Abstract
The duration of nocturnal pineal melatonin secretion transduces effects of day length (DL) on the neuroendocrine axis of photoperiodic rodents. Long DLs support reproduction, and short DLs induce testicular regression, followed several months later by spontaneous recrudescence; gonadal regrowth is thought to reflect development of tissue refractoriness to melatonin. In most photoperiodic species, pinealectomy does not diminish reproductive competence in long DLs. Turkish hamsters (Mesocricetus brandti) deviate from this norm: elimination of melatonin secretion in long-day males by pinealectomy or constant light treatment induces testicular regression and subsequently recrudescence; the time course of these gonadal transitions is similar to that observed in males transferred from long to short DLs. In the present study, long-day Turkish hamsters that underwent testicular regression and recrudescence in constant light subsequently were completely unresponsive to the antigonadal effects of short DLs. Other hamsters that manifested testicular regression and recrudescence in short DLs were unresponsive to the antigonadal effects of pinealectomy or constant light. Long-term suppression of melatonin secretion induces a physiological state in Turkish hamsters similar or identical to the neuroendocrine refractoriness produced by short-day melatonin signals (i.e., neural refractoriness to melatonin develops in the absence of circulating melatonin secretion). A melatonin-independent interval timer, which would remain operative in the absence of melatonin during hibernation, may determine the onset of testicular recrudescence in the spring. In this respect, Turkish hamsters differ from most other photoperiodic rodents.
Keywords: Turkish hamster, photoperiod, pineal, interval timer, testes
Seasonal rhythms of reproduction, social behavior, sleep, body weight, food intake, hormone secretion, and immune function are well characterized in several mammals (e.g., Martin et al., 2008; Paul et al., 2008; Prendergast et al., 2002). Variation in day length (DL) provides a reliable calendar used by many species to restrict reproduction to energetically favorable times of year (Bronson and Heideman, 1994; Goldman, 2001). Transduction of DL information to the neuroendocrine axis is mediated by variations in the duration of nocturnal pineal melatonin secretion (Bittman and Karsch, 1984; Carter and Goldman, 1983); elevated melatonin secretion is ~3 h longer in 14-h nights than in 8-h nights (Darrow et al., 1986). Neural and pituitary melatonin target tissues decode the duration of these signals, thereby controlling many seasonal traits (Bartness et al., 1991; Lincoln and Clarke, 1994; Maywood and Hastings, 1995).
In long-day (LD) breeders, the decrease in DL after the summer solstice initiates gonadal regression (Butler et al., 2007a). Reproductive quiescence endures for 3 to 4 months (Carter et al., 1982; Park et al., 2006; Reiter, 1980), during which time the antigonadal effect of short days wanes. Both in the field (Christian, 1980) and in simulated natural photoperiods (Butler et al., 2007a, 2007b), gonadal recrudescence begins during the short DLs of mid-winter. Rodents held in static short DLs also spontaneously revert to the spring reproductive phenotype after many weeks (Prendergast et al., 2000). This so-called spontaneous recrudescence, documented in a number of rodent species (Goldman et al., 2004), is universally attributed to the development of neuroendocrine refractoriness to long-duration pineal melatonin signals (e.g., Bartness et al., 1993; Bittman, 1978; Freeman and Zucker, 2001; Prendergast et al., 2002). Importantly, nocturnal melatonin secretion continues to faithfully encode day length in refractory hamsters (Rollag et al., 1980).
In most rodent species, the transition to the winter phenotype requires the pineal gland: pinealectomized animals in LDs sustain the spring phenotype and continue to do so even when transferred to short days (SDs; e.g., Siberian hamsters, Carter and Goldman, 1983; marsh rice rats, Edmonds et al., 2005; white-footed mice, Johnston et al., 1982; Syrian hamsters, Reiter, 1980; meadow voles, Rhodes, 1989; field voles, Versi et al., 1983). In contrast, pinealectomy of LD-housed Turkish hamsters (Mesocricetus brandti) induces a sequence of gonadal regression and recrudescence temporally indistinguishable from that induced by short DLs (Carter et al., 1982). A similar phenomenon has been described in only one other rodent, the European hamster, Cricetus cricetus (Masson-Pévet et al., 1987). The proximate causes of this type of gonadal regression and recrudescence are unknown. The sudden withdrawal of melatonin appears to trigger both gonadal regression and subsequent recrudescence via a melatonin-independent mechanism. Turkish hamsters experience long melatonin-free intervals during hibernation; a melatonin-independent interval timer may be necessary to ensure the onset of gonadal recrudescence at the appropriate time in the spring.
The properties of the postulated melatonin-independent mechanism, as well as its relation to melatonin-dependent seasonal processes, have not been studied. Hong et al. (1986, p 530) suggested that “two different mechanisms are responsible for testicular regression in this species [Turkish hamsters].” We tested the hypothesis that testicular regression and subsequent recrudescence induced by short day lengths (long-duration nocturnal melatonin secretion) and by constant light treatment, which eliminates melatonin secretion, are mediated by common or interconnected neuroendocrine substrates.
MATERIALS AND METHODS
A Turkish hamster colony was established locally in 2004 from stock generously supplied by Robert and Joan Johnston of Cornell University. The breeding room was maintained in a 16L photocycle (16 h light/day, light intensity at cage level of ~300 lux; lights on at 0200 h, Pacific Standard Time) at an ambient temperature of 22 ± 1 °C. Hamsters had continuous access to tap water and pellets of either Lab Diet Prolab 5P00 or 8664 Harlan Teklad F6 Rodent Diet. Diet had no effect on responses to photoperiod (data not shown). Hamsters were assigned to experimental groups at approximately 10 weeks of age. All procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley.
