Abstract
Hydrogen bonds in metalloproteins are key in directing reactivity yet to be achieved in synthetic systems. We have been developing a synthetic system that uses hydrogen-bonding interactions to modulate the secondary coordination around a transition metal ion. This was accomplished with the ligand bis[N-(6-pivalamido-2-pyridylmethyl)]benzylamine (H2pmb), which contains two carboxyamido units appended from pyridine rings. Several nickel complexes were prepared and structurally characterized. In particular, we found that the appended carboxyamido groups either provide intramolecular H-bond donors or can be converted to bind directly to a metal center. We established that the complex NiIIH2pmb(Cl)2 can be sequentially deprotonated with potassium tert-butoxide, causing coordination of the carboxyamido oxygen atoms and concomitant loss of the chloro ligands. The chloro ligands were also removed with silver(I) salts—in the presence of acetate ions, the complex NiIIH2pmb(κ2-OAc)(κ1-OAc) was isolated, in which an intramolecular H-bonding network occurs between the H2pmb ligand and the coordinate acetato ligands.
1. Introduction
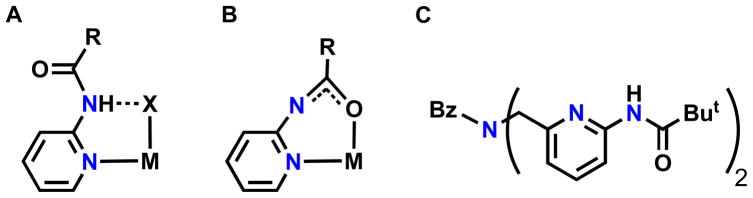
Hydrogen bonds (H-bonds) within the secondary coordination sphere are important in controlling the structure and function of metal complexes.i This is typified within the active sites of metalloproteins, wherein protein-derived H-bonding networks affect a variety of structural and physical properties that are ultimately correlated to activity.ii These findings have inspired the development of synthetic systems that attempt to create similar H-bonding networks within the secondary coordination sphere of transition metal complexes.iii The most common designs utilize multidentate ligands that contain a metal ion-binding pocket and appended functional groups that can position H-bond donors/acceptors proximal to bound metal ions. In addition, most of these ligands form relatively rigid cavities in order to promote formation of intramolecular H-bonds. Several systems have featured the carboxyamidopyridyl moiety, which has been successful in forming intramolecular H-bonds to other coordinated species (Fig. 1A). First introduced by Masuda within a symmetrical tripodal ligand,iv several derivatives now exist that are being used to probe H-bonding effects.v,vi
Fig. 1.
The carboxyamido unit used to form an intramolecular H-bond (A), to form a bidentate ligand (B), and the H2pmb ligand used in this study (C).
In the structure depicted in Fig. 1A, the pyridyl groups coordinate to the metal center and the carboxyamido portion serves as the H-bond donor to another ligand. However, another binding mode for the carboxyamidopyridyl moiety is also possible, in which the pyridyl nitrogen and the carbonyl oxygen atoms both bind to a metal ion (Fig. 1B).5,6,vii This bidentate chelation could be a convenient means for controlling access to a metal ion and modulating the properties of metal complexes. Toward these goals we have been exploring the Ni(II) coordination chemistry with the ligand bis(N-pivalamido-2-pyridylmethyl)benzylamine (H2pmb) that contains two carboxyamidopyridyl units (Fig. 1C). This report describes the syntheses, molecular structures, and physical properties for a series of Ni(II) complexes with this ligand that illustrate the utility of incorporating carboxyamidopyridyl units into the design of metal complexes.
2. Experimental
2.1. Preparative Methods and Syntheses
All reagents were purchased from commercial sources and used as received, unless otherwise noted. Solvents were purged with argon and dried over columns containing Q-5 (supported redox catalyst for dioxygen removal) and molecular sieves. Syntheses of air-sensitive metal complexes were conducted in a Vacuum Atmospheres, Co. (Hawthorne, CA) drybox under an argon atmosphere. The H2pmb ligand was prepared following a literature procedure.viii Elemental analyses for NiIIH2pmb and NiIIH2pmb(κ2-OAc)(κ1-OAc) consistently gave low values for the percentages of carbon and nitrogen. We thus report high-resolution mass spectral data for these complexes.
2.2. Preparation of Complexes
2.2.1. {(Bis[N-(6-pivalamido-2-pyridylmethyl)]benzylamine)(dichloro)}nickelate(II) (NiIIH2pmbCl2)
A solution of H2pmb (50 mg, 0.103 mmol) in 3 mL of methanol was treated with NiCl2·6H2O (25 mg, 0.052 mmol). The solution turned dark green and was stirred for 10 minutes. Orange crystals were obtained through diffusion with diethyl ether into methanol (46 mg, 72%). Found: C, 55.44; H, 6.22; N, 11.24%. NiIIH2pmbCl2·1/2MeOH (C29.5H39N5O2.5NiCl2) requires C, 55.95; H, 6.21: N, 11.06%. FT-IR/cm−1 (Nujol): ν(NH) = 3297; λmax (THF, nm (ε, M−1cm−1)) 20 °C: 461 (124), −100 °C: 429 (89), λmax (Acetone, nm (ε, M−1cm−1)) 20 °C: 457 (101) and −90 °C: 417 (53), λmax (MeCN, nm, (ε, M−1cm−1)) 20 °C: 452 (88) and −40 °C: 446 (79), λmax (DCM, nm (ε, M−1cm−1)) 20 °C: 452 (100) and −80 °C: 442 (42), λmax (MeOH, nm, (ε, M−1cm−1)) 20 °C: 597 (11) and −90 °C: 555 (32); μeff = 2.86.
