Abstract
Six weekly sessions of group cognitive-behavioral therapy for insomnia and osteoarthritis pain (CBT-PI), and for osteoarthritis pain alone (CBT-P) were compared to an education only control (EOC). Basic education about pain and sleep was comparable, so EOC controlled for information and group participation. Active interventions differed from EOC in training pain coping skills (CBT-P and CBT-PI) and sleep enhancement techniques (CBT-PI). Persons with osteoarthritis age 60 or older were screened for osteoarthritis pain and insomnia severity via mailed survey. Primary outcomes were pain severity (pain intensity and interference ratings from the Graded Chronic Pain Scale) and insomnia severity (Insomnia Severity Index). Secondary outcomes were arthritis pain (AIMS-2 symptom scale ) and sleep efficiency assessed by wrist actigraphy. Ancillary outcomes included: cognitive function, depression, and health care use. A clustered randomized design provided adequate power to identify moderate effects on primary outcomes (effect size ≥0.35). Modified intent to treat analyses, including all participants who attended the first session, assessed effects across CBT-PI, CBT-P, and EOC groups. Treatment effects were assessed post-intervention (2 months) and at 9 months, with durability of intervention effects evaluated at 18 months. The trial was executed in 6 primary clinics, randomizing 367 participants, with 93.2% of randomized patients attending at least 4 group sessions. Response rates for post-intervention and 9 month assessments were 96.7 % and 92.9% respectively. This hybrid efficacy-effectiveness trial design evaluates whether interventions yield specific benefits for clinical and behavioral outcomes relative to an education only control when implemented in a primary care setting.
Keywords: Cluster randomized trial; group intervention; efficacy, effectiveness; cognitive-behavioral intervention; insomnia; chronic pain
Chronic pain and insomnia are interrelated problems that are common and troublesome among older adults with osteoarthritis [1,2]. Effective behavioral interventions are available for insomnia [3] and for osteoarthritis pain [4]. These interventions have shared content that includes: education about causes and consequences of sleep disturbance or pain; setting behavioral goals; activity scheduling and pacing; progressive muscle relaxation; and modification of negative thoughts. Unique components of behavioral interventions for insomnia include sleep hygiene, stimulus control, sleep restriction techniques, and use of sleep diaries to guide individualized treatment [3]. There is preliminary evidence suggesting that behavioral sleep interventions have significant benefits for improving chronic pain outcomes [5-7], but this has yet to be evaluated in a large randomized trial.
We designed and implemented a randomized trial to determine whether integration of state-of-the-art cognitive-behavioral interventions for osteoarthritis pain and insomnia would improve pain, insomnia, and functional outcomes relative to an education only control, and relative to a cognitive-behavioral intervention for osteoarthritis pain alone. The education only condition controlled for information and non-specific effects of group participation. Assessments were augmented to provide ancillary data on clinically important outcomes related to osteoarthritis pain and insomnia, including cognition, depression, and health care use (visits for pain and non-specific symptoms, use of medications for pain and sleep problems, and overall ambulatory health care costs).
This paper describes the design and execution of the “Lifestyles” trial. The objectives of this paper are to: 1) explain the trial design; 2) evaluate success in implementing the design; and 3) consider strengths and limitations of a hybrid efficacy-effectiveness design for evaluating behavioral interventions for insomnia and osteoarthritis pain.
Methods
Conceptual framework
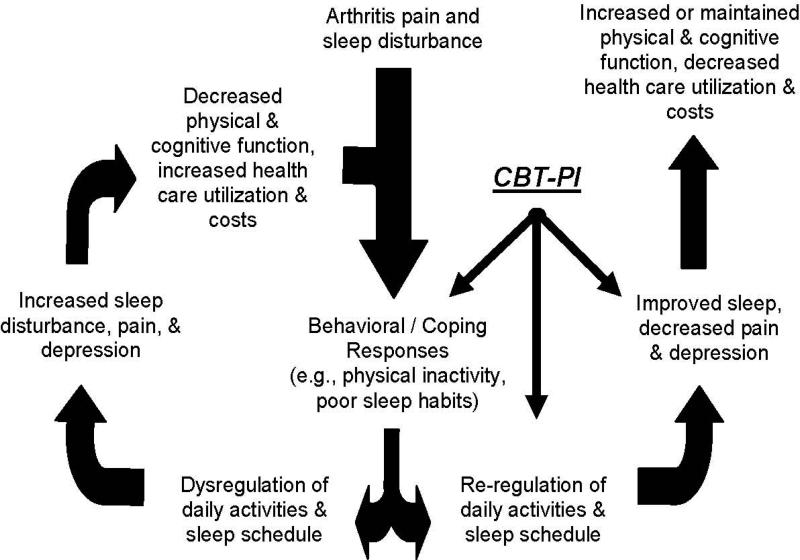
The Lifestyles trial was designed to test interventions based in a biobehavioral model of chronic pain dysfunction [8,9] and sleep disturbance [10] (see Figure 1). The combination of cognitive-behavioral pain coping skills training and insomnia intervention was expected to improve outcomes by enhancing coping responses to both osteoarthritis pain and insomnia, and re-regulating sleep patterns and daily activities. Thereby, restorative sleep was expected to be increased and pain threshold reduced, resulting in reduced pain, increased activity levels, and more positive emotions and cognitions.