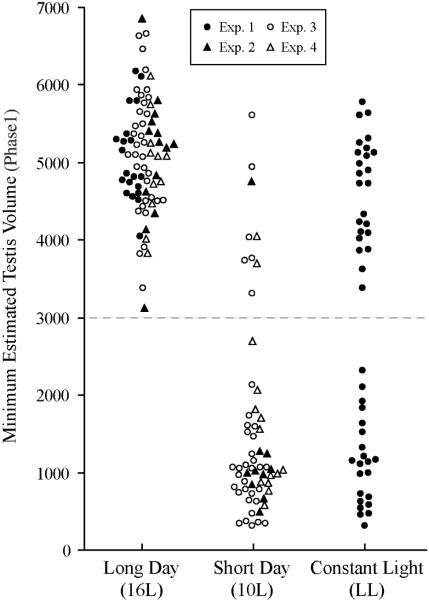
At regularly scheduled intervals, the length and width of the hamsters' right testes were measured externally under light anesthesia induced by isoflurane vapors. Estimated testis volume (ETV; testis length × width squared) is a noninvasive measure highly correlated with testis weight and reproductive competence (Watson-Whitmyre and Stetson, 1985). Long-day Turkish hamsters in reproductive condition and those that have undergone complete testicular regression have ETVs of ~5500 and 1500, respectively. Testes were measured by a single investigator within experiments (experiments 1, 3, 4: MB; experiment 2: KT). Measures differed slightly between experimenters. For purposes of identifying testicular regression across all hamsters (see Results and Fig. 2), minimum ETV values for experiment 2 were adjusted (ETVadjusted = 1.13 × ETVKT + 66.7) based on the mean difference between minimum ETV in 16L (ETVMB = ETVKT + 642) and in 10L hamsters with ETV <2000 (ETVMB = ETVKT + 168). All other analyses in experiment 2 were performed on unadjusted data.
Figure 2.
Minimum testis dimension of individual hamsters during maintenance in long or short days or constant light. Each point represents a different hamster. Values for experiment 2 are adjusted as described in Materials and Methods.
Experiment 1: Effects of Constant Light (LL) on Responsiveness to Short Days
Adult males were maintained in their natal 16L photoperiod (n = 27) or transferred to LL (n = 46) for 28 weeks (weeks 0–28; phase 1); thereafter, all hamsters were housed in a 10L photoperiod for 24 weeks (lights on at 0800 h; weeks 28–52; phase 2). See Figure 1 for the timeline of treatments in this and succeeding experiments. ETV was determined every 4 weeks. For 7 of the long-day control hamsters, ETV was not measured during phase 1. See Table 1 and Figure 3 legend for specific details about numbers of hamsters in each analysis.
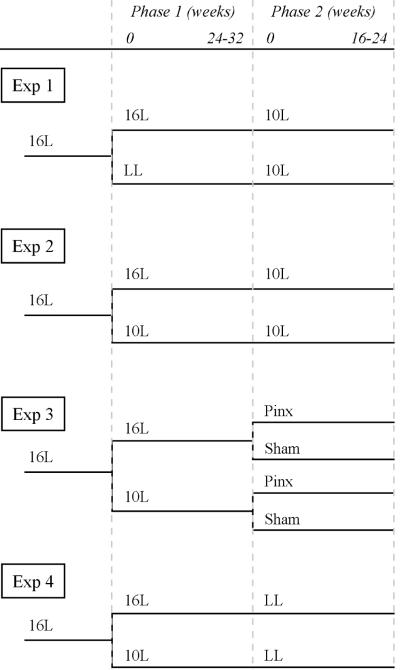
Figure 1.
Course of photoperiodic and surgical treatments during phases 1 and 2 (separated by dashed lines) for the 4 experiments. Number of weeks in each phase is indicated at the top but varied by experiment; phase 1 lasted 24 to 32 weeks, whereas phase 2 continued for either 16 or 24 weeks in different experiments.
Table 1.
Treatments in each phase (photoperiod or surgery) and the number of hamsters that did (Regr.) or did not (No Regr.) exhibit testicular regression. In experiment 3, phase 2, hamsters remained in their phase 1 photoperiod after pinealectomy (pinx) or sham-pinx. Chi-square tests compared the proportion of Regr. hamsters in a given group with the within-experiment control group.
| Phase 1 Treatment | Regr. | No Regr. | Phase 2 Treatment | Regr. | No Regr. | Chi-Square Test | |
|---|---|---|---|---|---|---|---|
| Experiment 1 | 16L | 0 | 20a | 10L | 10 | 16b | Control |
| LL | 22 | 24c | 10L | 2 | 20 | p < 0.05 | |
| Experiment 2 | 16L | 0 | 14 | 10L | 6 | 8 | Control |
| 10L | 9 | 1c | 10L | 0 | 9 | p < 0.05 | |
| Experiment 3 | 16L | 0 | 34 | Pinx in 16L | 8 | 14 | Control |
| Sham in 16L | 0 | 12 | p < 0.05 | ||||
| 10L | 29 | 6c | Pinx in 10L | 0 | 17 | p < 0.01 | |
| Sham in 10L | 1 | 11 | |||||
| Experiment 4 | 16L | 0 | 11 | LL | 5 | 6 | Control |
| 10L | 12d | 2d | LL | 0 | 9 | p < 0.02 |
ETV was not measured for 7 hamsters in phase 1.