2.2.2. {(Bis[N-(6-pivalamido-2-pyridylmethyl)]benzylamine)(diacetato)}nickelate(II) (NiIIH2pmb(κ2-OAc)(κ1-OAc))
A solution of NiIIH2pmbCl2 (58 mg, 0.081 mmol) in 3 mL of 1:1 MeCN:THF was treated with Me4NOAc (22 mg, 1.6 mmol). The orange solution was stirred for 15 minutes, then AgBF4 was added and allowed to stir for an additional 10 min then filtered over Celite to remove AgCl. The volitles were removed and the resulting blue solid was redissolved in THF. Blue crystals were obtained via pentane diffusion into THF solution (45 mg, 84%). FT-IR/cm−1 (Nujol): ν(NH) 3406, 3315; λmax (THF, nm (ε, M−1cm−1)) 20 °C: 651 (16) and −100 °C: 639 (39), λmax (MeCN, nm (ε, M−1cm−1)) 20 °C: 656 (20) and −40 °C: 645 (26), λmax (Acetone, nm (ε, M−1cm−1)) 20 °C: 646 (19) and −80 °C: 645 (33); μeff = 2.84; HRMS (ES+): Exact mass calcd for C31H40N5NiO4 [M − OAc], 604.2433. Found 604.2408.
2.2.3. {(Bis[N-(6-pivalamido-2-pyridylmethyl)][O-carboxyamido]benzylamine])(chloro)} nickelate(II) (NiIIHpmbCl)
Under argon, a solution of NiIIH2pmbCl2 (50 mg, 0.081 mmol) in 3 mL of THF was treated with KOBut (9.1 mg, 0.081mmol) and was allowed to stir for 20 minutes. Green crystals were obtained via pentane diffusion into a THF solution (46 mg, 98%). FT-IR/cm−1 (Nujol): ν(NH)3264, 3180; λmax (THF, nm (ε, M−1cm−1)) 20 °C: 655 (35) and −100 °C: 639 (39). λmax (DCM, nm (ε, M−1cm−1)) 20 °C: 635 (25) and −90 °C: 635 (44); μeff = 2.88; HRMS (APCI): Exact mass calcd for C29H36N5NiO2Cl [M + H], 580.1989. Found 580.1995.
2.2.4. {(Bis[N-(6-pivalamido-2-pyridylmethyl)-O-carboxyamido]benzylamine])}-nickelate(II) (NiIIpmb)
Under argon, a solution of NiIIH2pmbCl2 (25 mg, 0.041 mmol) in 3 mL of 1:1 THF:MeCN was treated with KOBut (9.1 mg, 0.081 mmol) and was allowed to stir for 20 min. Volatiles were removed under reduced pressure. The resulting green residue was dissolved in THF and crystals were obtained via pentane diffusion (13 mg, 59%). Found: C, 63.77; H, 7.58; N, 11.16%. NiIIpmb·THF (C33H43N5O3Ni) requires C, 64.30; H, 7.03: N, 11.36%. FT-IR/cm−1 (Nujol): ν(NH) 3347; λmax (THF, nm (ε, M−1cm−1)) 20 °C: 446 (29); μeff = 2.76; HRMS (ES+): Exact mass calcd for C29H35N5NiO2 [M + H], 544.222. Found 544.2215.
2.3. Physical Methods
NMR spectra were obtained on either Bruker DRX-400 MHz or Bruker Advance 500 MHz spectrometers. NMR solvents were used as received. Electronic spectra were recorded with an Agilent 8453 spectrophotometer. FT-IR spectra were collected on a Varian 800 Scimitar Series FT-IR spectrophotometer and are reported in wavenumbers. Mass spectra were recorded on a Waters LCT Premier mass spectrometer operated in EI or APCI mode. Magnetic moments were determined using Evan’s method in DMSO.ix Elemental analyses were performed on a Perkin-Elmer 2400 Series II CHNS analyzer.
2.4.1. Crystallographic Structural Determination
Intensity data for the complexes were collected using a Bruker SMART APEX II diffractometer using graphite-monochromated Mo Kα radiation (λ=0.71073 Å). The SMARTx program package was used to determine the unit-cell parameters and for data collection (25 s/frame scan time) for a sphere of diffraction data. The raw frame data was processed using SAINTxi and SADABSxii to yield the reflection data files. Subsequent calculations were carried out using the SHELXTLxiii program. The structure was solved by direct methods and refined on F2 by full-matrix least-squares techniques. The analytical scattering factorsxiv for neutral atoms were used throughout the analysis.