Figure 1. Conceptual model of the effects of Cognitive-Behavioral Therapy for ain and Insomnia.
Conceptual Model: Impact of CBT for Pain and Insomnia (CBT-PI)
Study questions
The primary study questions posed in this trial were whether:
An integrated group cognitive-behavioral pain coping skills training intervention for osteoarthritis pain [11] and insomnia (CBT-PI), and a group cognitive-behavioral intervention for chronic pain alone (CBT-P), differed in effectiveness for arthritis pain and sleep outcomes relative to a group education only control intervention (EOC).
An integrated behavioral intervention for osteoarthritis pain and insomnia differed in effectiveness for arthritis pain and insomnia relative to a behavioral intervention for osteoarthritis pain alone.
Experimental design
Eligible participants were assigned to CBT-PI; CBT-P; or EOC through a clustered randomization procedure. The clusters were groups of participants who received one of the three interventions in a class format. Using a computer algorithm, the project programmer (KS) randomly assigned sets of 9 groups to the three experimental conditions in one block of 3 and one block of 6. Blocking limited the chance that group assignments would be unbalanced across the 6 participating primary care clinics.. In the third set, 11 groups were randomly allocated: 3 to CBT-PI, 3 to CBT-P and 5 to EOC to equalize accrual across the three experimental conditions due to chance fluctuations in group size. To achieve sample size goals, one additional group beyond the 38 initially planned was formed. This group was assigned to CBT-PI, the condition with the smallest cumulative sample size across the first 38 groups.
Study setting
The Lifestyles trial was carried out by a multi-disciplinary team from the University of Washington (UW) and Group Health Research Institute. The study setting was primary care clinics of Group Health Cooperative, a non-profit, integrated healthcare system in Washington State. From January 2009 to November 2010, we recruited patients from 6 Group Health primary care clinics. The study protocol and methods were reviewed and approved by relevant Institutional Review Boards. Study interventions were delivered in the clinics where participants received primary care.
Recruitment
Participant screening and recruitment was population-based, employing Group Health's electronic health care records. We identified persons who had a diagnosis of osteoarthritis (715xx) on at least one health care visit in the three years prior to the date of screening initiation in each clinic. Persons eligible for screening were age 60 years or older and continuously enrolled at Group Health for at least one year. Screening was via mail survey with two mailings and a reminder telephone call. Initial mailing included a $2 cash incentive. To remain eligible for the randomized controlled trial, persons who reported significant pain and insomnia on the screening survey were asked to agree to permit medical records review for study purposes, and to allow their contact information to be sent to UW study staff.
Consenting subjects were then contacted by telephone to confirm eligibility and interest in participation. During this follow-up call, eligible participants were offered up to three alternative start dates for a Lifestyles class, with classes starting on a Tuesday, Wednesday, or Thursday afternoon. Neither the staff contacting participants nor the participants knew which class (EOC, CBT-P, or CBT-PI) was being offered on a particular date. Participants received a reminder telephone call from a group leader one or two days prior to the initial class session for their assigned start date.
Eligibility criteria
Persons with both clinically significant arthritis pain and clinically significant insomnia were initially eligible for enrollment. Significant arthritis pain was defined by Grade II, III or IV pain on the Graded Chronic Pain Scale [12]. Significant insomnia was defined by self-reported sleep difficulties (trouble falling asleep, difficulty staying asleep, waking up too early, or waking up unrefreshed) at least 3 nights/week during the past month with at least one daytime sleep-related problem (e.g. fatigue/malaise, social/vocational dysfunction) [13].
Exclusion criteria
Persons with the following conditions identified in the last three years from Group Health electronic health care data were excluded prior to initial screening: rheumatoid arthritis; obstructive sleep apnea, periodic leg movement disorder, restless leg syndrome, sleep-wake cycle disturbance, rapid eye movement behavior disorder; dementia or receiving cholinesterase inhibitors; Parkinson's disease; cancer diagnosis in the past year and receiving chemotherapy or radiation therapy in the past year; inpatient treatment for congestive heart failure within the prior 6 months. At the time of telephone contact by study staff, potentially eligible subjects with a score of 7 or greater on the Blessed Short Orientation Memory and Concentration Test [14], or with a score greater than 32 on the sleep apnea sub-scale of the Sleep Disorders Questionnaire [15] were also excluded. At that telephone call, those who self-reported any of the following limitations or chronic conditions were also excluded: unable to read a newspaper; difficulty hearing in a group situation; unable to walk across a room without help, as well as persons reporting the following chronic conditions: periodic leg movement disorder; rapid eye movement behavior disorder; sleep apnea; Parkinson's disease; rheumatoid arthritis.
Enrollment
Eligible participants were enrolled in the trial once they: a) signed the informed consent statement prior to the baseline assessment; b) completed the baseline assessment; and, c) signed in at the beginning of the first session of the group program to which they were assigned.
