run, a parameter set (transition probabilities between health states) was randomly selected from within the bounds covering all reasonably likely rates for each transition (17), producing a wide and thorough sampling of potential progression rate combinations and resulting pre-cancer health state prevalences and age-adjusted esophageal adenocarcinoma incidences.
Ten billion (1010) unique results from the simulations were generated and assessed. Two criteria were applied to select simulations that most accurately reflected the observed epidemiologic data in our calibration process. For the first selection criterion, we calculated the odds ratios (OR) of GERD symptom prevalence and EAC incidence between the Obese and Non-Obese groups from the model outputs. We selected simulations that produced odds ratios consistent to within a factor of two of the values from the published literature: GERD OR =1.94; EAC OR=2.780; the target odds ratios, ranges and references may be seen in Table 1. We also excluded any combinations with an odds ratio less than one, as this would have been inconsistent with published data and clinical plausibility
Table 1.
Model Inputs: Parameters
| Parameter | Value | Range | References |
|---|---|---|---|
| Odds Ratio (OR) for Effect of Obesity on Prevalence/ Incidence | |||
| GERD Symptoms Prevalence | 1.94 | 1.0–4.0 | (2–3, 6, 8, 24–26) |
| BE Prevalence | * | 0.99–4.0 | (9, 24, 27–33) |
| EAC Incidence | 2.78 | 1.0–5.4 | (6–7, 20, 34–38) |
| Population Prevalence (%) | |||
| GERD Symptoms Prevalence | 18.6 | 17.6–19.9 | (39–49) |
| BE Prevalence | 4.2 | 0.8–25 | (40, 50–58) |
| Annual Transition Rates Between Health States | |||
| Normal to GERD Symptoms | Derived From Calibration | Derived From Calibration | |
| Normal to BE | `` | `` | |
| GERD to BE | `` | `` | |
| BE to Undetected EAC | `` | `` | |
| Undetected EACs detected (per year) | 25% | 11–100% | (59–60) |
This table describes the literature-derived values and ranges used as model input parameters. Population prevalences of GERD and BE are not themselves model parameters, but they are used to derive values for transition or progression from the “Normal” state to the “GERD” and “BE” states, respectively.
A meta-analysis32 could not find any statistically significant association between the effect of obesity and BE Prevalence; the effect is uncertain as published results have produced a wide range of result. Consequently, we did not use the OR for the effect of obesity on developing BE as part of our simulation selection criteria, but checked the OR to ensure that it was within the published range.
Abbreviations GERD=gastroesophageal reflux disease; BE=Barrett’s esophagus; EAC=esophageal adenocarcinoma; OR=Odds Ratio
For the second selection criterion, the esophageal adenocarcinoma incidences of simulations were compared to SEER esophageal adenocarcinoma incidences averaged over the same period (1973 to 1977) and their chi-squared scores were calculated as a measure of their goodness of fit. The model was calibrated to the early years of the study period, or esophageal adenocarcinoma incidences prior to the sharp increase. This process was consistent with the goal of producing model parameters (transition probabilities) preceding the secular trends and effects of obesity and other risk factors, both known and unknown. The first five years of the study period (1973–1977) were used instead of the first year alone (1973) to provide enough data points and lessen the risk of statistical noise resulting from small samples. The 1,000 simulations with the lowest chi-squared scores which also met the first (odds ratio) criterion was deemed superior and therefore selected for use in the remainder of the analysis.
In Scenario 1, we envisioned a static US population where risk factors that would affect progression to esophageal adenocarcinoma in the population, including the prevalence of obesity stayed at its average between 1973 and 1977, our basal rate (approximately 11%), over the ensuing 33-year study period. We recombined the populations by weighting the Obese and Non-Obese populations by their respective proportions from the basal rate. Consequently, the resulting number of esophageal adenocarcinoma cases in Scenario 1 represented the expected number of esophageal adenocarcinomas had obesity prevalence remained static over the years analyzed.