2.4.1.1. NiIIH2pmbCl2
A green crystal of approximate dimensions 0.15 × 0.21 × 0.23 mm was used, giving diffraction symmetry of 2/m and systematic absences consistent with the monoclinic space group P21/c. The structure was solved by direct methods and refined on F2 by full-matrix least-squares techniques. Hydrogen atoms associated with atoms C(20), C(21), and C(22) were included using a riding model.
2.4.1.2 NiIIH2pmb(κ2-OAc)(κ1-OAc)·CH3CN
A blue crystal of approximate dimensions 0.07 × 0.26 × 0.27 mm was employed, giving diffraction symmetry of 2/m and the systematic absences consistent with the monoclinic space groups P2/ and Pc. It was later determined that space group Pc was correct. Hydrogen atoms were either located from a difference-Fourier map and refined (x,y,z and Uiso) or were included using a riding model. There were two molecules of the formula-unit and two molecules of acetonitrile solvent present. Attempts to solve the structure in space group P2/c were unsuccessful. Analysis of the data using the PLATONxv program package suggested the space group assignment was correct. The structure was refined using the TWIN instructionxvi, BASF = 0.372.
2.4.1.3. NiIIHpmbCl
A gold-green crystal of approximate dimensions 0.15 × 0.30 × 0.35 mm gave diffraction symmetry of 2/m and the systematic absences consistent with the monoclinic space group P21/n that was later determined to be correct. Hydrogen atoms were included using a riding model. There were two molecules of the formula-unit present (Z = 8).
2.4.1.4. NiIIpmb(THF)
The crystal of this complex isolated contained one coordinated THF molecule. A colorless crystal of approximate dimensions 0.13 × 0.13 × 0.22 mm was mounted on a glass fiber and transferred to the diffractometer. There were no systematic absences or any diffraction symmetry other than the Friedel condition. The centrosymmetric triclinic space group P1̄ was assigned and later determined to be correct. Hydrogen atoms were included using a riding model. Carbon atoms C(9), C(10), C(11), C(30), C(31), C(32) and C(33) were disordered and included using multiple components, partial site-occupancy-factors and isotropic thermal parameters. At convergence, wR2 = 0.1032 and Goof = 1.028 for 381 variables refined against 6753 data (0.78Å), R1 = 0.0399 for those 5622 data with I > 2.0σ(I).
3. Results and discussion
3.1. Synthesis and Solution Studies
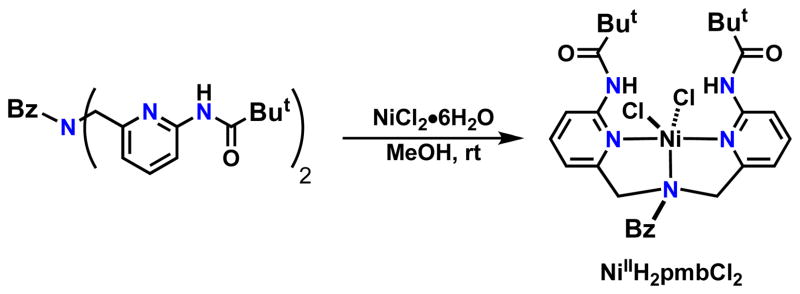
The ligand H2pmb is similar to other dipyridylamine ligands used in coordination chemistry.xvii The key difference is the appended carboxyamido groups that can affect both the primary and secondary coordination spheres. To explore this premise we have prepared a series of Ni(II) complexes, starting with the parent complex, NiIIH2 pmb(Cl2) (Scheme 1). This complex was readily prepared by treating a methanolic solution of H2pmb with NiIICl2. The complex was isolated via vapor diffusion of diethyl ether to afford orange crystals in a 72% yield.
Scheme 1.
Preparative route to NiIIH2pmb(Cl)2.
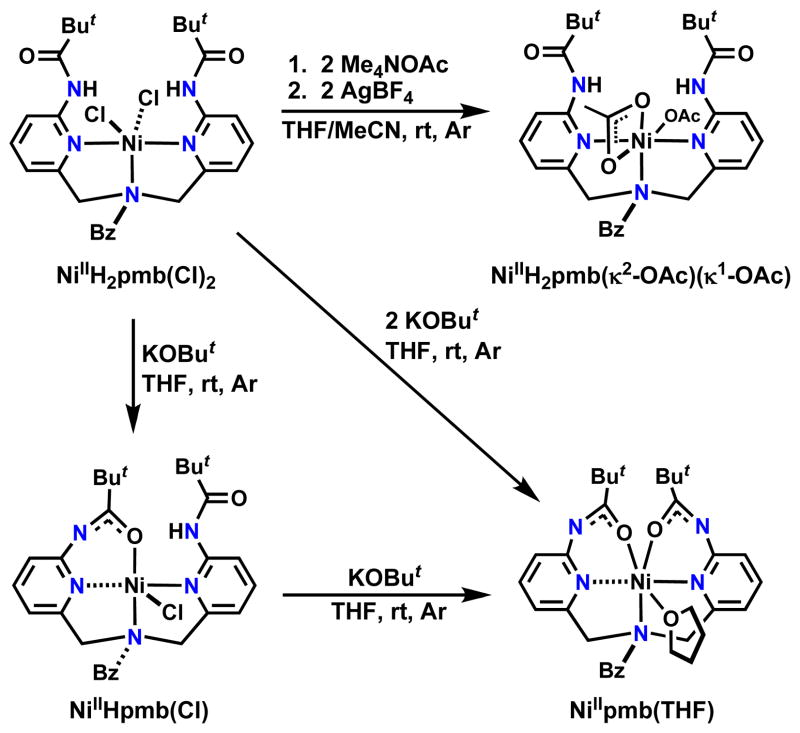
The appended carboxyamido groups in NiIIH2pmb(Cl)2 could be deprotonated under basic conditions, causing loss of the chloro ligands with concomitant amidate coordination to the nickel center (Scheme 2). For example, the monochloro complex, NiIIHpmb(Cl) was produced in nearly quantitative yield when NiIIH2 pmb(Cl2) was allowed to react with 1 equiv of KOBut in THF.