Primary outcomes
The Lifestyles trial assessed treatment effects on two primary outcomes. Arthritis pain severity was measured using the combined Characteristic Pain Intensity and Disability Score scales of the Graded Chronic Pain Scale (GCPS) [16]. The primary osteoarthritis pain outcome measure was the mean of six 0-10 ratings: pain intensity right now, average pain intensity in the prior 3 months, worst pain intensity in the prior 3 months, and interference with daily activities, interference with work and housework activities, and interference with family and social activities. Insomnia was assessed using the Insomnia Severity Index (ISI) total item score [17]. The ISI includes seven items (sleep-onset, sleep maintenance, early morning awakening, satisfaction with current sleep pattern, interference with daily functioning, impairment attributed to sleep problems, distress caused by sleep problems). Each item was rated for the prior 2 weeks on a five-point Likert scale (0= no problem, 4= severe problem). A score of 15 or greater indicates clinical insomnia of moderate or greater severity [17].
Secondary outcomes
Secondary outcomes included the symptom scale of the Arthritis Impact Measurement Scale (AIMS-2) [18] and Sleep Efficiency (percent) as assessed by wrist actigraphy [19]. Wrist actigraphy data (Actiwatch-2; Respironics, Inc., Bend, Oregon) were collected for one week at each assessment point with concurrent data from sleep diaries [20] to determine objective sleep/wake patterns. One-minute epochs were analyzed with Actiware software, version 5.59.0015, using sleep diary, light sensor, and actigraph event marker data to identify bed time and wake time. The symptom scale of the AIMS-2 asks respondents to rate whether severe pain, morning stiffness, and pain making it difficult to fall asleep were present on all days, most days, some days, a few days, or no days in the past month.
Ancillary outcomes
Measures of depression, cognition, and health care utilization and costs were employed to assess potential intervention effects on a broader set of clinically relevant outcomes. The Lifestyles trial was not powered to assess effects on ancillary outcomes, but will provide data regarding the following variables:
Depression
Clinical depression was measured by the Geriatric Depression Scale (GDS) [21]. The GDS is a 30-item depression questionnaire for older persons with yes/no ratings. Scores of 11-13 indicate mild depression while 14 or greater indicates moderate to severe depression.
Cognition
Four different but comparable versions of a brief cognitive test battery were administered in counterbalanced order, at baseline, 2 month follow-up, and at 9 and 18-month follow-up visits. A laptop touch-screen notebook computer-administered test battery took approximately 30 minutes to complete and targeted cognitive domains deemed susceptible to effects of pain and sleep disturbance, including sustained and selective attention, executive function, short-term memory, and processing speed. All but one of the cognitive tests (Modified Mini-Mental Status Exam [22]) were selected from a laptop-administered cognitive test battery used in clinical trials [23]. Cognitive testing occurred in the participants’ homes. The battery included the following tests: Sustained Attention; the Stroop; Auditory Number Sequencing; Word List Memory; Symbol Digit Substitution ; Semantic Fluency Test; and the Modified Mini-Mental Exam (3MSE). The 3MSE was administered only at the baseline and 18 month assessments. See Appendix 1 for descriptions of the cognitive tests.
Health care use and costs
Information on health care visits and medication use of study participants from Group Health electronic health care data will be analyzed for two years after enrollment. Permission was obtained to track health care and medicine use data for five years after enrollment. Group Health electronic health care data have been used in numerous studies evaluating effects of behavioral interventions on health care use [24]. Intervention group differences in rates of health care visits for pain, sleep and other non-specific symptoms, and rates of filling prescriptions for pain and sleep medications will be compared, as well as differences in overall costs of ambulatory health care.
Baseline and follow-up assessments
Each assessment included two home visits. At the initial baseline visit, a research assistant/assessor obtained signed informed consent, measured blood pressure, and administered the Modified Mini-Mental State Exam (at baseline and 18 month assessments only). At the first baseline assessment (only), the assessor introduced the participant to an abbreviated practice set of the computer-administered cognitive tests (as described above). The practice cognitive testing was employed to train participants in cognitive assessment procedures. Data from practice testing will not be used for research purposes. The assessor then explained how to use the wrist actigraph and daily sleep diary over the following week, and left a self-administered questionnaire booklet to be completed before the next visit.
The assessor made a second visit to the participant's home seven days later to administer the full set of computerized cognitive tests, and to retrieve the actigraph, daily diary, and completed self-administered questionnaires. After the participant completed the computerized cognitive assessment, the assessor reviewed the daily diary and questionnaire booklet, and obtained missing information when possible. The assessor then conducted an interview that included the pain, sleep and depression questionnaires. In addition,, the assessor asked the participant about current medication use for pain, sleep, and mood, and when these medications were last taken. Assessors looked at medication bottles to verify name, prescription schedule, and dose.
Participants received a $50 incentive payment after completing the baseline assessment and attending the first group session. Follow-up assessments were completed approximately 2 months after enrollment (post-intervention assessment) and 9 months after enrollment. While the classes typically lasted six weeks, the post-intervention assessment occurred two months after enrollment to permit leeway for a session delayed due to holiday or other reason. Long-term follow-up to assess durability of intervention effects was carried out 18 months after enrollment. The procedure for follow-up assessments was the same as the baseline assessment, except informed consent and practice cognitive testing were not repeated.