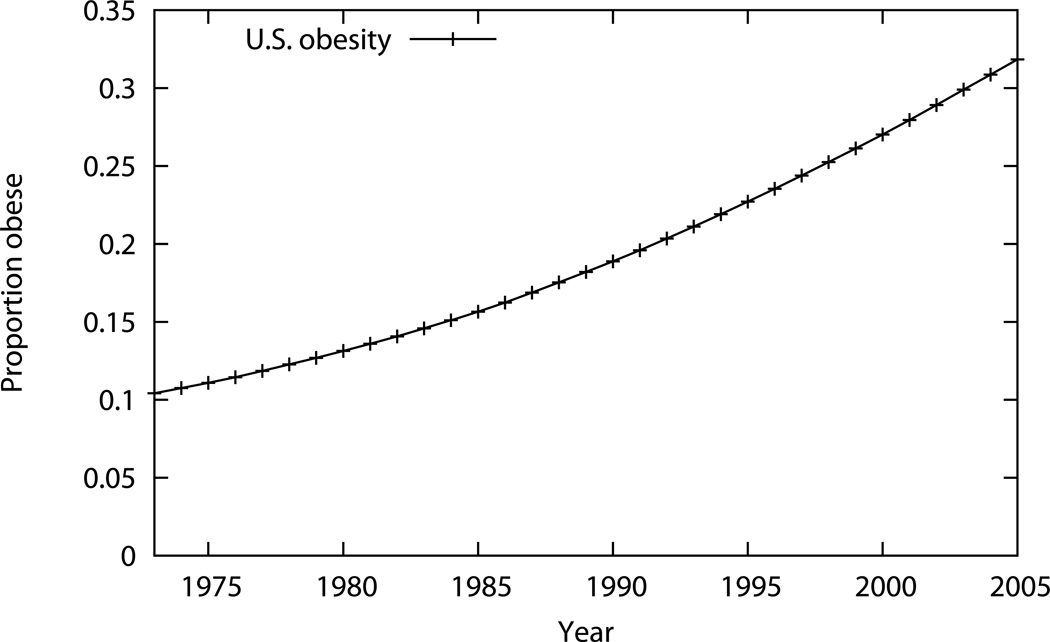
For Scenario 2, we incorporated secular trends of the rising obesity prevalence, while everything else in the model remained the same as in Scenario 1. The populations were recombined by weighting, as in Scenario 1, but weighted by their observed obesity prevalences for the corresponding calendar year as seen in Figure 1. These obesity prevalences were derived using National Health and Nutrition Examination Surveys (NHANES; 1971 to 2006) which were fit to regression models to create continuous body mass index values between surveys (18).
Figure 1. Obesity Prevalences from 1973–2005 for US White Males.
Obesity prevalences used for Scenario 2, which incorporates the rising prevalences over the study period (1973–2005) are plotted. The prevalence of obesity, defined as body mass index (BMI) >30kg/m2, rises from 11% of the population in 1973 to 32% in 2005.
Each scenario’s incidence and SEER incidence was weighted with the US white male population for the corresponding year, providing the number of esophageal adenocarcinoma cases each year, and summed over the study period, providing the total number of esophageal adenocarcinomas over the study period.
This difference in number of cases between Scenarios 1 and 2 was the contribution of obesity towards the rise in esophageal adenocarcinoma incidence. The difference between SEER data and Scenario 1 was the total rise in esophageal adenocarcinoma incidence. The ratio of the two differences, the increase from obesity divided by the full increase, was taken to be obesity’s fractional contribution to the rise in esophageal adenocarcinoma incidence: (Scenario 2 – Scenario 1) / (SEER – Scenario 1). This contribution was calculated for both the full study period and the final year, 2005, alone.