Scheme 2.
Preparative routes to NiIIHpmb(Cl), NiIIpmb(THF), and NiIIH2pmb(κ2-OAc)(κ1-OAc).
In addition, NiIIpmb was isolated (59% yield) when 2 equiv of KOBut were reacted with NiIIH2pmb(Cl)2. Note too that NiIIpmb was prepared from NiHpmbCl and 1 equiv of the base. Removal of the chloro ligands was also achieved using AgBF4 in the presence of acetate. In this case, two acetate ions coordinate to the nickel center, affording the complex NiIIH2pmb(κ2-OAc) (κ1-OAc) in a yield of 84%.
All the NiII complexes are paramagnetic at room temperature with solution effective magnetic moments between 2.8–2.9 μBM. These values are near the spin-only value of 2.83 μBM expected for an S = 1 spin system.xviii The chloro complexes and NiIIH2pmb(κ2-OAc)(κ1-OAc) have temperature-dependent optical properties, in which a red-shift in the absorbance bands occurred with decreased temperature. For instance, the 20°C absorbance spectrum of NiIIH2 pmb(Cl)2 in acetone has a peak at λmax = 452 nm that shifts to at λmax = 442 nm at −80°C.
3.2. Molecular Structures
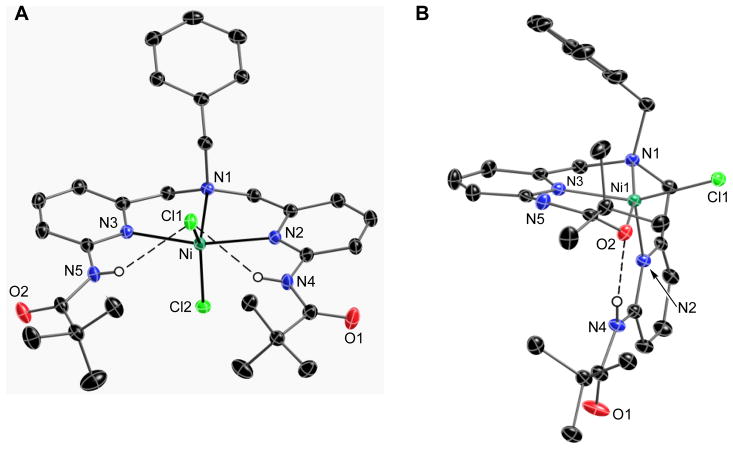
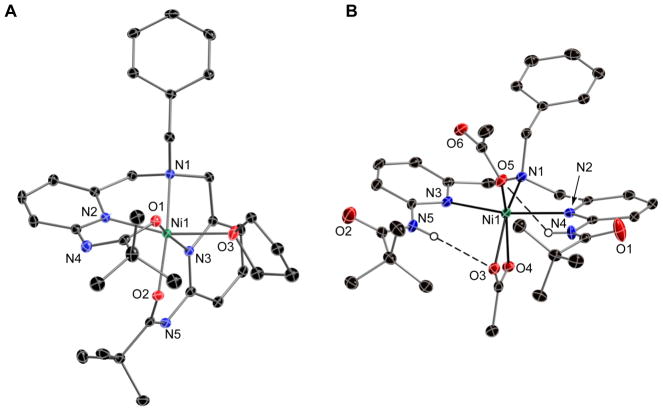
The molecular structures of the nickel complexes were examined in the solid-state using X-ray diffraction methods. Thermal ellipsoid diagrams of NiIIH2pmb(Cl)2 and NiIIHpmb(Cl) are found in Fig. 2, and NiIIpmb(THF) and NiIIH2pmb(κ2-OAc)(κ1-OAc) are shown in Fig. 3. Crystal, data collection, and refinement parameters are found in Table 1. Selected angles and distances are given in Tables 2 and 3.
Fig. 2.
Thermal ellipsoid diagrams of NiIIH2 pmb(Cl2) (A) and NiIIHpmb(Cl) (B). The ellipsoids are drawn at the 50% probability level and all non-carboxyamido hydrogen atoms are removed for clarity.