Interventions
The Lifestyles trial compared a six session cognitive-behavioral pain coping skills intervention for osteoarthritis pain and insomnia (CBT-PI) to a six session cognitive-behavioral intervention for osteoarthritis pain alone (CBT-P) [11,25], with both active interventions compared to a six session education only control (EOC) that presented non-directive chronic pain and sleep education. Each of the six weekly group sessions was approximately 1.5 hours in duration.
The content of the three intervention protocols is summarized in Table 1. The EOC intervention contained similar educational content to that of the two active interventions, but without treatment implementation components believed necessary to yield clinically meaningful benefits. These active components included modeling, in-session practice, goal-setting, problem-solving techniques, and homework in both the CBP-P and CBT-PI interventions, and sleep enhancement techniques (sleep hygiene, stimulus control, and sleep restriction) in the CBT-PI intervention. The intent was for EOC to be credible to participants, blinding them to whether they were receiving an active or control intervention, thereby controlling for expectations of benefit. The EOC also controlled for non-specific effects of group participation (e.g., social support) and for general information about osteoarthritis pain and sleep. The rationale for comparing CBT for pain and insomnia to CBT for pain alone was to isolate specific effects of CBT for insomnia on pain and sleep outcomes, relative to an otherwise comparable CBT intervention for pain alone. This strategy was possible because CBT for pain and for insomnia have considerable shared content, while CBT for insomnia has unique techniques that target sleep problems (e.g. sleep restriction).
Table 1.
Content of the six 1.5 hour intervention sessions for CBT-PI, CBT-P and EOC*.
| Session | Active Treatment Domain | CBT-P | CBT-PI | EOC |
|---|---|---|---|---|
| 1 | Educational rationale | Pain and sleep management rationale | Pain and sleep management rationale Sleep hygiene education, stimulus control and sleep restriction |
Pain and sleep management rationale |
| 2 | Altering activity patterns / attention diversion techniques | Activity goal setting and relaxation | Sleep and activity goal setting and relaxation | Medication education for pain and sleep |
| 3 | Pleasant activity scheduling and guided imagery | Sleep and pleasant activity scheduling | Complementary and alternative treatments for pain and sleep | |
| 4 | Activity pacing | Activity pacing and sleep schedule review | Nutrition, pain, and sleep | |
| 5 | Automatic thoughts / alternative treatments | Automatic thoughts and willingness | Automatic thoughts and willingness Problem solving: sleep and activity goals | Memory and communicating with health care providers |
| 6 | Maintenance | Maintenance plan | Maintenance plan | Maintenance plan |
| Treatment implementation components | Education In-session practice Goal setting Problem-solving Homework | Education In-session practice Goal setting Problem-solving Homework | Education | |
CBT-PI = Cognitive-behavioral therapy for pain and insomnia; CBT-P – Cognitive-behavioral therapy for pain; EOC = Education only control
Intervention implementation
Each group was co-led by a single pair of mental health professionals (1 Masters-level family counselor, 1 PhD psychologist). These group leaders were informed whether they would be leading the CBT-P, CBT-PI, or EOC group to facilitate preparation, but they did not communicate this information to participants at any time (including during the group sessions). Both interventionists were experienced in working with older persons, but neither had prior experience with cognitive behavioral interventions for insomnia or osteoarthritis pain. Use of co-interventionists facilitated adherence to the intervention protocols and treatment individualization. In the CBT-PI sessions, a study assistant scored participants’ weekly sleep diaries at the beginning of each session to facilitate their use by the interventionists.
Intervention fidelity
Based in Lichstein's treatment implementation model [26], three intervention phases were differentiated: delivery by the provider; receipt by the participant; and enactment by the participant. Within each intervention phase, attention was paid to both induction and assessment of intervention fidelity. Table 2 summarizes steps taken to induce and assess intervention fidelity. Interventionists received 6 weeks of training by clinical psychologist co-investigators with substantial expertise and experience in protocol-based cognitive-behavioral interventions for insomnia (SM) and osteoarthritis pain (BB). One of the psychologist co-investigators (SM) observed all six sessions of CBT-P, CBT-PI and EOC the first time they were delivered. The audiotapes for ten percent of all subsequent group sessions were completely reviewed by a psychologist co-investigator (BB). Throughout the trial, the interventionists participated in weekly supervision sessions with the psychologist co-investigators (SM and BB), and received additional feedback as needed regarding areas where fidelity to intervention protocols could be enhanced based on audiotape review. For all three conditions, audiotape reviews were monitored to ensure that interventionists adhered to key protocol elements appropriate to each active intervention and that active treatment techniques for insomnia or pain were not employed in the EOC groups.
Table 2.