Acknowledging the uncertainty in the published odds ratios of esophageal adenocarcinoma incidence in obese individuals compared to individuals who are not obese, a sensitivity analysis was performed to explore the impact on the model’s projections with varying odds ratios. In particular, we calculated the odds ratios necessary to reproduce SEER esophageal adenocarcinoma incidence (both cumulative incidence over the study period and for the final year of study, 2005).
Results
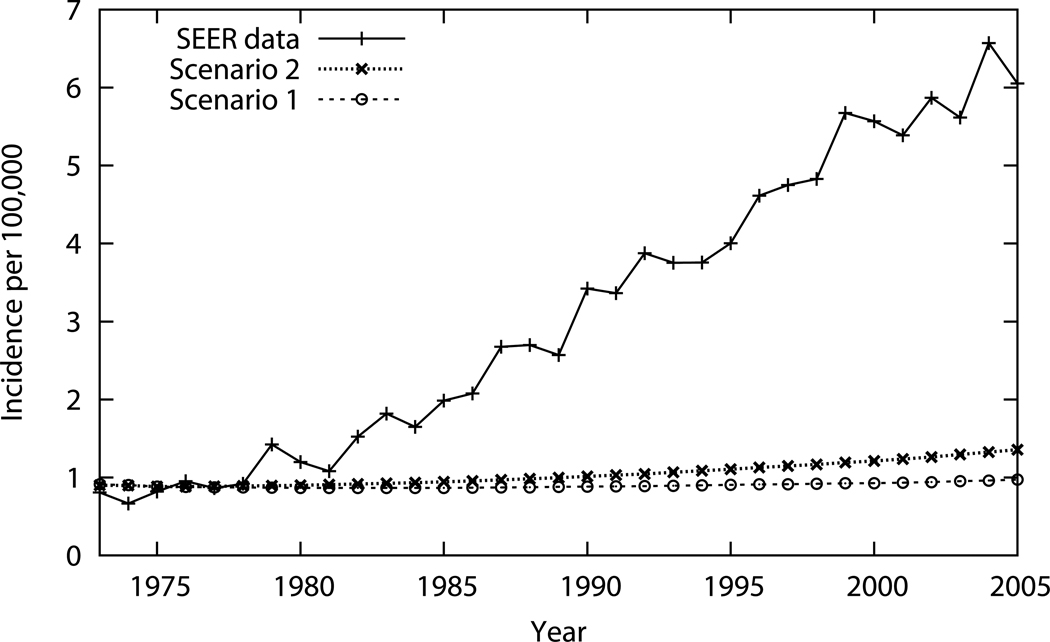
Table 2 and Figure 2 display the primary results of the analysis, esophageal adenocarcinoma incidence estimates for Scenarios 1 and 2, along with SEER registry data are presented over the study time period. The juxtaposed plots of the respective data in Figure 2 succinctly summarize the study results, with differences visually highlighted. Both results from Table 2 and Figure 2 are the weighted average of the 1,000 superior simulations. Scenario 1, where all risk factors remained constant, resulted in 30,555 esophageal adenocarcinoma cases cumulatively from 1973 to 2005 and 1,151 cases in 2005; as expected, esophageal adenocarcinoma incidences remained relatively flat. Scenario 2, where secular trends in obesity were incorporated into the model projected, resulted in 35,767 cases cumulatively and 1,608 in 2005; these figures are higher reflecting obesity’s effect on the progression rates to esophageal adenocarcinoma. SEER data estimates (incidence weighted by population figures) show 111,223 cases cumulatively and 7,173 cases in 2005. In Table 2, the fifth column is the difference between Scenario 2 and 1 which is the estimated number of cases attributable to obesity. The sixth and final column is the difference between SEER and Scenario 1, or the total rise in the number of cases. Using the calculation detailed in the Methods section, or dividing the number in fifth column by the number in the sixth column, these data show that obesity accounts for 6.5% (5212/80668) of esophageal adenocarcinoma cases in the US between 1973 and 2005, and 7.6% (457/6022) of cases in 2005.
Table 2.