One hamster died after 4 weeks of phase 2.
Nonresponders were removed from phase 2 analyses.
Three responders and 2 nonresponders were removed for a separate experiment before phase 2.
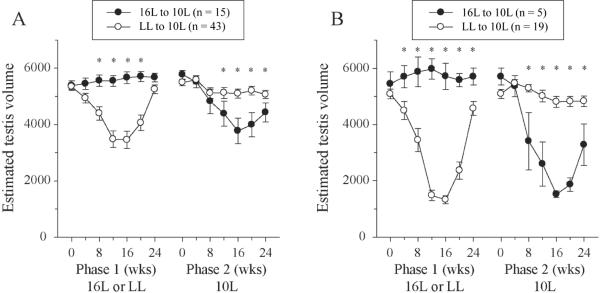
Figure 3.
Testis dimensions (mean ± SE) in constant light (LL) or long days (16L) during 24 weeks in phase 1 and subsequently during maintenance for 24 weeks in short days (10L, phase 2). Week 28 of phase 1 is equivalent to week 0 of phase 2. Data in A are for all hamsters for which estimated testis volume (ETV) measures were obtained at all 14 time points; of the 27 hamsters in 16L, 7 hamsters were not measured in phase 1, 2 others missed isolated measurements, 1 died after 4 weeks of phase 2, and 2 more died after 16 weeks of phase 2. Three of the 46 hamsters originally in LL died after 16 weeks of phase 2; their data are not plotted. Data in B are restricted to hamsters with minimum ETV < 3000 either in phase 1 LL or in phase 2 10L. Of the ten 10L-responsive hamsters (Table 1), only 5 are plotted: 3 hamsters were missing ETV measures, and 2 others died between weeks 16 and 20 of phase 2. *Significant pairwise differences between groups (Tukey test; p < 0.05).
Experiment 2: Effects of 10L on Responsiveness to Short Days
This experiment assessed whether hamsters that have undergone gonadal regression and recrudescence in 10L will undergo a second testicular involution with continued maintenance in short DLs. During phase 1, hamsters randomly assigned to 16L (n = 14) and 10L (n = 10) were maintained in these photoperiods (weeks 0–24) and then moved to 10L for 16 weeks in phase 2 (weeks 24–40); ETV was measured every 4 weeks.
Experiment 3: Effects of Refractoriness to Short Days on Responsiveness to Pinealectomy
Constant light and pinealectomy each cause gonadal regression in long-day photosensitive Turkish hamsters (Carter et al., 1982), but it was not known whether short-day refractory hamsters would respond in a similar fashion to these interventions. Groups of adult males from the 16L colony were randomly assigned to this photoperiod (n = 34) or to 10L (n = 35) for 24 weeks (phase 1, weeks 0–24). ETVs were obtained every 4 weeks. Three hamsters in 16L had small testes at a single phase 1 time point together with a substantial reduction in body weight attributable to defective water bottles; these and the next ETV points were removed from all analyses. At week 28, hamsters from each photoperiod were equated with respect to prior gonadal history and either pinealectomized (pinx) or sham-pinx. Hamsters remained in their presurgery photoperiods; the resulting groups were SD-pinx, SD-sham, LD-pinx, and LD-sham. ETVs were monitored for the next 16 weeks (phase 2, weeks 28–44). This experiment was performed in 3 balanced replications (n = 20, 20, and 29) and combined for analysis.
For surgical procedures, the hamsters were held in a stereotaxic apparatus equipped with a Model 906 rat anesthesia mask for the delivery of isoflurane vapors (David Kopf Instruments, Tujunga, CA). The lambda suture was exposed by a midline incision on the head, and a hole was drilled in the skull either lateral to or on lambda. The pineal gland was removed with fine iris forceps, and bleeding from the superior sagittal sinus was staunched. The skull opening was filled with Gelfoam (Pharmacia, Kalamazoo, MI) and the scalp incision closed with wound clips (Mik Ron Auto Clip 9 mm, Becton Dickinson, Franklin Lakes, NJ). In replicate 1, hamsters were deeply anesthetized with equithesin (2.5–3.0 mL/kg intraperitoneally [IP]) and treated postoperatively with buprenorphine (0.2 mL of 0.015-mg/mL solution, injected subcutaneously [SC], Hospira, Lake Forest, IL). In replicate 2, hamsters were treated preoperatively with 0.2 mL buprenorphine SC followed 10 min later by pentobarbital sodium (10 mg/kg IP); surgical anesthesia was then maintained by isoflurane. Hamsters in replicate 3 were treated as those in replicate 2 but were not injected with pentobarbital sodium. All hamsters in replicates 2 and 3 were injected with 5 to 10 mL of sterile saline SC postoperatively. Apple and mush were provided daily for 5 days after surgery.
Experiment 4: Effects of Short Days on Responsiveness to Constant Light
Groups of adult males were kept in the colony 16L photoperiod (n = 11) or transferred to 10L (n = 14) (phase 1, weeks 0–32). All hamsters were then transferred to LL for 16 weeks (weeks 32–48). ETV was measured every 4 weeks except for week 28 of phase 1.
