Abstract
Short day lengths increase the duration of nocturnal melatonin (Mel) secretion, which induces the winter phenotype in Siberian hamsters. After several months of continued exposure to short days, hamsters spontaneously revert to the spring-summer phenotype. This transition has been attributed to the development of refractoriness of Mel-binding tissues, including the suprachiasmatic nucleus (SCN), to long-duration Mel signals. The SCN of Siberian hamsters is required for the seasonal response to winter-like Mel signals, and becomes refractory to previously effective long-duration Mel signals restricted to this area. Acute Mel treatment phase shifts circadian locomotor rhythms of photosensitive Siberian hamsters, presumably by affecting circadian oscillators in the SCN. We tested whether seasonal refractoriness of the SCN to long-duration Mel signals also renders the circadian system of Siberian hamsters unresponsive to Mel. Males manifesting free-running circadian rhythms in constant dim red light were injected with Mel or vehicle for 5 days on a 23.5-h T-cycle beginning at circadian time 10. Mel injections caused significantly larger phase advances in activity onset than did the saline vehicle, but the magnitude of phase shifts to Mel did not differ between photorefractory and photosensitive hamsters. Similarly, when entrained to a 16-h light/8-h dark photocycle, photorefractory and photosensitive hamsters did not differ in their response to Mel injected 4 h before the onset of the dark phase. Activity onset in Mel-injected hamsters was masked by light but was revealed to be significantly earlier than in vehicle-injected hamsters upon transfer to constant dim red light. The acute effects of melatonin on circadian behavioral rhythms are preserved in photorefractory hamsters.
Keywords: phase shift, photoperiod, SCN, circadian, melatonin, refractoriness
Photoperiodic rodents monitor time of year by means of nocturnal pineal melatonin (Mel) secretion, which provides a neuroendocrine representation of day length (Prendergast et al., 2002). In Siberian hamsters, Phodopus sungorus, transfer from long day lengths (short-duration Mel signals) to short day lengths (long-duration Mel) initiates a transition from the spring-summer phenotype indicated by reproductive competence, to the fall-winter pheno-type of reproductive quiescence. Mel acts on receptors in Mel-binding central nervous system sites to induce these seasonal effects (Freeman and Zucker, 2001; Lincoln and Clarke, 1994; Maywood and Hastings, 1995; Weaver et al., 1989).
A marked change in Mel signal processing occurs when mammals are maintained indefinitely in short days. Hamsters become refractory to long-duration Mel signals of winter after ~4 months, and the spring phenotype spontaneously reemerges (Bittman, 1978; Hoffmann, 1973). Nightly Mel secretion during this period continues to faithfully represent ambient day length (Rollag et al., 1980). A key question in explicating refractoriness is the extent to which responsiveness to Mel depends on the signal duration. Summer-like short-duration Mel signals administered over the course of 6 or more weeks break short-day photorefractoriness (Kauffman et al., 2003), demonstrating that nonresponsiveness of refractory hamsters is specific to a particular range of Mel signal durations.
The suprachiasmatic nucleus (SCN) has been proposed as the principal target tissue through which Mel influences seasonal rhythms of Siberian hamsters (Bartness et al., 1991). Ablation of the SCN, but not that of the adjacent anterior hypothalamus, eliminates the ability of long-duration Mel signals to induce the winter phenotype (Bartness et al., 1991; Song and Bartness, 1996). Chronic Mel implants in the SCN, but not in adjacent tissue, initially induce gonadal regression but eventually their effect wanes, and despite continued presence of Mel in the SCN, the testes undergo recrudescence (Freeman and Zucker, 2001). This suggests that refractoriness can be locally induced in the SCN of this species. Notably, neither maximum binding nor affinity of Mel receptors in the SCN differ between photosensitive and photorefractory hamsters (Weaver et al., 1991).