Number of EAC Cases in the US by Year
| Annual EAC Incidence (Number of Cases) | |||||
|---|---|---|---|---|---|
| Year | Scenario 1 | Scenario 2 | SEER Data | From Obesity | Total Rise |
| 1975 | 806 | 806 | 746 | 0 | −60 |
| 1980 | 825 | 857 | 1,138 | 32 | 314 |
| 1985 | 855 | 929 | 1,958 | 75 | 1,103 |
| 1990 | 903 | 1,038 | 3,507 | 135 | 2,604 |
| 1995 | 979 | 1,197 | 4,334 | 218 | 3,355 |
| 2000 | 1,056 | 1,378 | 6,332 | 322 | 5,276 |
| 2005 | 1,151 | 1,608 | 7,173 | 457 | 6,022 |
| Total | 30,555 | 35,767 | 111,223 | 5,212 | 80,668 |
Numbers of esophageal adenocarcinoma (EAC) cases from Scenarios 1 and 2 and estimated from SEER data are presented by sampled year and cumulatively in the final row. The fifth column, “From Obesity,” is the difference between Scenario 2 and Scenario 1, which is the number of cases attributed to the rise in obesity. The final column, entitled “Total Rise,” is the difference between SEER and Scenario 1.
Figure 2. EAC Incidence by Year: Simulation Results Compared to SEER Data.
In this figure, EAC incidences over the study period (1973–2005) are plotted for Scenarios 1 and 2 next to SEER data for direct visual comparison.
Because there was uncertainty regarding model inputs, particularly obesity’s effect on progression risk to esophageal adenocarcinoma, sensitivity analysis was performed to explore and delineate parameter’s effect on model results or projections. Specifically, we aimed to determine the point or threshold value for the obesity-related odds ratios in order for Scenario 2 to reproduce SEER esophageal adenocarcinoma incidence. We found the odds ratio would have to be at least 77 (or >87 for 2005 esophageal adenocarcinoma incidence), substantially higher than the odds ratio of 2.78 used in our base case analysis and reported in the literature (see Table 3).
Table 3.
Obesity Effects, Sensitivity Analysis
| Odds Ratio | Cumulative Cases | 2005 Cases |
|---|---|---|
| Percent of SEER Cases | ||
| 2.8 | 6.5% | 7.6% |
| 5 | 17% | 15% |
| 10 | 26% | 24% |
| 20 | 35% | 33% |
| 50 | 57% | 53% |
| 77 | 100% | 95% |
| 87 | 106% | 100% |
| 100 | 115% | 108% |
The results of the sensitivity analysis are presented where the odds ratio (OR) for obesity’s impact on esophageal adenocarcinoma, which was 2.8 in the base case analysis, is varied and the threshold for the OR where Scenario 2 reproduces SEER data is determined. Column 2 is cumulative cases and Column 3 is incidence in 2005.
Discussion
The goal of our study was to estimate the impact of obesity on the witnessed rise in esophageal adenocarcinoma incidence since the early 1970s. The etiology of the dramatic increase in esophageal adenocarcinoma incidence has not been explained, although many have postulated that the concomitant and parallel trend in obesity prevalence is suggestive of a causal relationship (7, 10–11). However, these studies only qualitatively compared the rise in EAC incidence with obesity. Our analysis is unique in that we integrate the obesity prevalence as a model input to produce a quantitative estimation of obesity’s contribution.
Within the context of the limited data and means to study this hypothesis, we used a previously validated simulation disease (EACMo) and performed a thought experiment. We imagined a world where obesity and esophageal adenocarcinoma incidence remained static since the early 1970s (Scenario 1). This world served as a baseline to introduce a single, and therefore isolated, factor into the model: changes in obesity prevalence over the study period (Scenario 2). Comparisons between Scenarios 1 and 2 and to actual SEER EAC incidence data allowed us to estimate what percentage of the rise in esophageal adenocarcinoma incidence was due to obesity. Short of a modeling and simulation methodology, it would not have been possible to perform this analysis.