Statistics
Effects of photoperiod treatment and time were assessed with repeated-measures analysis of variance (ANOVA), with each experimental phase analyzed separately. The results were largely unchanged by the inclusion of nonresponders (e.g., Fig. 3), so for clarity, only the data from responsive hamsters are shown in Figures 4 to 6. When analyses were limited to responsive hamsters, all effects of photoperiod, time, surgery, and their interactions were highly significant (p < 0.001). The results were robust and remained significant (p < 0.05) when all nonresponders were included, save for experiment 3, phase 2, as noted below in the Results section. Pairwise differences between groups were tested with the post hoc Tukey test (indicated in the figures). Differences in the proportions of hamsters responding to different manipulations were assessed with chi-square tests. Within experiments, sample sizes differed for different statistical tests: ETV values were unavailable for some hamsters (missing values or death; see Results), so data from these hamsters were neither included in ANOVA tests nor plotted in line graphs (Figs. 3–6). Nevertheless, whether or not these hamsters underwent testicular regression could generally be determined, so they were included in the chi-square tests of independence (Table 1). Analyses of photoresponsiveness were restricted to hamsters that survived until at least week 16 of phase 2 in each experiment. In experiment 3, between-group differences at specific time points were analyzed by 2-way ANOVA followed by post hoc Tukey tests. All statistical tests were performed using the StatView program (Version 5.0.1, SAS Institute, Cary, NC), with significance level set to p = 0.05. Means and standard errors are reported.
Figure 4.
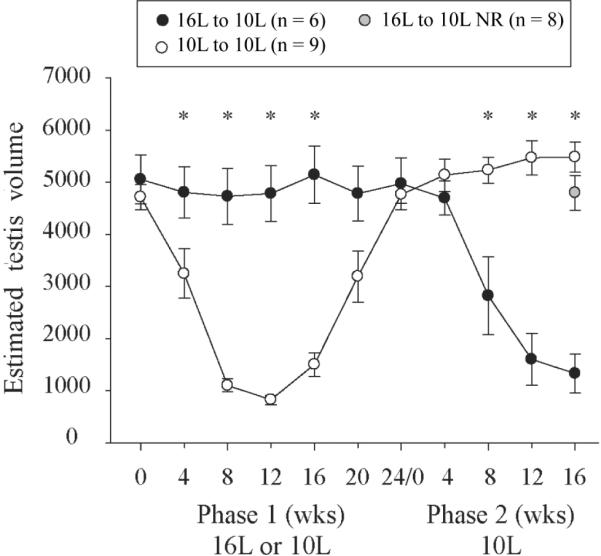
Testis dimensions (mean ± SE) of control hamsters in 16L and hamsters that underwent gonadal regression during phase 1 maintenance in 10L; all animals were maintained in 10L during phase 2. The final mean ETV for 16L to 10L nonresponders (NR) is plotted as a shaded gray circle. *Significant pairwise differences between groups (Tukey test; p < 0.05).
Figure 6.
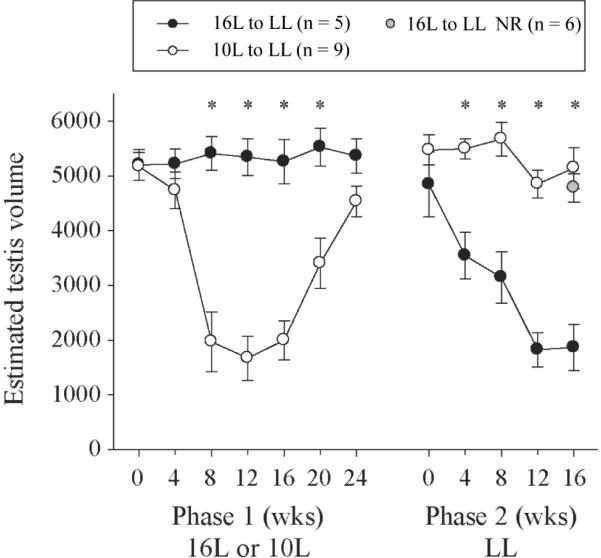
Testis dimensions (mean ± SE) of responsive hamsters maintained in 16L or 10L (phase 1) and then transferred to LL (phase 2). *Significant pairwise comparisons, Tukey test, p < 0.05. Responsiveness of 10L and 16L hamsters was determined by their response to 10L in phase 1 and LL in phase 2, respectively. The final mean ETV for 16L nonresponders (NR) is plotted as a gray shaded circle.
RESULTS
Photoperiodic Nonresponsiveness
In every well-studied photoperiodic rodent species, some individuals fail to undergo gonadal regression during maintenance in short day lengths; they are designated nonresponders (reviewed in Prendergast et al., 2001). Rates of nonresponsiveness to both short days and constant light (LL) were higher than previously reported for this species (Carter et al., 1982). Based on phase 1 data, hamsters were considered photoresponsive if minimum ETV fell below 3000; this threshold discriminates between regressed and nonregressed testes (Fig. 2). Mean minimum ETV of 16L hamsters was 5096 ± 83 (range, 3124–6859; Fig. 2). The number of hamsters that exhibited regression or maintained large testes is shown in Table 1. During phase 1, nonresponsiveness was more prevalent in LL (30 of 55, 55%) than in short days (9 of 59, 15%; chi-square test: p < 0.001). The same threshold (ETV < 3000) defined responsiveness to 10L, LL, and pinealectomy during phase 2.