Although there is no evidence that Mel plays a role in entrainment or generation of mammalian circadian rhythms under physiological conditions, it is in widespread use as a chronobiotic to phase shift circadian rhythms and ameliorate the effects of jet lag (Arendt, 2006). Mel injections phase shift the circadian rhythms of several rodents, including the Siberian hamster (Armstrong, 1989; Duffield et al., 1998). Several lines of evidence support direct effects of Mel on the SCN. Mel injections inhibit SCN metabolism as measured by 2-deoxyglucose uptake (Cassone et al., 1987). This effect is most pronounced at times when melatonin causes phase shifts, and is SCN specific, there being no metabolic suppression in several other hypothalamic tissues (Cassone et al., 1988). Mel affects spike rate of SCN neurons in Syrian and Siberian hamsters (Margraf and Lynch, 1993; Mason and Rusak, 1990) and phase shifts the circadian rhythms of electrical activity in Siberian hamster SCN slices (Weaver et al., 1996).
Previous work suggested a correlation between seasonal photorefractoriness and responsiveness of circadian rhythms to Mel. Injections of Mel 4 h before onset of darkness in Siberian hamsters housed in 16 h light per day induced gonadal regression and molt, and also advanced the onset of circadian wheel running activity and expanded the duration of locomotor activity (α) (Puchalski and Lynch, 1988b). α shortened when hamsters became seasonally photorefractory after ~27–29 weeks of Mel treatment. The initial α expansion may be due in part to daily phase advances of the circadian system in response to Mel injections, whereas the later α contraction may indicate that circadian phase shifts to Mel are attenuated in refractory hamsters.
Mel's acute effects appear to be preserved at the cellular level in refractory animals (Johnston et al., 2003; Weaver et al., 1991). These studies suggest that refractoriness is encoded downstream of the Mel receptor-containing neurons; nevertheless, where and how Mel signaling pathways are compromised in the refractory state is unknown. To address this, we analyzed a behavioral endpoint of Mel signaling that is mediated by a tissue important for both circadian and seasonal rhythms. We probed responsiveness of the circadian system to Mel in photorefractory Siberian hamsters by measuring phase advances of wheel-running activity under free-running and entrained conditions. If seasonal and circadian effects of Mel share common pathways, then circadian phase shifts should be attenuated in photorefractory hamsters. If, instead, separate pathways subserve these two effects and these pathways' sensitivity to Mel can be independently controlled, then the circadian responses of photorefractory and photosensitive hamsters to acute Mel treatment may be indistinguishable.
MATERIALS AND METHODS
Male and female hamsters were paired and maintained in long days (14 h light, 10 h darkness per day: 14L), or transferred to short days (10L) on the day of pairing. Male offspring were weaned at 18–21 days of age and designated as photosensitive (14L-reared) and photorefractory (10L-reared) subjects in the present experiments. Hamsters were housed in their natal photoperiod, 1–4 per clear polypropylene cage (18 × 28 × 12 cm), furnished with Harlan Tek-Fresh bedding (Harlan Teklad, Madison, WI). Tap water and Lab Diet 5015 Mouse Diet (PMI Nutrition International, Brentwood, MO) were available ad libitum. Estimated testis volume (ETV) was assessed at several time points in hamsters under light isoflurane-induced anesthesia: the left testis was palpated into the scrotum and its width (W) and length (L) measured with calipers to ±3 mm. ETV, defined by W2L, correlates highly with testis mass (Butler et al., 2007). Pelage color was assigned on the 4-point scale (1 = summer, 4 = winter) of Duncan and Goldman (1984) with the addition of half steps.