Induction and assessment of intervention fidelity across delivery, reception and enactment phases.
| Fidelity Induction | Fidelity Assessment | |
|---|---|---|
| Delivery | Comprehensive treatment manual Intervention delivery checklist Interventionist training |
Supervision Audiotape review |
| Receipt | Multiple channels for conveying key intervention elements to participants Repetition and practice of key intervention content with participants |
Participant post-session ratings of intervention delivery |
| Enactment | Explicit behavioral goals set Intervention activity scheduling Problem-solving enactment barriers Environmental cues employed as reminders |
Participant checklist for completing activities on schedule |
Blinding
At all assessments, assessors were blinded to participants’ intervention group assignment. The participants were never told to which group they had been assigned. The informed consent statement described the three different classes in general terms as alternative approaches to improving sleep and controlling pain. Participants were instructed not to discuss their class experience with the assessor to preserve assessor blinding. We did not ask participants to guess which class they had received in follow-up assessments, since describing the three types of classes could have compromised the blinding of the participants as well as the assessors. Since both participants and assessors were blind to which class participants received, the trial was double-blinded. While participants became aware of the content of the intervention they received, they were not told whether they were receiving an active or a control intervention, nor were they told anything about the content of the alternative classes. Each set of classes addressed pain and sleep issues to preserve this blinding .The principal investigators not involved in clinical supervision of study trainers (MVV, MVK), project staff involved in baseline and follow-up data collection, and staff responsible for data preparation remained blinded to the group assignments throughout recruitment, intervention, and follow-up.
Data analyses
Following CONSORT guidelines [27,28], evaluation of study hypotheses employed modified intent to treat analyses, including all individuals in the analyses who attended the first class session regardless of the number of sessions they completed over the six week class. We refer to the design as a modified intent to treat analysis because participants were enrolled in the trial when they signed in for the initial class session, rather than when they signed the consent form. Thus, a minimum level of protocol adherence was required for enrollment in the trial.
Null hypotheses of no difference across the three intervention arms were tested for two primary outcomes (pain severity as measured by the GCPS and insomnia severity as measured by the ISI). Experimental effects at post-intervention and nine month follow-ups were tested using repeated measures linear regression models, adjusted for baseline measures of age, baseline depression, baseline modified mini-mental status, an indicator for if the subject used opioids, an indicator for if the subject used hypnotics, clinic at which the intervention was delivered, and an indicator for if the outcome was measured at the nine-month follow up. Subsequent analyses evaluate the durability of intervention effects at long-term follow-up (18 months).
Regression models were estimated using generalized estimating equations (GEE) with an independence working correlation matrix structure [29]. The overall effect of intervention status for each outcome was estimated using the main effect of intervention group status (CBT-PI, CBT-P, EOC). The hypothesis of no-treatment effects was assessed using the modified Wald test [30], with a small-sample adjustment to the estimated sandwich covariance matrix to account for any within group correlation and within person correlation over time. A small-sample adjustment was employed because standard error estimates based on the traditional sandwich estimator using fewer than 40 groups are biased downwards [31,32]. Primary analyses included individuals at every follow-up visit at which a patient provided information. Since there was differential drop-out across among treatment groups, we used weighted GEE to account for differential drop out in analyses.
For outcome measures where the null hypothesis of no difference across the three experimental groups was rejected, we estimated confidence intervals and reported p-values for each of three pairwise comparisons (CBT-PI vs. EOC, CBT-PI vs. EOC, and CBT-PI vs. CBT-P).
Evaluation of secondary and ancillary outcomes were carried out in a similar fashion, but interpretation was informed by results of primary outcome analyses. When robust effects on the primary outcomes were observed, analyses of secondary and ancillary outcomes were employed to assess broader intervention effects. When primary outcome analyses did not reject the null hypothesis of no intervention effect, then subsequent analyses were considered exploratory and were presented within the context of the negative results for primary outcomes.
A planned set of secondary analyses examined differences across the three experimental conditions among the sub-group of participants with more severe arthritis pain, defined by having a Graded Chronic Pain severity score of 5.0 or greater.
Evaluation of statistical power
Power for the post-intervention and 9 month primary outcomes analysis was calculated using an intraclass correlation of 0.022, estimated from prior data on the GCPS pain severity measure. Since we did not have comparable data for the ISI, sleep efficiency, or the AIMS symptom scale, we assumed an equal intraclass correlation (0.022). Additionally, we assumed the within person correlation over time to be 0.5, such that using both post-intervention and 9 month outcome assessments increases effective sample size by one third in each treatment arm as compared to an analysis using 9 month data alone. Considering intraclass correlation, within person correlation over time, and a 90% retention rate, Lifestyles had an effective sample size of 127 in each of the treatment arms allowing the detection of a standardized effect size of approximately 0.35 with 80% power and alpha=0.05 for a two-sided test of significance. These estimates do not consider gains in efficiency potentially realized through adjustment for baseline scores.
Evaluation of detectable standardized effect size did not adjust for multiple comparisons. The two primary hypotheses regarding insomnia severity and pain severity are independent and measure different outcomes. Conclusions about the intervention effect can be made using only one of the outcomes. The success or failure of the intervention does not require a statistically significant effect in both outcomes. For this reason we did not adjust for multiple comparisons, in line with one school of thought about when adjustment for multiple comparisons is appropriate [33]. Given differing opinions on whether or not adjustment for multiple comparisons is appropriate in a trial with two primary outcomes, exact p-values will be reported for all tests performed regardless of significance level.