The rate of nonresponsiveness to short days increased with age, from 15% in phase 1 to 59% in phase 2 (experiments 1 and 2: chi-square test, p < 0.001). Nonresponsiveness to melatonin suppression remained unchanged at 55% in phase 1 LL (experiments 1 and 2, n = 55), 55% in phase 2 LL treatment (experiment 4, n = 11), and 64% after phase 2 pinealectomy (experiment 3: n = 22) (omnibus chi-square test: p > 0.7).
Experiment 1: Effects of Constant Light (LL) on Refractoriness
Phase 1
Hamsters in 16L maintained large gonads, while those in LL exhibited regression and recrudescence (all hamsters, Fig. 3A; responders only, Fig. 3B). Asignificantly higher proportion of LL hamsters exhibited regression (Table 1, chi-square test, p < 0.001). Between weeks 16 and 20, testes of LL hamsters began to undergo recrudescence and achieved long-day values by week 24. This confirms findings of Carter et al. (1982) that LL induces testicular regression and, after ~16 weeks, permits testicular redevelopment.
Phase 2
A significantly larger proportion of hamsters from 16L than from LL responded to 10L with gonadal regression, indicating that LL induced refractoriness to short days (Table 1). Hamsters from LL maintained large gonads during the short-day (10L) challenge, whereas 16L hamsters underwent substantial gonadal regression before initiating spontaneous recrudescence (Fig. 3). Note that the time course of regression and recrudescence is similar in LL and 10L. The inclusion of nonresponders (Fig. 3A) attenuates but does not obscure the general pattern evident among the responders (Fig. 3B); when analysis is limited to responders, between-group differences are detected at 3 additional time points (Tukey test, Fig. 3).
Experiment 2: Effects of 10L on Responsiveness to Short Days
Phase 1
The testes of 9 of 10 hamsters underwent regression and recrudescence in 10L but remained large in all 14 hamsters in 16L (chi-square test, p < 0.001).
Phase 2
Hamsters that had experienced regression in 10L failed to undergo a second regression in response to the continuation of the 10L challenge. In contrast, photosensitive hamsters from 16L experienced gonadal regression (Fig. 4; responders only are plotted). Including all hamsters, there was a significant difference in the proportion of 10L and 16L hamsters that exhibited regression in 10L during phase 2 (Table 1).
Experiment 3: Effects of Refractoriness to Short Days on Response to Pinealectomy
Phase 1
Twenty-nine of 35 hamsters in 10L but none of 34 hamsters in 16L underwent testicular regression (Table 1; chi-square test, p < 0.001). The 6 SD males that failed to undergo gonadal regression were removed from the experiment.
Phase 2
Pinealectomy caused gonadal regression in 16L-photosensitive but not 10L-photorefractory hamsters (chi-square test, p < 0.05, Table 1). Testicular regression, recrudescence, and the response to pinealectomy are shown in Figure 5 for responsive hamsters (i.e., those exhibiting regression in 10L or after pinealectomy in 16L). The time course of response to SDs and to pinx was comparable: minimum ETV values indicative of complete gonadal regression occurred at week 12 of both phases (Fig. 5). When data analysis was restricted to responders, the effects of photoperiod, surgery, time, and all interactions were significant (repeated-measures ANOVA, p < 0.001, Fig. 5). ETVs for LD-pinx hamsters differed significantly from those of the other 3 groups on weeks 8 to 16 after surgery (phase 2; Tukey test, p < 0.05). Unlike in the other experiments, the significance of the statistical tests was altered when data for nonresponders were included, but trends remained unchanged (effect of photoperiod, p = 0.052; effect of surgery, p = 0.13; time × photoperiod × surgery interaction, p = 0.067; all other effects, p < 0.05). Significant pairwise differences in phase 2, as illustrated in Figure 5, were nevertheless preserved when all LD-pinx nonresponders were included in the analysis.
Figure 5.
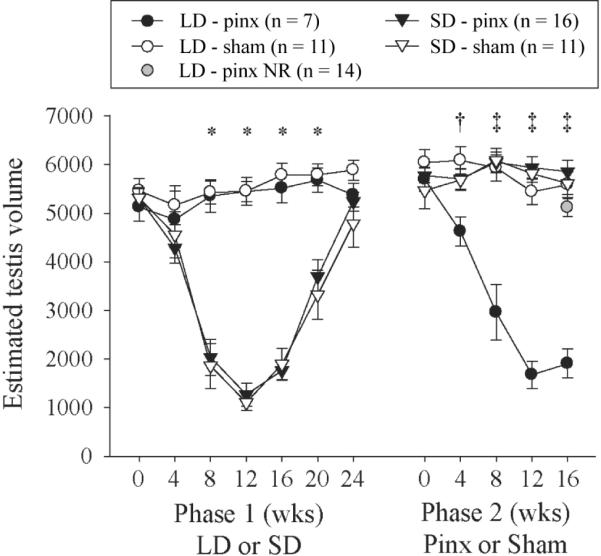
Testis dimensions (mean ± SE) for hamsters undergoing regression in short days (SDs) in phase 1 or after pinealectomy in phase 2. Hamsters with intact pineal glands were maintained in 16L or 10L in phase 1 and then either pinx or sham-pinx at phase 2, week 0 (= phase 1, week 28). *Tukey test: all long-day (LD) versus all SD hamsters (p < 0.05). †Tukey test: LD-pinx different from LD-sham (p < 0.05). ‡Tukey test: LD-pinx different from all other groups (p < 0.05). The final mean ETV for the LD-pinx nonresponders (NR) is shown for comparison (gray shaded circle).