Juvenile Siberian hamsters grow rapidly and attain puberty in ~5 weeks in long days; those in short days gain body weight much more slowly and delay puberty for up to 5 months (Hoffmann, 1978). Thereafter, they remain reproductively competent despite short days, indicating refractoriness. Photoperiodic nonresponders are well described for this species; despite short days, their development is indistinguishable from 14L-housed pups and they reach sexual maturity at a young age (Gorman and Zucker, 1997; Puchalski and Lynch, 1988a). In experiment 1, all 10L experimental animals were verified responders: they had small testes (ETV < 400) and winter pelage (score ≥ 2) at ~100 days of age and large testes (ETV > 500) at ~300 days of age (for treatment time line, see Fig. 1). Hamsters for experiment 2, originally part of a separate study (Paul et al., 2006), were born in 14L or 10L and either sham-castrated or unilaterally castrated at 20 or 60 days of age; ETV was measured biweekly after surgery. After testicular development or recrudescence was verified (ETV > 400), remaining testes were removed at ~29 weeks of age, and the hamsters randomly assigned to groups for the present experiment at 10–11 months of age (Fig. 1). All 10L-reared hamsters had ETV < 400 at 11–13 weeks of age, indicative of photoresponsiveness. All procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley.
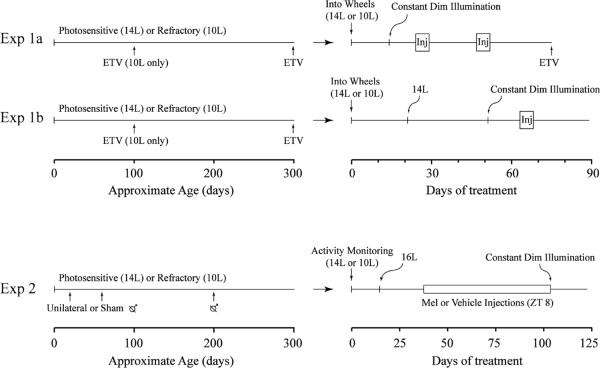
Figure 1.
Time line of treatments for each experiment. Inj denotes the 5-day interval during which hamsters were injected; in experiment 2 the 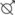 symbol denotes time of castration. In experiment 2, estimated testis volume (ETV) was measured biweekly after surgery.
symbol denotes time of castration. In experiment 2, estimated testis volume (ETV) was measured biweekly after surgery.
Experiments 1a, 1b: Phase Shifting in Photosensitive and Photorefractory Hamsters
In experiment 1a, 10-month-old photosensitive (14L, n = 20) and photorefractory (10L, n = 20) hamsters were randomly assigned to two groups and individuals were transferred to large clear polypropylene cages (48 × 25 × 21 cm) equipped with running wheels (17 cm diameter, 8.3 cm width) attached to the wire cage lids. A strip of blue polyethylene plastic was woven through the rungs of the wheels to prevent leg injuries. All hamsters were housed in light-tight boxes in their previous photoperiod, but with additional continuous dim red illumination from a Bright Lab Jr. Safelight (6W, minimum transmitted wave length of 610 nm; Delta 1/CPM, Inc., Dallas, TX). Nocturnal illuminance at cage level varied between 0.2 and 0.5 lx (IL1700 Research Radiometer; International Light, Newburyport, MA).
Stable entrainment to the natal photoperiod was verified by monitoring wheel running activity for 2 weeks, after which the main lights were turned off and hamsters were housed continuously in constant dim red light. After stable free-running rhythms were confirmed (10 days), hamsters were injected subcutaneously with Mel (1 mg/kg in 3% ethanolic saline) or vehicle for 5 days on a 23.5-h T-cycle beginning at circadian time 10 (CT10; CT12 defined as predicted time of activity onset), following a procedure of Duffield et al. (1998). Five consecutive daily injections were administered to sum the otherwise small daily phase shifts to nonphotic stimuli. The 23.5-h T-cycle ensures that the injections remain roughly within the Mel-sensitive period (late subjective day). Injections were performed out of the housing boxes under dim red light (maximum illuminance, 0.6 lx). Wheel running was monitored for 18 days after the end of the first injection sequence; hamsters with clear activity onsets were then injected in the same manner as above, but with the opposite solution. Thus, hamsters received both Mel and vehicle injections in a crossover design. Figure 1 indicates the time line.