Fig. 3.
Thermal ellipsoid diagrams NiIIpmb(THF) (A) and NiIIH2 pmb(κ2-OAc)(κ1-OAc) (B). The ellipsoids are drawn at the 50% probability level and all non-carboxyamido hydrogen atoms are removed for clarity.
Table 1.
Crystallographic data for NiIIH2pmb(Cl)2, NiIIHpmb(Cl), NiIIpmb(THF), and NiIIH2pmb(κ2-OAc)(κ1-OAc)·CH3CN.
| Complex | NiIIH2pmb(Cl)2 | NiIIHpmb(Cl) | NiIIpmb(THF) | NiIIH2pmb(κ2-OAc)(κ1- OAc)2·CH3CN |
|---|---|---|---|---|
| molecular formula | C29H37NiN5O2Cl2 | C29H36NiN5O2Cl | C33H43NiN5O3 | C35H46NiN6O6 |
| formula weight | 617.25 | 580.79 | 616.43 | 705.49 |
| T (K) | 153(2) | 148(2) | 98(2) | 153(2) |
| space group | P21/c | P21/n | P1̄ | Pc |
| a (Å) | 9.3786(5) | 17.9869(18) | 9.8123(5) | 13.452(2) |
| b (Å) | 18.1064(9) | 11.6153(12) | 11.8641(6) | 14.834(3) |
| c (Å) | 17.4684(9) | 28.718(3) | 13.4619(7) | 17.896(3) |
| α (deg) | 90 | 90 | 82.8894(7) | 90 |
| β (deg) | 93.4637(6) | 102.5698(14) | 82.2732(7) | 102.073(2) |
| γ (deg) | 90 | 90 | 88.6757(7) | 90 |
| Z | 4 | 8 | 2 | 4 |
| V (Å3) | 2960.9(3) | 5856.0(10) | 1540.95(14) | 3492.4(10) |
| δcalc (Mg/m3) | 1.385 | 1.318 | 1.329 | 1.342 |
| Ra | 0.0382 | 0.0627 | 0.0399 | 0.0363 |
| Rwb | 0.1028 | 0.1494 | 0.0969 | 0.0735 |
| GOFc | 1.053 | 1.098 | 1.028 | 1.004 |
| Largest diff. peak (eÅ3) | 1.803 | 1.450 | 1.011 | 0.447 |
R = [Σ|ΔF|/Σ|Fo|].
Rw = [Σω(ΔF)2/ΣωFo2].
goodness of fit on F2.
Table 2.
Selected bond distances and angles for NiIIH2pmb(Cl)2 and NiIIHpmb(Cl).
| Complex | NiIIH2 pmbCl2 | NiIIHpmbCl |
|---|---|---|
| Distances (Å) | ||
| Ni1—N1 | 2.031(2) | 2.081(3) |
| Ni—N2 | 2.125(2) | 2.067(3) |
| Ni—N3 | 2.134(2) | 2.006(3) |
| Ni—Cl1 | 2.291(1) | 2.315(1) |
| Ni—Cl2 | 2.269(1) | – |
| Ni—O2 | – | 2.007(3) |
| Cl1···N4 | 3.211(6) | – |
| Cl1···N5 | 3.230(6) | – |
| O2···N4 | – | 2.978(1) |
| Angles (deg) | ||
| N1—Ni1—N2 | 81.64(7) | 80.71(13) |
| N1—Ni1—N3 | 81.69(7) | 82.89(13) |
| N2—Ni1—N3 | 163.24(7) | 102.26(13) |
| N1—Ni1—Cl1 | 114.31(5) | 94.24(9) |
| N1—Ni1—Cl2 | 101.50(5) | – |
| N2—Ni1—Cl1 | 92.81(5) | 101.76(9) |
| N2—Ni1—Cl2 | 92.82(5) | – |
| N3—Ni1—Cl1 | 92.44(5) | 155.00(10) |
| N3—Ni1—Cl2 | 92.20(5) | – |
| Cl1—Ni1—Cl2 | – | |
| N1—Ni1—O2 | – | 169.56(12) |
| N2—Ni1—O2 | – | 105.94(12) |
| N3—Ni1—O2 | – | 87.76(12) |
| Cl1—Ni1—O2 | – | 92.27(8) |
Table 3.
Selected bond distances and angles for NiIIpmb(THF) and NiIIH2pmb(κ2-OAc)(κ1-OAc)·CH3CN.