Results
Screening survey response
The screening survey was mailed to 8057 potentially eligible Group Health enrollees, among whom 1972 were found to be ineligible. The most common reason for ineligibility was not having osteoarthritis (N=1790). Usable screening surveys were obtained from 3321 individuals among 6025 initially eligible persons (53%). Among those completing the screening survey, 1210 (36%) met study severity criteria for clinically significant arthritis pain and insomnia defined by Grade II-IV pain on the GCPS and reporting sleep difficulties (trouble falling asleep, difficulty staying asleep, waking up too early, or waking up unrefreshed) at least 3 nights per week during the past month with at least one daytime sleep-related problem. Among these 1210 persons, 998 (82.5%) agreed to medical records review and contact by the study. As reported previously, participation in the groups was unrelated to participant clinical characteristics, but was related to factors affecting ability to attend a daytime class (age and retirement status) [34].
Regression to the mean from screening to baseline
Since patients became eligible based on elevated pain and insomnia severity scores at the time of screening assessment, regression to the mean was expected from screening to baseline. The median interval between the screening and baseline assessments was 65.8 days (SD=41.8) among patients who were subsequently enrolled in the trial. Among trial participants, from screening to baseline, the average pain severity score decreased from 5.0 (SD=1.50) to 4.3 (SD=4.59) (t=8.5, df=362, p<.0001). The mean Insomnia Severity Index rating decreased from 12.8 (SD=4.82) to 11.5 (SD=4.97) (t=5.43, df=366, p<.0001).
Trial enrollment
Baseline assessment was initiated for 393 persons, of whom 367 were enrolled in the trial when they signed in at the start of the first meeting of their assigned class. The attendance rate at the initial group session among those who were approached for baseline assessment was 91.1% for the EOC class (123/135); 96.1% for the CBT-P class (122/127); and 93.1% for the CBT-PI class (122/131), differences that were non-significant (X2=2.62, df=2, p=0.27). Among those who attended the first class session, 93.2% attended four or more of the six sessions (94.3% for EOC, 91.8% for CBT-P, and 93.4% for CBT-PI).
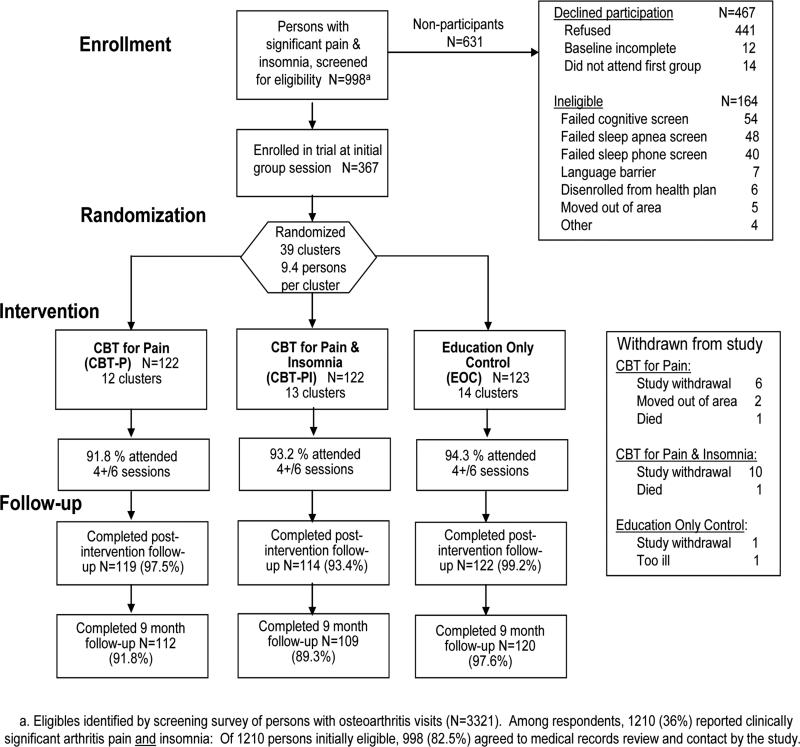
CONSORT flow chart
The CONSORT flow chart including participation in the baseline and follow-up assessments is provided as Figure 2 to summarize trial enrollment and follow-up procedures. There were 39 classes (clusters) assigned to the three experimental conditions by the study randomization procedure, with an average of 9.4 participants per class. The largest class had 12 participants, while the smallest had 5 participants.
Figure 2.
Consort Flow Diagram for enrollment of potentially eligible participants
Among those enrolled, the response rates at post-intervention follow-up were: 99.2% for EOC participants, 97.5% for CBT-P participants, and 93.4% for CBT-PI participants. At nine months, the response rates were 97.6 % for EOC participants, 91.8 % for CBT-P participants, and 89.3 % for CBT-PI participants. The response rates at nine months differed by greater than chance expectation (X2=6.63, df=2, p=0.036).