Experiment 4: Effects of Short Days on Responsiveness to Constant Light
Phase 1
Twelve of 14 hamsters in 10L underwent gonadal regression and recrudescence. Four hamsters were sacrificed for a separate experiment after 14 weeks, and another died during the experiment, leaving 9 responders for phase 2. All 11 hamsters in 16L maintained large gonads (chi-square test, p < 0.001, compared with those in 10L).
Phase 2
All 9 hamsters from 10L maintained large gonads during the LL challenge, whereas significantly more hamsters from 16L exhibited regression (Table 1, Fig. 6). With the inclusion of the 6 LL nonresponders, all pairwise comparisons indicated in Figure 6 remained significant, and additional differences were detected at weeks 4 and 24 of phase 1 (not illustrated).
DISCUSSION
In Turkish hamsters, a melatonin-independent timer coordinates a pattern of gonadal regression and recrudescence in the absence of melatonin, culminating in a physiological state indistinguishable from that induced by short day lengths. Experiments 1 and 2 demonstrate that prolonged exposure to constant light, which suppresses melatonin secretion in Turkish hamsters (Hong et al., 1993), renders males unresponsive to the antigonadal effects of subsequent short-day exposure. These hamsters are refractory to long-duration melatonin signals despite never once experiencing such a signal. Experiments 3 and 4 establish the converse relation: short days that induce gonadal regression and later gonadal recrudescence render hamsters unresponsive to the antigonadal effects produced by cessation of melatonin secretion. The state induced by prolonged exposure to short-day melatonin signals appears to be isomorphic with that produced by long-term absence of melatonin.
Turkish hamsters exposed to low ambient temperatures spend much of the winter in deep hibernation, during which melatonin secretion is suppressed, except during periodic arousals of 1 to 3 days' duration (Darrow et al., 1986). A melatonin-independent timer that induces refractoriness to short days would both ensure appropriate timing of spring gonadal growth and prevent photoinhibition by the short days of late winter and early spring. We propose that decreases in day length from mid to late summer initiate testis regression, a melatonin-dependent process that may also be accelerated by decreasing ambient temperatures (cf. Larkin et al., 2002). The decrease in testosterone secretion is a prerequisite for entry into hibernation in male Turkish hamsters (Hall and Goldman, 1980). Once this occurs, we propose that an interval timer that continues to run in the absence of melatonin during bouts of torpor terminates hibernation and initiates spring gonadal recrudescence. Although likely unavoidable in the field, decreasing day lengths may not be a strict requirement to initiate the process: in Turkish hamsters maintained in long days, decreases in ambient temperature from 22 to 6 °C provoke testicular regression (Jagiello et al., 1992), followed by spontaneous gonadal recrudescence several months later (Hall et al., 1982). We surmise that these hamsters would subsequently be unresponsive to antigonadal effects of SDs because melatonin secretion is abrogated at low ambient temperatures.
Faithful encoding of day length by melatonin is sufficient to initiate gonadal regression in Siberian hamsters, but here too, additional weeks of melatonin secretion are dispensable. Thus, 6 weeks of short DLs trigger refractoriness many weeks later without the necessity of intervening melatonin secretion (Prendergast et al., 2000); 6 weeks of long days also terminate refractoriness several months later, again without intervening melatonin availability (Kauffman et al., 2003). In these instances, melatonin signals initiate processes that, once begun, later induce and break photorefractoriness, respectively, in the absence of melatonin. The interval timers that govern these events are at least partially independent of sustained melatonin secretion; melatonin-independent processes may be common among seasonal species. It is unknown whether the breaking of short-day-induced refractoriness by long day lengths can occur in the complete absence of melatonin, but we consider this unlikely (cf. Bittman and Zucker, 1981).
Only nocturnal melatonin durations that fall within a narrow range, corresponding to day lengths of 15 to 17 h (Hong et al., 1986), maintain reproduction; all other melatonin durations induce and maintain reproductive quiescence until refractoriness ensues. Such biphasic responses to photoperiods have been shown in 2 other species to various degrees (Siberian hamsters, Hoffmann, 1982; Soay sheep, Wagner et al., 2008). In sheep, physiological responses to photoperiod correlated with amplitude of several photoperiodism-related genes, suggesting that very long and very short melatonin durations may be transduced in the same way at the molecular level (Wagner et al., 2008). There may be little selection pressure to sustain gonadotropin secretion for melatonin durations outside a restricted range, including those generated in very short or very long day lengths or after suppression of melatonin secretion by constant light or pinealectomy (B. D. Goldman, personal communication, 2008). This view dispenses with the distinction between melatonin-dependent and melatonin-independent interval timers; differences between congeneric Syrian and Turkish hamsters may reflect only the range of long days sufficient to maintain the long-day phenotype (12.5–24 h in the former and 15–17 h in the latter; Elliott, 1976; Hong et al., 1986).
The present results suggest a close relation between the mechanisms that underlie Type I seasonal rhythms, which rely on exogenous cues to trigger and reset endogenous timers, and Type II rhythms that are completely endogenous (Goldman et al., 2004; Zucker et al., 1991; Zucker, 2001). Turkish and European hamsters are the only Type I photoperiodic rodents in which the pineal gland is necessary to maintain the long-day reproductive phenotype (Carter et al., 1982; Hoffman and Reiter, 1965; Masson-Pévet et al., 1987; Prendergast and Freeman, 1999). That Turkish hamsters have both Type I and Type II traits suggests flux in seasonality substrates or coexistence of different seasonal pheno-types (Nelson, 1987).