To control for possible photoperiod history effects given the different pre-experiment photoperiods, the study was replicated after allowing animals to entrain to a common photoperiod (experiment 1b). At 10–12 months of age, photosensitive and refractory hamsters were transferred to running wheel cages and stable entrainment was verified for 3 weeks in their natal photoperiods of 14L or 10L. The photoschedule was then adjusted to 14L for all animals (dark onset held constant), and running wheel activity monitored for 30 days. Red lights were turned on for the last 10 days of 14L, after which all hamsters were maintained in constant dim red light. A month of 14L is not sufficient to break refractoriness (Kauffman et al., 2003). These hamsters received only 1 round of injections that commenced after 12 days of constant dim red light treatment. The experiment was terminated 21 days after the last injection (Fig. 1).
Experiment 2: α Expansion in 16L-Housed Photosensitive and Photorefractory Hamsters
Hamsters were singly housed in cages (28 × 18 × 12 cm) equipped with passive infrared motion detectors to measure locomotor activity (Quest PIR; Electronics Line USA, Boulder, CO) mounted on the cage lids (described in Butler et al., 2007). After entrainment to the natal photoperiod was verified (17 days; Fig. 1 time line), photoperiod was lengthened to 16L (lights-on 0200 h PST). Once entrainment to 16L was verified (26 days), photosensitive and photorefractory hamsters were injected once daily 4 h before dark onset with Mel (25 μg in 0.1 mL of 1% ethanolic saline) or the vehicle. Injections continued for 10 weeks. From dark onset on the day of the last injection, the boxes housing the cages were maintained in constant dim red illumination and free-running circadian rhythms were monitored for 23 days (Fig. 1). Note that 26 days of 16L before injections are insufficient to break photorefractoriness (Kauffman et al., 2003), but refractoriness was probably broken in most vehicle-injected hamsters by the end of the injections, due to ~14 weeks of exposure to 16L. Late afternoon Mel injections lengthen the duration of circulating Mel and thus produce a short-day-like signal that should prevent the breaking of refractoriness (Bartness et al., 1993).
To test whether light masked advances of locomotor activity onset, the endogenous phase angle of entrainment on the last day of injections in 16L was estimated by extrapolating back from the activity onsets in the free-running hamsters in constant dim red light. Actograms for each hamster were single-plotted from the first day of constant conditions. A line was drawn by eye through the daily onsets immediately after release into dim red light by an observer uninformed about both group and injection status of the hamsters. Extension of this line back to the last day of the light-dark cycle defined the onset of the endogenous circadian activity rhythm.
Activity Monitoring
Running wheel revolutions or deflections of infrared beams were collected as counts per 10-min bin with DataQuest III (Mini-Mitter, Sun River, OR) and analyzed with ClockLab (Actimetrics, Wilmette, IL). Regression lines fitted to activity onset were calculated from the onsets defined by ClockLab. In the few cases in which this analysis yielded obviously incorrect onset values, onsets were adjusted by eye by an observer uninformed about the treatments for any specific animal. These regressions were used to predict activity onset on the first day of injections. Phase shifts were calculated on the last day of injections as the difference between the extrapolated regression lines fit to the preinjection and postinjection period. In experiment 2, activity onset and offset were calculated from the average daily activity profile over 2 weeks. Onset was scored as the first point >100% of the mean activity, after which this level was sustained in 3 of the following 6 bins. Similarly, offset was the last point >100% of mean activity, before which activity had exceeded this threshold in 3 of 6 bins (Gorman, 2001). a (offset minus onset time) was measured in 14L and 10L, again in the last 2 weeks of 16L before Mel or vehicle injections, and then during the last 2 weeks of injections.
Statistics
Group differences were assessed by t test or ANOVA followed by post-hoc Fisher's PLSD. Changes in α from the beginning to the end of the injection period in experiment 2 were analyzed by repeated-measures ANOVA with time, injection (Mel or vehicle), and condition (sensitive or refractory) as independent factors. All statistical tests were performed using Statview 5.0.1 (SAS Institute, Cary, NC). Significance level was set at p = 0.05.