Our findings suggest that the increase in obesity in the US population only accounts for a small portion of the concurrent increase in esophageal adenocarcinoma incidence. Our data affirms our hypothesis, estimating that 6.5%, a relatively small proportion, of the increase in esophageal adenocarcinoma incidence in the US is attributable to rising obesity prevalence in the US over the three-decade period studied.
Our finding that obesity’s contribution was minor was consistent with a few observations. First, the rise in both obesity and esophageal adenocarcinoma seemed to occur simultaneously, whereas if obesity were indeed playing a pivotal causal role, we would expect a temporal lag between the rises in obesity and esophageal adenocarcinoma, as observed in other examples such as the effect of aspirin on colorectal cancer (19). One of the suggested mechanism behind the proposal of obesity causing esophageal adenocarcinoma is that obesity increases abdominal pressure on the stomach leading to more gastroesophageal reflux disease symptoms, more cases of Barrett’s esophagus, and ultimately more esophageal adenocarcinoma cases, a sequence of events involving several steps and that would take at least a few years to occur (20). In fact, a recent publication suggests that the esophageal adenocarcinoma incidence was rising a full decade prior to significant increases in obesity prevalence in the US (21). Second, esophageal adenocarcinoma continues to be significantly more common in men than in women; however, the rise in obesity is more marked in the latter group (22). Some have postulated that gender differences in the distribution of adipose tissue within the body plays a role in this phenomenon (8), with men more likely to have central abdominal obesity than women. The slope of the increase in esophageal adenocarcinoma incidence appears too precipitous for obesity to be the only explanation. Our data suggests that other causes, such as hormonal differences between genders (8), synergies between factors, or other factors hitherto unsuspected or unstudied are contributing significantly to the rise in esophageal adenocarcinoma incidence.
Prior studies that have analyzed obesity's contribution to the increase in esophageal adenocarcinoma were observational, mainly case-control studies. These types of analyses have limited abilities to estimate the role of obesity in the rise of esophageal adenocarcinoma incidence due to their retrospective nature and the presence of potential bias and competing risks. Additionally, a cohort study with sufficient sample size and long enough follow-up period has not been performed previously because of the low incidence of esophageal adenocarcinoma in the general population. Currently a large clinical trial (AspECT) is underway in the United Kingdom that has recruited over 2,000 patients with Barrett’s esophagus and plans to follow the cohort for over 8 years (23). This study will provide important observational data regarding progression to esophageal adenocarcinoma from Barrett’s esophagus and may provide additional prospective data regarding obesity’s effect on esophageal adenocarcinoma incidence. However, AspECT was primarily designed and statistically powered to assess the effects of aspirin and acid suppression on progression rates, rendering any obesity analyses secondary or post-hoc and, even with the large numbers and follow-up period, underpowered. Using simulation modeling techniques, we were able to examine the uncertainty surrounding the estimates and provided a way to test and evaluate a wide range of potential transition probabilities and see which correspond to various natural histories.