Nonresponsiveness to 10L increased with age, from 15% at ~10 to 34 weeks to 59% at ~34 to 52 weeks of age. In contrast, age did not increase nonresponsiveness rates in LL (55% to 61% for age ranges above). Increases in short-day nonresponsiveness have been documented in older Siberian hamsters and prairie voles (Bernard et al., 1997; Horton and Yellon, 2001; Nelson, 1987). For short-lived species, advancing age and reduced future reproductive potential may favor continued breeding with the approach of winter, compared with ceasing breeding until the next season.
The present data suggest a degree of melatonin independence in seasonal timing and in the development of neuroendocrine refractoriness to melatonin. This may be peculiar to Turkish hamsters, or it may be a hitherto unrecognized mechanism that underlies seasonal timing in many photoperiodic species but that remains inaccessible without the pineal gland. The Turkish hamster may be a valuable species with which to dissect the interplay of photoperiod, melatonin, and endogenous interval timers in photoperiodism.
ACKNOWLEDGMENTS
We are grateful to Chris Tuthill, Kim Pelz, and Elanor Schoomer for assistance in maintaining the Turkish hamster colony. MPB was a Howard Hughes Predoctoral Fellow and was also supported by the Robert Katz Fellowship and Wang Family Fellowship. This research was supported by grant MH-61171 from the National Institute of Mental Health.
Footnotes
Dedication: This paper is dedicated to Bruce Goldman, in acknowledgment of his pioneering research on mammalian photoperiodism, on the occasion of his retirement from the Department of Physiology and Neurobiology, University of Connecticut, Storrs.
REFERENCES
- Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Losee-Olson S, Turek FW. Age-related changes in the photoperiodic response of Siberian hamsters. Biol Reprod. 1997;57:172–177. doi: 10.1095/biolreprod57.1.172. [DOI] [PubMed] [Google Scholar]
- Bittman EL. Hamster refractoriness: The role of insensitivity of pineal target tissues. Science. 1978;202:648–650. doi: 10.1126/science.568311. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Karsch FJ. Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biol Reprod. 1984;30:585–593. doi: 10.1095/biolreprod30.3.585. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Zucker I. Photoperiodic termination of hamster refractoriness: Participation of the pineal gland. Biol Reprod. 1981;24:568–572. doi: 10.1095/biolreprod24.3.568. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Heideman PD. Seasonal regulation of reproduction in mammals. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2nd ed. vol. 2. Raven; New York: 1994. pp. 541–583. [Google Scholar]
- Butler MP, Turner KW, Park JH, Butler JP, Trumbull JJ, Dunn SP, Villa P, Zucker I. Simulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters. Am J Physiol. 2007a;293:R402–R412. doi: 10.1152/ajpregu.00146.2007. [DOI] [PubMed] [Google Scholar]
- Butler MP, Trumbull JJ, Turner KW, Zucker I. Timing of puberty and synchronization of seasonal rhythms by simulated natural photoperiods in female Siberian hamsters. Am J Physiol. 2007b;293:R413–R420. doi: 10.1152/ajpregu.00216.2007. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): Duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Carter DS, Hall VD, Tamarkin L, Goldman BD. Pineal is required for testicular maintenance in the Turkish hamster (Mesocricetus brandti) Endocrinology. 1982;111:863–871. doi: 10.1210/endo-111-3-863. [DOI] [PubMed] [Google Scholar]
- Christian JJ. Regulation of annual rhythms of reproduction in temperate small rodents. In: Steinberger A, Steinberger E, editors. Testicular Development, Structure, and Function. Raven; New York: 1980. pp. 367–380. [Google Scholar]
- Darrow JM, Tamarkin L, Duncan MJ, Goldman BD. Pineal melatonin rhythms in female Turkish hamsters: Effects of photoperiod and hibernation. Biol Reprod. 1986;35:74–83. doi: 10.1095/biolreprod35.1.74. [DOI] [PubMed] [Google Scholar]
- Edmonds K, Riggs L, Masden T. Effects of photoperiod, melatonin, and the pineal gland on compensatory gonadal hypertrophy during postnatal development in the marsh rice rat (Oryzomys palustris) Zoolog Sci. 2005;22:763–774. doi: 10.2108/zsj.22.763. [DOI] [PubMed] [Google Scholar]
- Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–2346. [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci USA. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Goldman BD, Gwinner E, Karsch FJ, Saunders D, Zucker I, Ball G. Circannual rhythms and photoperiodism. In: Dunlap JC, Loros JJ, DeCoursey PJ, editors. Chronobiology: Biological Timekeeping. Sinauer Associates; Sunderland, MA: 2004. pp. 107–144. [Google Scholar]
- Hall VD, Bartke A, Goldman BD. Role of the testis in regulating the duration of hibernation in the Turkish hamster, Mesocricetus brandti. Biol Reprod. 1982;27:802–810. doi: 10.1095/biolreprod27.4.802. [DOI] [PubMed] [Google Scholar]
- Hall VD, Goldman BD. Effects of gonadal steroid hormones on hibernation in the Turkish hamster (Mesocricetus brandti) J Comp Physiol. 1980;135:107–114. [Google Scholar]