RESULTS
Experiment 1
In experiment 1a, the testes regressed significantly in photoresponsive hamsters held in constant dim red light, but not in photorefractory hamsters, thereby confirming our previous designations (Fig. 2).
Figure 2.
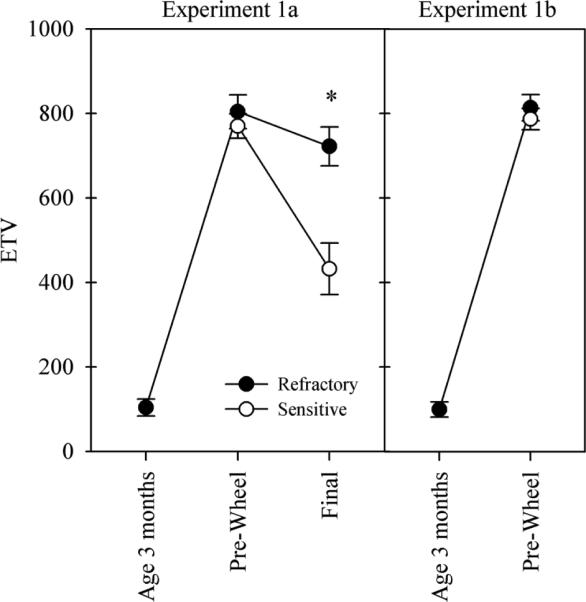
Mean ETV (± SE) of hamsters in experiments 1a and 1b. 10L fully inhibited gonadal growth at 3 months of age (●). All hamsters had large gonads before they were transferred to wheels at ~10 months of age (pre-wheel; 1a) or 10–12 months of age (pre-wheel; 1b). Photosensitive hamsters (◯) underwent significantly greater gonadal regression during housing in constant dim light (1a). *t test, p < 0.05.
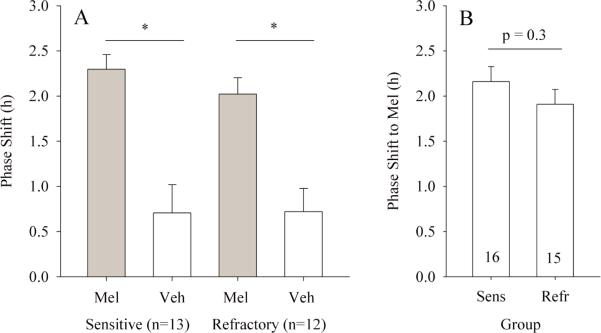
During the first round of injections, circadian rhythms were sufficiently stable to permit assessment of circadian time in 33 of 40 hamsters and calculation of phase shift in 30. In the second round, phase shifts were calculated for 32 of 33 injected hamsters. Mel injections caused significantly larger phase advances than did vehicle injections in photosensitive and photorefractory hamsters (paired t tests, both p < 0.01; Figs. 3, 4A). The magnitude of phase shifts to Mel did not differ between photosensitive and photorefractory hamsters, either in the subset of hamsters treated in the crossover design (t test, p = 0.3; Fig. 4A) or across all hamsters (t test, p = 0.3; Fig. 4B).
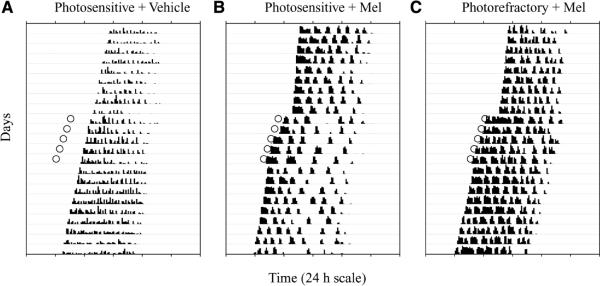
Figure 3.