There were a few limitations in our model which could be addressed in future research and analyses. First, there are limited data to inform model inputs. The annual rates of progression from gastroesophageal reflux disease to Barrett’s esophagus and from there to esophageal adenocarcinoma are the most salient examples, and there remains significant uncertainty regarding the effect of obesity on progression to esophageal adenocarcinoma. Second, our analysis focused on white males. We chose to perform our analysis in the patient group for which the most empiric clinical data existed, and that group is white males. As previously mentioned, esophageal adenocarcinoma incidence in the past and present, as well as the aforementioned sharp increase therein, is most notable in white males. Furthermore, while specific empirical data regarding precursor health states such as Barrett’s esophagus are limited in white males, they are even more lacking for females and non-whites. Therefore, including females and non-whites in the model would introduce yet another level or dimension of uncertainty. The purpose of our study was to test a scientific hypothesis, not to guide clinical practice or inform public health policy and in this context we believe building a model of white males is justifiable because of the precision we gain in our analysis. Additionally, some analyses suggest that waist circumference may be superior to body mass index as a predictor of esophageal adenocarcinoma among males and females (20, 24). Ideally our model would have included such markers of central obesity; however, our analysis only simulated males, where there is a strong correlation between high BMI values and the presence of central obesity. Therefore, using BMI alone may have been adequate. Another limitation to our analysis was that we modeled obesity dichotomizing the population using a BMI of 30 as a cut off value; consequently, those with intermediate BMI values, or overweight, were not incorporated, potentially leading to an underestimate of the impact of obesity and raising concerns about the validity of the conclusion. Although the incorporation of waist circumference and an overweight category into our model would have been interesting and potentially worthwhile, the lack of sufficient data to adequately inform model inputs made this unfeasible. Finally, our sensitivity analysis that explores the association between obesity and EAC provides some insight into how model projections would change with differing estimates of the impact of obesity. To estimate the impact of the increase in obesity on esophageal adenocarcinoma, we isolated obesity as a specific risk factor and then incorporated it into Scenario 2. We acknowledge that this methodology has the limitation of assuming an overly simplified system where no potential interactions exist. However, an advantage of these simplifying assumptions is an analysis that is more transparent and readily comprehensible.
Future trial data from studies such as AspECT (23) that carefully track the natural history of those with Barrett’s esophagus could improve understanding of obesity’s impact on progression to esophageal adenocarcinoma, confirming or contradicting the odds ratios we derived from prior published studies. However, our sensitivity analysis finds that the odds ratio would have to be unrealistically high to reproduce the actual increase in esophageal adenocarcinoma incidence with our model framework. Future epidemiologic and modeling analyses should focus on searching for additional risk factors, synergies between risk factors, and identifying potential subsets of the population that are at highest risk for this cancer that still affects a relatively small percentage of the population, but is undergoing a dramatic rise in incidence.
The message of our analysis is not that obesity is acceptable because the associated increased risk of EAC is not substantial but instead that we need to go back to the drawing board and search for other hypotheses to better explain and understand the alarming rise in esophageal adenocarcinoma incidence. We chose to focus on and isolate a single putative contributing factor. Our conclusion is that unless there is significant synergy with other hypothesized contributors or substantial heterogeneity of effect within a subgroup of the population, obesity is not a major cause of the 500% rise in esophageal adenocarcinoma.
Supplementary Material
Acknowledgements
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers U01CA152926 and R01CA140574 to C.H., K25CA133141 to C.Y.K, and R00CA126147 to P.M.M).
Abbreviations
- EAC
esophageal adenocarcinoma
- GERD
gastroesophageal reflux disease
- BE
Barrett’s esophagus
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the form for Disclosure of Potential Conflicts of Interest and the following were reported:
Stuart Spechler:
AstraZeneca Pharmaceuticals: Grant/Research Support
Ironwood Pharmaceuticals Inc.: Consulting fee
Takeda Pharmaceutical Company Ltd: Grant/Research Support
XenoPort, Inc.: Consulting fee
Torax Medical: Consulting fee
BARRX Medical, Inc.: Grant/Research Support
G Scott Gazelle:
GE Healthcare: Consulting fee
The remaining authors disclosed no potential conflicts of interest.