| Complex | NiIIpmb(THF) | NiIIH2pmb (κ2 -OAc) (κ1-OAc))·CH3CN |
|---|---|---|
| Distances (Å) | ||
| Ni—N1 | 2.166(2) | 2.079(2) |
| Ni—N2 | 2.022(2) | 2.117(2) |
| Ni—N3 | 2.052(2) | 2.120(2) |
| Ni—O1 | 2.001(2) | – |
| Ni—O2 | 2.013(2) | – |
| Ni—O3 | – | 2.110(2) |
| Ni—O4 | – | 2.144(2) |
| Ni—O5 | – | 1.999(2) |
| Ni—O3(THF) | 2.175(2) | – |
| O3···N5 | – | 3.063(8) |
| O5···N4 | – | 3.000(8) |
| Angles (deg) | ||
| N1—Ni—N2 | 77.72(7) | 80.49(9) |
| N1—Ni—N3 | 77.09(7) | 82.59(9) |
| N2—Ni—N3 | 109.08(7) | 163.07(8) |
| N1—Ni—O1 | 107.91(6) | – |
| N2—Ni—O1 | 81.83(7) | – |
| N3—Ni—O1 | 168.93(6) | – |
| O1—Ni—O2 | 89.72(6) | – |
| O2—Ni—N2 | 97.14(6) | – |
| O2—Ni—N3 | 87.08(6) | – |
| N1—Ni—O3 | – | 154.16(8) |
| N1—Ni—O4 | – | 92.09(8) |
| N1—Ni—O5 | – | 110.99(8) |
| N2—Ni—O3 | – | 98.27(8) |
| N2—Ni—O4 | – | 91.54(8) |
| N2—Ni—O5 | – | 89.70(8) |
| N3—Ni—O3 | – | 97.21(8) |
| N3—Ni—O4 | – | 89.67(8) |
| N3—Ni—O5 | – | 95.81(8) |
| O3—Ni—O4 | – | 62.08(7) |
| O3—Ni—O5 | – | 94.78(8) |
| O4—Ni—O5 | – | 156.75(8) |
3.2.1. Molecular Structures of NiIIH2pmb(Cl)2 and NiIIHpmb(Cl)
The complexes NiIIH2pmb(Cl)2 and NiIIHpmb(Cl) crystallized in the monoclinic space groups P21/c and P21/n, respectively. The asymmetric unit for NiIIHpmb(Cl) contained two crystallographically independent, but chemically identical, molecules. Only the molecule containing Ni1 will be included in this discussion.
The complex NiIIH2pmb(Cl)2 is five-coordinate with a coordination geometry that is best described as distorted trigonal bipyramidal (Fig. 2A). The nickel center is positioned nearly within (0.007 Å) the trigonal plane defined by the chloro ligands and the apical nitrogen atom N1. The H2pmb ligand binds in a meridional manner, with N1 and pyridyl nitrogen (N2, N3) atoms coordinated to the nickel center. The Ni–N1 bond distance of 2.031(2) Å is greater than those of the Ni–Npy bonds, which have an average bond distance of 2.129(2) Å. The binding of H2pmb to NiIIpmb(Cl)2 is comparable to that reported for the NiII complexes of the ligand, N,N-bis[(phenylpyridin-2-yl)methyl] benzylamine) (1).xvii Ligand 1 is similar to H2pmb, differing only in having appended phenyl groups instead of carboxyamido units. For instance, in the NiII(1)(CH3CN)(H2O) complex, 1 also binds meridionally and has similar Ni N bond lengths.
The chloro ligands occupied the remaining coordinate sites with Ni–Cl1 and Ni–Cl2 bond distances of 2.291(1) and 2.269(1) Å. The difference in the Ni–Cl bond length is attributed to the presence of an intramolecular H-bonding network that surrounds Cl1. Two intramolecular H-bonds are present involving Cl1 and NH groups of the appended carboxyamido units. The N4–H and N5–H bonds vectors are directed toward Cl1 producing N···Cl1 distances of 3.211(3) and 3.230(3) Å, which are within the heavy atom distances normally associated with H-bonds.xix
Our structural analyses confirmed that treating NiIIH2pmb(Cl)2 with a base caused deprotonation of one of the carboxyamido groups to form NiIIHpmb(Cl). A comparison of the molecular structures of these two complexes showed that a large structural change occurs upon deprotonation. NiIIHpmb(Cl) has a molecular structure that is closer to square pyramidal (Fig. 2B). The N1, N2, and N3 nitrogen atoms of [Hpmb]− ligand coordinates to the nickel center, similar to what was found in NiIIH2pmb(Cl)2, but in a facial manner. Both the N1–N2 and N1–N3 bond distances of 2.067(3) and 2.006(3) Å are significantly shorter in NiIIHpmb(Cl) compared to those in NiIIH2pmb(Cl)2. Moreover, the carboxyamidato oxygen atom O2 in NiIIHpmb(Cl) coordinates to the nickel center in NiIIHpmb(Cl) with a Ni1–O2 bond distance equal to 2.007(3) Å. The final coordination site is filled by the lone chloro ligand that binds at a Ni1–Cl1 bond length of 2.315(1) Å. Unlike in NiIIH2pmb(Cl)2 the chloro ligand does not form a H-bond—rather, a intramolecular H-bond is formed between the appended carboxyamido groups (the N4···O2 distance is 2.978(3) Å).
3.2.2. Molecular Structures of and NiIIpmb(THF) and NiIIH2pmb(κ2-OAc)(κ1-OAc)·CH3CN
The complexes NiIIpmb(THF) and NiIIH2pmb(κ2-OAc)(κ1-OAc) crystallized in the triclinic space group P1̄ and the monoclinic space group Pc, respectively. For NiIIH2pmb(κ2-OAc)(κ1-OAc)·CH3CN, there were two independent, but chemically equivalent, molecules in the asymmetric unit. Only the molecule containing Ni1 will be discussed.