Representative actograms of running wheel activity in a photosensitive hamster injected with vehicle (A), a photosensitive hamster injected with Mel (B), and a photorefractory hamster injected with Mel (C). Open circles indicate the time of injection: Mel, but not vehicle injections, caused phase advances. Hatch marks along each horizontal line represent the amount of activity (revolutions/10 min bin); each horizontal line represents 24 h, and activity records from consecutive days are plotted beneath each other. Each hamster has a free-running period <24 h, as indicated by the earlier onset of activity on consecutive days.
Figure 4.
Mean phase shifts (±SE) to Mel or vehicle (Veh) in hamsters that received both injections (A). Numbers receiving both injections in a crossover design indicated in parentheses. *paired t test, p < 0.01. Among all hamsters that received Mel injections, there was no difference in the magnitude of phase advance between photosensitive (Sens) and photorefractory (Refr) hamsters (B). Sample size is indicated within the bars in B.
Photoperiod history (experiment 1b) did not influence the magnitude of the phase shift to Mel. As above, all 10L-raised hamsters had small testes at ~3 months of age, and ETV did not differ between photosensitive and photorefractory hamsters at the time of transfer to running wheel cages (ETV > 600 for all; Fig. 2). Rhythms were sufficiently stable to allow injection of 35 of 40 hamsters and calculation of phase shifts for 34. Mel caused significantly larger phase advances than vehicle across all hamsters (ANOVA: main effect of injection, p < 0.01; main effect of condition, n.s.). Mel-induced phase advances did not differ between photosensitive and photorefractory hamsters (2.4 ± 0.3 h and 2.1 ± 0.4 h, respectively; t test, p = 0.5).
Experiment 2
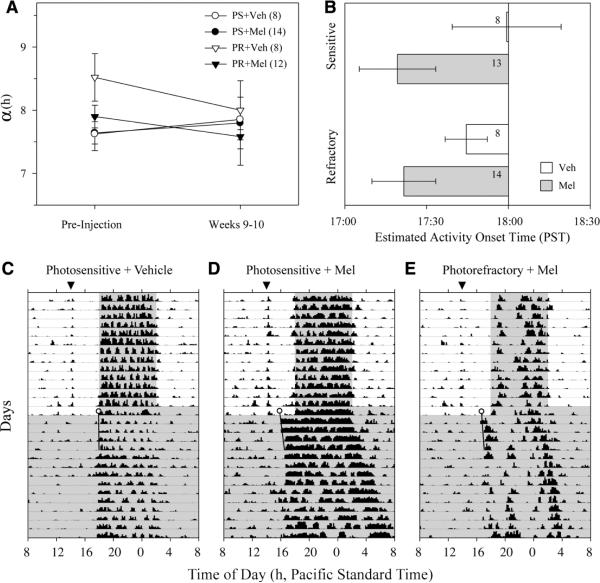
Initially, α was significantly longer in refractory short-day (10L, n = 24) than photosensitive long-day (14L, n = 23) hamsters (11.8 ± 0.4 vs. 9.5 ± 0.2 h, respectively; t test, p < 0.001). This difference disappeared by the last 2 weeks of 16L before injections (refractory: 8.0 ± 0.2 h, vs. photosensitive: 7.6 ± 0.1 h; t test, p > 0.1). Neither Mel nor vehicle injections affected α or activity onset in either photosensitive or photorefractory hamsters entrained to the 16L photocycle (α: Fig. 5A, repeated-measures ANOVA, all effects n.s.; activity onset: not illustrated, ANOVA, all effects n.s.).
Figure 5.