REFERENCES
- 1.Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–555. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329–338. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan TL, Kristal AR, Blount PL, Levine DS, Galipeau PC, Prevo LJ, et al. Nonsteroidal anti-inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2002;11:745–752. [PubMed] [Google Scholar]
- 4.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 5.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 6.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA., Jr Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340–2348. doi: 10.1056/NEJMoa054391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Jeon J, Luebeck EG, Moolgavkar SH. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States) Cancer Causes Control. 2006;17:971–981. doi: 10.1007/s10552-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 11.Kort EJ, Sevensma E, Fitzgerald TL. Trends in esophageal cancer and body mass index by race and gender in the state of Michigan. BMC Gastroenterol. 2009;9:47. doi: 10.1186/1471-230X-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151:738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 14.Hur C, Hayeck TJ, Yeh JM, Richards EB, Spechler SJ, Gazelle GS, et al. Development, calibration, and validation of a U.S. white male population-based simulation model of esophageal adenocarcinoma. PLoS One. 2010;5:e9483. doi: 10.1371/journal.pone.0009483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of aspirin chemoprevention for Barrett's esophagus. J Natl Cancer Inst. 2004;96:316–325. doi: 10.1093/jnci/djh039. [DOI] [PubMed] [Google Scholar]
- 16.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett's esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–457. doi: 10.1111/j.1442-2050.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY, Goldie SJ. Development of an empirically calibrated model of gastric cancer in two high-risk countries. Cancer Epidemiol Biomarkers Prev. 2008;17:1179–1187. doi: 10.1158/1055-9965.EPI-07-2539. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will All Americans Become Overweight or Obese? Estimating the Progression and Cost of the US Obesity Epidemic. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 19.Imperiale TF. Aspirin and the prevention of colorectal cancer. N Engl J Med. 2003;348:879–880. doi: 10.1056/NEJMp030005. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams JA, Sharaiha RZ, Gonsalves L, Lightdale CJ, Neugut AI. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 20:183–186. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring) 2007;15:2855–2865. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 23.Das D, Chilton AP, Jankowski JA. Chemoprevention of oesophageal cancer and the AspECT trial. Recent Results Cancer Res. 2009;181:161–169. doi: 10.1007/978-3-540-69297-3_15. [DOI] [PubMed] [Google Scholar]
- 24.Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 25.Lee HL, Eun CS, Lee OY, Jeon YC, Sohn JH, Han DS, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42:672–675. doi: 10.1097/MCG.0b013e31806daf64. [DOI] [PubMed] [Google Scholar]
- 26.Dore MP, Maragkoudakis E, Fraley K, Pedroni A, Tadeu V, Realdi G, et al. Diet, lifestyle and gender in gastro-esophageal reflux disease. Dig Dis Sci. 2008;53:2027–2032. doi: 10.1007/s10620-007-0108-7. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett's esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 28.Stein DJ, El-Serag HB, Kuczynski J, Kramer JR, Sampliner RE. The association of body mass index with Barrett's oesophagus. Aliment Pharmacol Ther. 2005;22:1005–1010. doi: 10.1111/j.1365-2036.2005.02674.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith KJ, O'Brien SM, Smithers BM, Gotley DC, Webb PM, Green AC, et al. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481–2486. doi: 10.1158/1055-9965.EPI-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama T, Yoneda M, Inamori M, Iida H, Endo H, Hosono K, et al. Visceral obesity and the risk of Barrett's esophagus in Japanese patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2009;9:56. doi: 10.1186/1471-230X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson BC, Chan AT, Giovannucci EL, Fuchs CS. Body mass index and Barrett's oesophagus in women. Gut. 2009;58:1460–1466. doi: 10.1136/gut.2008.174508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook MB, Greenwood DC, Hardie LJ, Wild CP, Forman D. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett's esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 33.Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett's esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–842. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown LM, Swanson CA, Gridley G, Swanson GM, Schoenberg JB, Greenberg RS, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995;87:104–109. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 35.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–294. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 36.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, Stanford JL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 37.El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci. 2008;53:2307–2312. doi: 10.1007/s10620-008-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lofdahl HE, Lu Y, Lagergren J. Sex-specific risk factor profile in oesophageal adenocarcinoma. Br J Cancer. 2008;99:1506–1510. doi: 10.1038/sj.bjc.6604701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 40.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 41.El-Serag HB. Time Trends of Gastroesophageal Reflux Disease: A Systematic Review. Clinical Gastroenterology and Hepatology. 2007;5:17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Terry P, Lagergren J, Wolk A, Nyren O. Reflux-Inducing Dietary Factors and Risk of Adenocarcinoma of the Esophagus and Gastric Cardia. Nutrition and Cancer. 2000;38:186–191. doi: 10.1207/S15327914NC382_7. [DOI] [PubMed] [Google Scholar]
- 43.Collen MJ, Abdulian JD, Chen YK. Gastroesophageal Reflux Disease in the Elderly: More Severe Disease That Requires Aggressive Therapy. American Journal of Gastroenterology. 1995;90:1053–1057. [PubMed] [Google Scholar]
- 44.Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in gastro-oesophageal reflux disease: a twin study. Gut. 2003;52:1085–1089. doi: 10.1136/gut.52.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiocca JC, Olmos JA, Salis GB, Soifer LO, Higa R, Marcolongo M. Prevalence, clinical spectrum and atypical symptoms of gastro-oesophageal reflux in Argentina: a nationwide population-based study. Alimentary Pharmacology and Therapeutics. 2005;22:331–342. doi: 10.1111/j.1365-2036.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- 46.El-Serag HB, Petersen NJ, Carter J, Graham DY, Richardson P, Genta RM, et al. Gastroesophageal Reflux Among Different Racial Groups in the United States. Gastroenterology. 2004;126:1692–1699. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 47.Isolauri J, Laippala P. Prevalence of Symptoms Suggestive of Gastroesophageal Reflux Disease in an Adult Population. Annals of Internal Medicine. 1995;27:67–70. doi: 10.3109/07853899509031939. [DOI] [PubMed] [Google Scholar]
- 48.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and Dyspepsia Subgroups: A Population-Based Study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 49.Diaz-Rubio M, Moreno-Elola-Olaso C, Rey E, Locke GR, 3rd, Rodriguez-Artalejo F. Symptoms of gastro-oesophageal reflux: prevalence, severity, duration and associated factors in a Spanish population. Alimentary Pharmacology and Therapeutics. 2004;19:95–105. doi: 10.1046/j.1365-2036.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 50.Clark GW, Ireland AP, Peters JH, Chandrasoma P, DeMeester TR, Bremner CG. Short-segment Barrett's esophagus: A prevalent complication of gastroesophageal reflux disease with malignant potential. J Gastrointest Surg. 1997;1:113–122. doi: 10.1016/s1091-255x(97)80098-4. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 52.Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 53.Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett's esophagus? A meta-analysis. Am J Gastroenterol. 2003;98:2390–2394. doi: 10.1111/j.1572-0241.2003.08702.x. [DOI] [PubMed] [Google Scholar]
- 54.Westhoff B, Brotze S, Weston A, McElhinney C, Cherian R, Mayo MS, et al. The frequency of Barrett's esophagus in high-risk patients with chronic GERD. Gastrointest Endosc. 2005;61:226–231. doi: 10.1016/s0016-5107(04)02589-1. [DOI] [PubMed] [Google Scholar]
- 55.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology. 2002;123:461–467. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 56.Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RK, Vasudeva RS, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 57.Pera M. Trends in incidence and prevalence of specialized intestinal metaplasia, barrett's esophagus, and adenocarcinoma of the gastroesophageal junction. World Journal of Surgery. 2003;27:999–1008. doi: 10.1007/s00268-003-7052-2. [DOI] [PubMed] [Google Scholar]
- 58.Cameron AJ, Lomboy CT. Barrett's esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992;103:1241–1245. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- 59.Guanrei Y, Songliang Q, He H, Guizen F. Natural history of early esophageal squamous carcinoma and early adenocarcinoma of the gastric cardia in the People's Republic of China. Endoscopy. 1988;20:95–98. doi: 10.1055/s-2007-1018145. [DOI] [PubMed] [Google Scholar]
- 60.Provenzale D, Kemp JA, Arora S, Wong JB. A guide for surveillance of patients with Barrett's esophagus. Am J Gastroenterol. 1994;89:670–680. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.