The molecular structure of NiIIpmb(THF) corroborated that deprotonation of both appended carboxyamido groups of H2pmb caused complete loss of the chloro ligands and coordination of the carbonyl oxygen atoms to the nickel center (Fig. 3A). Facial coordination of the N1, N2, and N3 atoms was observed in NiIIpmb(THF), a binding of the [pmb]2− ligand that is similar to the way [Hpmb]− binds in NiIIHpmb(Cl). In NiIIpmb(THF) the carbonyl oxygen atoms O1 and O2 are also coordinated with Ni1–O1 and Ni1–O2 bond distances of 2.011(2) and 2.013(2) Å. Note that unlike in the molecular structures of the NiII–chloro complexes, NiIIpmb(THF) is six-coordinate, with a THF molecule occupying the final coordination site (Ni1–O3(THF) = 2.175(2) Å).
We have also found that the chloro ligands in NiIIH2pmb(Cl)2 are removed with AgI salts in the presence of acetate ions. The resulting complex, NiIIH2pmb(κ2-OAc)(κ1-OAc) is six-coordinate (Fig. 3B), in which the H2pmb ligand is bonded to the nickel ion in a meridional manner similar to what was observed in NiIIH2pmb(Cl)2. The N1–N2 and N1–N3 bond lengths are statistically the same at 2.117(2) and 2.120(2) Å, respectively and the Ni1–N1 bond distance is 2.079(2) Å. The two acetate ions coordinate differently: one is bidentate, having Ni1–O3 and Ni1–O4 bond distances of 2.110(2) and 2.144(2) Å, whereas the other is monodentate, having a Ni1–O5 bond length of 1.999(2) Å. Intramolecular H-bonds were also found in NiIIH2pmb(κ2-OAc)(κ1-OAc) between the acetate ions and the H2pmb ligand. The heavy atom N4···O5 and N5···O3 distances are approximately at 3.0 Å, which is within the range considered for H-bonds.xx
4. Summary
Previous studies have shown the utility of the pyridylcarboxyamido unit in ligand design. We have further demonstrated its usefulness in controlling both the primary and secondary coordination spheres in transition metal complexes. Both NiIIH2pmb(Cl)2 and NiIIH2pmb(κ2-OAc)(κ1-OAc) have H-bonding networks involving the H2pmb and bound chloro or acetato ligands. Sequential deprotonation of NiIIH2pmb(Cl)2 led to the binding of the carboxyamido oxygen atoms with concurrent loss of the chloro ligands. Alteration in the H-bonding networks was also observed upon carboxyamido binding and was completely absent in NiIIpmb(THF). The ability to affect structural changes within both coordination spheres could translate into a method for regulating functional properties, such as substrate binding, in a manner reminiscent of what is found in metalloproteins.
Supplementary Material
Acknowledgments
We thank the NIH (GM50781) and NSF ((0738252)) for financial support of this work. The NSF funded the purchase of the X-ray instrumentation. We thank M. Takase for help in the molecular structure determinations.
Footnotes
CIF format for X-ray analyses of NiIIH2pmb(Cl)2, NiIIHpmb(Cl), NiIIpmb(THF), and NiIIH2pmb(κ2-OAc)(κ1-OAc)·CH3CN.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.(a) Lu Y, Valentine JS. Curr Opin Struct Biol. 1997;7:495–500. doi: 10.1016/s0959-440x(97)80112-1. [DOI] [PubMed] [Google Scholar]; (b) Natale D, Marque-Rivas JC. Chem Comm. 2008:425–437. doi: 10.1039/b709650j. [DOI] [PubMed] [Google Scholar]; (c) Marque-Rivas JC. Curr Org Chem. 2007;11:1434–1449. [Google Scholar]; (d) Steiner T. Angew Chem Int Ed. 2002;41:48–76. doi: 10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (e) Miller AF. Acc Chem Res. 2008;41:501–510. doi: 10.1021/ar700237u. [DOI] [PubMed] [Google Scholar]; (f) Jackson TA, Brunold TC. Acc Chem Res. 2004;37:461–470. doi: 10.1021/ar030272h. [DOI] [PubMed] [Google Scholar]
- ii.Representative examples: Lipscomb WN, Strater N. Chem Rev. 1996;96:2375–2433. doi: 10.1021/cr950042j.Bhaumik D, Medin J, Gathy K, Coleman MS. J Biol Chem. 1993;268:5464–5470.Sideraki V, Mihamedali KA, Wilson DK, Chang Z, Kellems RE, Quiocho FA, Rudolph FB. Biochemistry. 1996;35:7862–7872. doi: 10.1021/bi952920d.
- iii.Reviews: Shook RL, Borovik AS. Chem Comm. 2008:6095–6107. doi: 10.1039/b810957e.Borovik AS. Acc Chem Res. 2005;38:54–61. doi: 10.1021/ar030160q.Marque-Rivas JC, Hinchley SL, Metteau L, Parsons S. Dalton Trans. 2006:2316–2322. doi: 10.1039/b516234c.