(A) Duration of locomotor activity (α) before and at the end of a 10-week period of daily injections of either Mel or vehicle (Veh) in photosensitive (PS) and photorefractory (PR) hamsters. n in parentheses. (B) Activity onset on the last day of injections, as estimated by the subsequent free-running rhythms, occurred significantly earlier in Mel-injected than Veh-injected hamsters. Dark onset at 1800 h PST. n within panel. Although the main effect of melatonin versus vehicle injection was significant across all animals, post-hoc tests limited to within the photosensitive or within the photorefractory condition fell short of significance (Fisher's PLSD: photosensitive, p = 0.11; photorefractory, p = 0.19), likely due to small sample sizes. Representative actograms of locomotor activity used to calculate the onsets in B are plotted for a photosensitive hamster injected with Veh (C), a photosensitive hamster injected with Mel (D), and a photorefractory hamster injected with Mel (E). Data for the last 14 days of injections and the first 14 days of constant dim red illumination are plotted; gray shading indicates time of darkness or dim red light. The target time of injection is designated by the filled triangle at the top (1400 h). A line fitted by eye through the onsets of the first 4 days in constant conditions (black line) was extended back to estimate the endogenous circadian phase angle of entrainment on the last day of injections (circle). Hatch marks represent gross loco-motor activity measured by passive infrared detectors; other conventions as in Figure 3.
Although Mel injections did not affect the pattern of locomotor activity under entrained conditions, this appears to be due to masking by light (see Figs. 5C–5E). The endogenous activity onset, extrapolated back to the last day of 16L, was significantly earlier in hamsters treated with Mel than vehicle (Fig. 5B; ANOVA: main effect of injection, p < 0.05; condition effect, p = 0.7; interaction, p = 0.6). Considering only the hamsters injected with Mel, estimated phase of locomotor activity onset did not differ between photosensitive and photorefrac-tory groups (Fig. 5B; t test, p = 0.9).
DISCUSSION
Melatonin injections produced equal phase advances in the circadian rhythms of both photosensitive and photorefractory hamsters, indicating that this Mel signaling pathway remains responsive in the photorefractory state. Thus, seasonal refractoriness is not a consequence of global insensitivity to Mel, but may be restricted to particular Mel target systems or Mel durations. Refractoriness that is specific to particular durations of Mel is in keeping with previous results that Mel target tissues of photorefractory hamsters are unresponsive to long-duration but responsive to short-duration Mel signals (e.g., Kauffman et al., 2003). The Mel output pathways that subserve circadian and seasonal functions nevertheless remain to be specified (Lincoln et al., 2005).
Mel binds to several brain areas in the Siberian hamster, all of which appear to be locally and independently rendered refractory by targeted intracranial implants of Mel (Freeman and Zucker, 2001). Of the several Mel binding sites in the Siberian hamster brain, the SCN is of particular interest because ablation of this tissue, but not of the others, blocks seasonal reproductive inhibition by short days or by short-day-like subcutaneous Mel infusions (Bartness et al., 1991; Purvis and Duncan, 1997). The Siberian hamster is therefore an excellent model species in which to examine Mel effects on the circadian and seasonal systems and their interrelation.
The refractory hamster's preserved sensitivity to short Mel durations has long been recognized (e.g., Bittman and Zucker, 1981). Nevertheless, previous results suggested changes in Mel's effects on the refractory SCN. In SCN explants from long-day Siberian hamsters, local Mel application changes spike rates of a subset of SCN neurons in a dose-dependent and reversible manner (Margraf and Lynch, 1993). In photorefractory Syrian hamsters, fewer SCN neurons responded to Mel with a change in spike rate compared to photosensitive hamsters (Mason and Rusak, 1990). Because SCN neuronal responses were compared between refractory hamsters in short days and photosensitive hamsters in long days, direct effects of short-day length, unrelated to refractoriness, cannot be discounted. Mel-independent correlates of photoperiod have been documented in the SCN. The duration of SCN neural activity differs in long-day versus short-day lengths in Syrian hamsters (Mrugala et al., 2000) and other rodents (VanderLeest et al., 2007); this difference appears to be pineal independent and melatonin independent (Jacob et al., 1997).