Discussion
The Lifestyles trial is a hybrid design combining features of efficacy and effectiveness trials. Assessors and participants were both blind to whether the participant was assigned to an active intervention or to a control condition. Participants were identified through a population screening survey, enhancing generalizability. Interventions were delivered in study participants’ primary care clinics, enhancing the relevance of the trial to dissemination in general health care settings. The education only control permits differentiation of the efficacy of the CBT-PI and CBT-P interventions from non-specific effects of participation in a support group that presents basic information about osteoarthritis pain and insomnia. Educational content of the EOC group might have clinical benefits for pain and insomnia outcomes, and participation in a group intervention per se might have non-specific effects on study outcomes. However, prior research assessing basic educational interventions have found them to be credible but ineffective for improving insomnia and chronic pain outcomes [35-38].
The multi-stage recruitment process employed in this study, and implementation of group interventions in widely distributed primary care clinics, necessarily involved difficulties that limit the generalizability and interpretation of study results. The multi-stage recruitment process meant that patients who were not motivated to participate in a group intervention were unlikely to enroll. Analyses comparing participants to non-participants found that participants were older and more likely to be retired than non-participants, but that no differences in pain severity, insomnia severity, depression, or use of prescription medications for pain or sleep were observed [34]. Since eligibility for the trial in terms of pain and insomnia severity was determined at the time of initial screening, improvements in outcome measures prior to group enrollment were observed. This reduced potential for improvement post-intervention, but also reduced potential for non-specific improvement in all three study groups. While it was not possible to blind the group leaders to intervention assignment, the assessors were blinded to patient group assignment. The participants were not informed which of the three interventions they were receiving. Participation rates in all three groups were high, as were follow-up response rates. The nine-month response rates in the two groups which received CBT were somewhat lower than the response rates for the EOC group, possibly reflecting greater demands of intervention participation in the CBT groups.
We considered a design with four arms in which cognitive-behavioral therapy for insomnia (only) was also provided. We decided against this design because of the substantial overlap of intervention content between CBT for pain and CBT for insomnia, the added sample size requirements, and the logistical difficulties of implementing four different group programs.
In summary, the Lifestyles trial design assessed the effectiveness of group cognitive-behavioral interventions for osteoarthritis pain and insomnia, and for osteoarthritis pain alone, relative to an education only control group. Group support and exposure to basic pain and sleep education was comparable between the active intervention and control groups. The research design evaluated intervention effects on primary study outcomes (pain severity and insomnia severity), secondary measures of pain severity and insomnia, intervention process outcomes, and on ancillary outcomes (cognition, depression, and health care utilization and costs). Group participation rates were high, as were follow-up rates post-intervention and at 9 months. This hybrid efficacy-effectiveness trial design permits assessment of intervention effects in primary health care settings while evaluating whether the group behavioral interventions yield specific effects relative to an education only control.
Acknowledgments
Supported by PHS grant R01-AG031126 (Drs. Vitiello, Von Korff and McCurry, Principal Investigators). We acknowledge the extraordinary efforts of Shirley Meyer, Fredda Jaffe and Janyce Vick.
Appendix 1
Assessments in the Cognitive Test Battery
All but one of the constituent cognitive tests (Modified Mini-Mental Status Exam) represent a subset of the Cogtest™ library (www.cogtest.com) developed by The Cognition Group. Cognitive testing occurs in the participants’ homes. The battery includes the following tests:
1. Sustained Attention Test
A computerized test of sustained attention requiring the participant to make a forced choice response to a visually presented 6-item alphanumeric array about whether a specified target character is present. A lengthy series of arrays, each requiring such a response is presented. Outcome measures include accuracy and response time (RT).
2. Stroop
A computerized test of selective attention that requires the participant to attend to two stimulus features at once (word content, font color) and respond when specified conditions are met (e.g., when word content matches font color). Responses to match stimuli (the word “red” painted red) are typically faster than responses to mismatch stimuli (e.g., the word “red” painted blue) as mismatch stimuli provide competing and therefore distracting information that impedes the decision and ultimate response. Faster RTs and fewer errors reflect more efficient attentional processing.
3. Auditory Number Sequencing
A computer-assisted test to assess verbal working memory capacity. In this test, a series of numbers is presented auditorily, and the participant is asked to repeat the list but in ascending order. The string of numbers is progressively increased until the participant is unable to successfully complete two consecutive trials. For each trial, the examiner will record the participant's response using the computer. The outcome measure for this task is maximum number of items successfully manipulated.
4. Word List Memory
A computer-assisted test of verbal learning that permits quantification of learning capacity over a number of trials and retention of this information over time. In this test, 16 words are auditorily presented (via computer) and the participant is asked to immediately recall the list. For the second learning trial, only those items that were omitted from recall are re-presented to the participant. The participant is then instructed to recall all of the words on the list, including those thatwere recalled but not repeated when the list was re-presented. This selective reminding procedure, whereby participants are reminded only of the words that were omitted from the previous recall, is repeated for an additional 3 trials. Following a delay (15 minutes), the participant is asked to recall the words on the list. For each trial, the examiner will record the participant's response using the computer. Outcome measures for this test include number correct on the learning trials and number correct following the delay.