- iv.Harata M, Hasegawa K, Jitsukawa K, Masuda H, Einaga H. Bull Chem Soc, Jpn. 1998;71:1031–1038.Harata M, Jitsukawa J, Masuda H, Einage H. J Am Chem Soc. 1994;116:10817–10819.Berreau LM, Mahapatra S, Halfen JA, Young VG, Jr, Tolman WB. Inorg, Chem. 1996;35:6339–6342.
- v.Mareque-Rivas JC, Salvagni E, Parsons S. Dalton Trans. 2004:4185–4192. doi: 10.1039/b414223c.Natale D, Mareque-Rivas JC. Chem Commun. 2008:425–437. doi: 10.1039/b709650j.Mareques Rivas JC, de Rosales RTM, Parsons S. Dalton Trans. 2003:2156–2163.Mareques Rivas JC, Salvagni E, de Rosales RTM, Parsons S. Dalton Trans. 2003:3339–3349.(e)
- vi.(a) Ingle GK, Makowska-Grzyka MM, Arif AM, Berreau LM. Eur J Inorg Chem. 2007:5262–5269. [Google Scholar]; (c) Rudzka K, Arif AM, Berreau LM. J Am Chem Soc. 2006;128:17018–17023. doi: 10.1021/ja0601336. [DOI] [PubMed] [Google Scholar]; (c) Berreau LM, Makowska-Grzyska MM, Arif AM. Inorg Chem. 2000;39:4390–4391. doi: 10.1021/ic001190h. [DOI] [PubMed] [Google Scholar]; (d) Szajna E, Makowska-Grzyska MM, Wasden CC, Arif AM, Berreau LM. Inorg Chem. 2005;44:7595–7605. doi: 10.1021/ic050750f. [DOI] [PubMed] [Google Scholar]; (e) Rudzka K, Arif AM, Berreau LM. Inorg Chem. 2005;44:7234–7242. doi: 10.1021/ic0508122. [DOI] [PubMed] [Google Scholar]; (f) Ingle GK, Makowska-Grzyska MM, Szajna-Fuller E, Sen I, Price JC, Arif AM, Berreau LM. Inorg Chem. 2007;46:1471–1480. doi: 10.1021/ic062020t. [DOI] [PubMed] [Google Scholar]; (g) Grubel K, Fuller AL, Chambers BM, Arif AM, Berreau LM. Inorg Chem. 2010;49:1071–1081. doi: 10.1021/ic901981y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vii.Shook RL, Gunderson WA, Greaves J, Ziller JW, Hendrich MP, Borovik AS. J Am Chem Soc. 2009;130:8888–8889. doi: 10.1021/ja802775e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- viii.Shook RL, Borovik AS. Inorg Chem. 2010;49:0000. doi: 10.1021/ic901550k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ix.(a) Evans DF. J Chem Soc. 1959:2003. [Google Scholar]; (b) Ostfeld D, Cohen IA. J Chem Educ. 1972;49:829. [Google Scholar]
- x.Data Collection: SMART Software Reference Manual. Bruker-AXS; Madison, WI: 1994. [Google Scholar]
- xi.Data Reduction: SAINT Software Reference Manual. Bruker-AXS; Madison, WI: 1995. [Google Scholar]
- xii.Sheldrick GM. SADABS, Version 2.10. Bruker Analytical X-Ray Systems, Inc; Madison, WI: 2002. [Google Scholar]
- xiii.Sheldrick GM. SHELXTL Version 6.12. BrukerAnalytical X-Ray Systems, Inc; Masdison, WI: 2001. [Google Scholar]
- xiv.International Tables for X-ray Crystallography. C. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- xv.Speck AL. PLATON, a multipurpose crystallographic tool. Utrecht University; Utrecht, The Netherlands: 2001. [Google Scholar]
- xvi.Sheldrick GM. SADABS, Version 2008/1. Bruker AXS, Inc; Madison, WO: 2008. [Google Scholar]
- xvii.Kunishita A, Doi Y, Kubo M, Ogura T, Sugimoto H, Itoh S. Inorg Chem. 2009;48:4997–5004. doi: 10.1021/ic900059m. [DOI] [PubMed] [Google Scholar]
- xviii.Drago R. Physical Methods in Inorganic Chemistry. chapter 11 Saunders; Philadelphia: 1977. [Google Scholar]
- xix.(a) Steiner T. Acta Crystallogr, Sec B. 1998;54:456–463. doi: 10.1107/s090744499701500x. [DOI] [PubMed] [Google Scholar]; (b) Aullón G, Bellamy D, Brammer L, Bruton EA, Orpen AG. Chem Commun. 1998:653–654. [Google Scholar]
- xx.(a) Jeffrey GA. An Introduction to Hydrogen Bonding. Oxford University Press, Inc; New York: 1997. [Google Scholar]; (b) Desiraju GR, Steiner T. The Weak Hydrogen Bond in Structural Chemistry and Biology. Oxford University Press Inc; New York: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.