- Hoffman RA, Reiter RJ. Pineal gland: Influence on gonads of male hamsters. Science. 1965;148:1609–1611. doi: 10.1126/science.148.3677.1609. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. The critical photoperiod in the Djungarian hamster Phodopus sungorus. In: Aschoff J, Daan S, Groos G, editors. Vertebrate Circadian Systems. Springer-Verlag; Berlin: 1982. pp. 297–304. [Google Scholar]
- Hong SM, Rollag MD, Ramirez J, Stetson MH. A single injection of adrenergic agonists enhances pineal melatonin production in Turkish hamsters. J Pineal Res. 1993;14:138–144. doi: 10.1111/j.1600-079x.1993.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Hong SM, Rollag MD, Stetson MH. Maintenance of testicular function in Turkish hamsters: Interaction of photoperiod and the pineal gland. Biol Reprod. 1986;34:527–531. doi: 10.1095/biolreprod34.3.527. [DOI] [PubMed] [Google Scholar]
- Horton TH, Yellon SM. Aging, reproduction, and the melatonin rhythm in the Siberian hamster. J Biol Rhythms. 2001;16:243–253. doi: 10.1177/074873040101600307. [DOI] [PubMed] [Google Scholar]
- Jagiello GM, Fang JS, Sung WK, Hembree WC, Ducayen MB. Genetic characteristics of spermatogenesis in the Turkish hamster (Mesocricetus brandti) subjected to reduced temperature or light. J Therm Biol. 1992;17:175–184. [Google Scholar]
- Johnston PG, Boshes M, Zucker I. Photoperiodic inhibition of testicular development is mediated by the pineal gland in white-footed mice. Biol Reprod. 1982;26:597–602. doi: 10.1095/biolreprod26.4.597. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Freeman DA, Zucker I. Termination of neuroendocrine refractoriness to melatonin in Siberian hamsters (Phodopus sungorus) J Neuroendocrinol. 2003;15:191–196. doi: 10.1046/j.1365-2826.2003.00966.x. [DOI] [PubMed] [Google Scholar]
- Larkin JE, Jones J, Zucker I. Temperature dependence of gonadal regression in Syrian hamsters exposed to short day lengths. Am J Physiol. 2002;282:R744–R752. doi: 10.1152/ajpregu.00299.2001. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Clarke IJ. Photoperiodically-induced cycles in the secretion of prolactin in hypothalamo-pituitary disconnected rams: Evidence for translation of the melatonin signal in the pituitary gland. J Neuroendocrinol. 1994;6:251–260. doi: 10.1111/j.1365-2826.1994.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Philos Trans R Soc Lond B Biol Sci. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson-Pévet M, Pévet P, Vivien-Roels B. Pinealectomy and constant release of melatonin or 5-methoxytryptamine induce testicular atrophy in the European hamster (Cricetus cricetus, L.) J Pineal Res. 1987;4:79–88. doi: 10.1111/j.1600-079x.1987.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Photoperiod-nonresponsive morphs: A possible variable in microtine population-density fluctuations. Am Nat. 1987;130:350–369. [Google Scholar]
- Park JH, Kauffman AS, Paul MJ, Butler MP, Beery AK, Costantini RM, Zucker I. Interval timer control of puberty in photoinhibited Siberian hamsters. J Biol Rhythms. 2006;21:373–383. doi: 10.1177/0748730406292315. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: The internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA. Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus) J Biol Rhythms. 1999;14:62–71. doi: 10.1177/074873099129000452. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Flynn AK, Zucker I. Triggering of neuroendocrine refractoriness to short-day patterns of melatonin in Siberian hamsters. J Neuroendocrinol. 2000;12:303–310. doi: 10.1046/j.1365-2826.2000.00452.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: Neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol. 2. Elsevier Science; New York: 2002. pp. 93–156. [Google Scholar]
- Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Rhodes DH. The influence of multiple photoperiods and pinealectomy on gonads, pelage and body weight in male meadow voles, Microtus pennsylvanicus. Comp Biochem Physiol A. 1989;93:445–449. doi: 10.1016/0300-9629(89)90064-9. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Panke ES, Reiter RJ. Pineal melatonin content in male hamsters throughout the seasonal reproductive cycle. Proc Soc Exp Biol Med. 1980;165:330–334. doi: 10.3181/00379727-165-40981. [DOI] [PubMed] [Google Scholar]
- Versi E, Chiappa SA, Fink G, Charlton HM. Pineal influences hypothalamic GnRH content in the vole, Microtus agrestis. J Reprod Fertil. 1983;67:365–368. doi: 10.1530/jrf.0.0670365. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Johnston JD, Clarke IJ, Lincoln GA, Hazlerigg DG. Redefining the limits of day length responsiveness in a seasonal mammal. Endocrinology. 2008;149:32–39. doi: 10.1210/en.2007-0658. [DOI] [PubMed] [Google Scholar]
- Watson-Whitmyre M, Stetson MH. A mathematical method for estimating paired testes weight from in situ testicular measurements in three species of hamster. Anat Rec. 1985;213:473–476. doi: 10.1002/ar.1092130313. [DOI] [PubMed] [Google Scholar]
- Zucker I. Circannual rhythms. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian Clocks, vol. 12 of Handbook of Behavioral Neurobiology. Kluwer Academic/Plenum; New York: 2001. pp. 509–528. [Google Scholar]
- Zucker I, Lee TM, Dark J. The suprachiasmatic nucleus and the annual rhythms of mammals. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. Oxford University Press; New York: 1991. pp. 246–259. [Google Scholar]