5. Symbol Digit Substitution
A computerized test modeled after the subtest of the standardized Wechsler Adult Intelligence Scale with the same name, to assess speed of information processing. In this task, participants are provided with a legend of paired numbers and symbols. Symbols (unpaired) are presented in series and in response, participants are asked to touch the associated number on the legend as quickly as possible. The outcome measure for this task is the number of correct responses within 90 seconds.
6. Semantic Fluency Test
A computer-assisted test assessing semantic access and verbal processing speed; abilities that are characteristically impaired for older adults with mild cognitive impairments relative to their age-matched peers. The outcome measure is the number of category exemplars listed in 60 seconds. The examiner will keep a tally of the number of correct responses using the computer.
7. The Modified Mini-Mental Exam (3MSE)
A screening test commonly administered in clinical trials to assess general cognitive functioning. This test represents an expanded version (100 points) of the shorter and well-known Mini-Mental Status Exam (MMSE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Moffitt PF, Kalucy EC, Kalucy RS, Baum FE, Cooke RD. Sleep difficulties, pain and other correlates. J Intern Med. 1991;230(3):245–9. doi: 10.1111/j.1365-2796.1991.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein K. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998-2004). Sleep. 2006;29(11):1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 4.Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain management in adults: A meta-analysis. Health Psychol. 2007;26(3):241–250. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 5.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5(4):355–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain interrelate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 7.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin SF, Von Korff M, Le Resche L. Epidemiologic studies of chronic pain: A dynamic-ecologic perspective. Ann Behav Med. 1992;14:3–11. [Google Scholar]
- 9.Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 10.Espie CA. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Ann Rev of Psychol. 2002;53:215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 11.Keefe JF, Caldwell DS, Williams DA, Gil KM, Mitchell D, et al. Pain coping skills training in the management of osteoarthritis knee pain: II. Follow-up results. Behav Ther. 1990;21:435–447. [Google Scholar]
- 12.Von Korff M, Ormel J, Keefe F, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 13.Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of Research Diagnostic Criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 14.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 15.Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone VP, Jr, et al. The Sleep Disorders Questionnaire: I. Creation and multivariate structure of SDQ. Sleep. 1994;17:160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 16.Von Korff M. Assessment of chronic pain in epidemiological and health services research: Empirical bases and new directions. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment Second Edition Third Edition. Guilford Press; New York: 2011. pp. 455–473. [Google Scholar]
- 17.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2: the content and properties of a revised and expanded Arthritis Impact Measurement Scales health status questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 19.Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, et al. Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep. 2003;26(3):337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 21.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatric Res. 1982-1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-mental state examination (MMSE) and the modified MMSE (3MS): a psychometric comparison and normative data. Psychol Assess. 1996;8:48–59. [Google Scholar]
- 23.The Cognitive Group website [July 13, 2011]; at http://www.cogtest.com/cog_lib.html.
- 24.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative of Puget Sound. In: Strom BL, editor. Pharmacoepidemiology. 3rd Edition John Wiley and Sons; New York: 2000. pp. 247–262. [Google Scholar]
- 25.Keefe FJ, Aberenethy AP, Campbell LC. Psychological approaches to understanding and treating disease-related pain. Ann Rev Psychol. 2005;56:601–630. doi: 10.1146/annurev.psych.56.091103.070302. [DOI] [PubMed] [Google Scholar]
- 26.Lichstein KL, Redel BW, Grieve R. Fair test of clinical trials: A treatment implementation model. Adv Behav Res Ther. 1994;16:1–29. [Google Scholar]
- 27.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1:2. doi: 10.1186/1471-2288-1-2. doi: 10.1186/1471-2288-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell MK, Elbourne DR, Altman DG, CONSORT group CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702–708. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;1986;42(1):121–30. [PubMed] [Google Scholar]
- 30.Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Transactions Am Mathematical Soc. 1943;54(3):426–482. [Google Scholar]
- 31.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Public Health. 2004;94(3):423–32. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veazie PJ. When to combine hypotheses and adjust for multiple tests. Health Services Research. 2006;41:804–818. doi: 10.1111/j.1475-6773.2006.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancl LA, DeRouen TA. DeRouen. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57(1):126–134. doi: 10.1111/j.0006-341x.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 34.McCurry SM, Von Korff M, Vitiello MV, Saunders K, Balderson BH, Moore AL, et al. Frequency of co-morbid insomnia, pain, and depression in older adults with osteoarthritis: Predictors of enrollment in a randomized treatment trial. J Psychosom Res. 2011 doi: 10.1016/j.jpsychores.2011.05.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: Results of a randomized, controlled trial. J Am Geriatr Soc. 2011 doi: 10.1111/j.1532-5415.2011.03519.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linton SJ, Kamwendo K. Low back schools. A critical review. Phys Ther. 1987;67(9):1375–1383. doi: 10.1093/ptj/67.9.1375. [DOI] [PubMed] [Google Scholar]
- 37.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine Report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 38.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26:177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]