Puchalski and Lynch (1988b) also showed that Mel's effect on the circadian system varied with seasonal phenotype: in response to Mel injections, α expanded and contracted with testicular involution and recrudescence, respectively. This suggested that photosensitive and photorefractory hamsters might differ in their circadian responses to Mel; that is, daily phase advances were attenuated or eliminated in the refractory state. In replicating this experiment, we were unable to detect differences in circadian organization (experiment 2), nor did we detect differences in a more direct assessment in free-running hamsters (experiment 1). It is unlikely that the a expansion observed by Puchalski and Lynch (1988b) is a seasonal trait under the proximate control of Mel duration rather than photoperiod because when different photoperiods are juxtaposed with different Mel infusion durations, α is determined by ambient photoperiod, not Mel duration (Gorman, 2003). It is unresolved why phase advances were masked by light in the present study, but not in that of Puchalski and Lynch (1988b). Procedural differences included the method for inducing photorefractoriness (supplementary Mel injections in long days vs. long-term exposure to short days), the type of activity measured (running wheels vs. passive infrared motion detectors), and gonadal condition (intact vs. castrated). We note, however, that the changes in locomotor activity observed by Puchalski and Lynch (1988b) could reflect differences in phase shift magnitude to Mel between the summer and winter phenotypes that are unrelated to photosensitivity or photorefractoriness.
There is no established dose-response curve for phase shifting of circadian rhythms by Mel in Siberian hamsters. Our doses (1 mg/kg in experiment 1, 25 μg in experiment 2, administered to ~40 g hamsters) were identical to those used previously in circadian studies of this species (Duffield et al., 1998; Puchalski and Lynch, 1988b). The ED50 (5 μg/kg) reported for Mel's ability to entrain circadian rhythms of rats (Cassone et al., 1986) is much lower. In that study, there was no graded response to the dose of Mel; rats either entrained or did not; all effective doses raised circulating Mel concentrations to supraphysiological concentrations (Cassone et al., 1986). We note that our measured phase advances could represent a ceiling effect, and that lower doses might reveal differences in phase shift magnitude between photosensitive and photorefractory hamsters.
Although refractoriness is well documented at the systems level, the underlying cellular mechanisms remain unknown. Mel signal production is not implicated: Mel secretory profiles do not change in the photorefractory state (Rollag et al., 1980), and target tissues become unresponsive when a fixed short-day-like Mel treatment is administered to Syrian hamsters (Bittman, 1978). Photorefractoriness also does not appear to reflect disrupted signaling at the level of the Mel receptor. Receptor function (maximum binding and affinity) in the SCN, and second messenger systems (Mel receptor–G protein coupling and adenylyl cyclase inhibition by Mel) in the pars tuberalis (PT), are normal in photorefractory hamsters (Weaver et al., 1991). Mel's genomic effects are likewise preserved, at least with respect to its effects on the circadian “clock” genes in the PT (Johnston et al., 2003; Lincoln et al., 2005). In Syrian hamsters, the diurnal patterns of clock gene expression in the PT and SCN differ in long and short photoperiods; their expression in the PT but not in the SCN is abolished by pinealectomy (Messager et al., 2001). Refractoriness may therefore depend on changes in Mel signal propagation to downstream targets, but given our results, this must be target specific: in our refractory hamsters, the brain circuits mediating Mel's circadian effects are functional, whereas those that mediate Mel's short-day seasonal effects have been compromised.
Incidental evidence supports a seasonally important functional partitioning of the Siberian hamster SCN. Complete SCN lesions block seasonal responses (e.g., testicular involution) to long-duration subcutaneous Mel infusions; lesions that destroy only the rostral SCN also blocked responses to Mel (Bartness et al., 1991), but damage to the adjacent anterior hypothalamus that spared SCN tissue was ineffective (Song and Bartness, 1996). This suggests that the rostral SCN is critical for mediating the effects of Mel on seasonal changes in reproductive function. The rostrocaudal extent of the SCN may also be important for accurate encoding of light information under various photoperiods (Hazlerigg et al., 2005).
In an attempt to localize Mel's effects within the SCN, we assessed Fos induction after a single injection of Mel at CT10. Whether Mel induces Fos in the SCN is equivocal (Kilduff et al., 1992; Kumar et al., 1997); Siberian hamsters may be a better model organism than the previously studied rats and Syrian hamsters, because Siberian hamsters are responsive to both seasonal variations in Mel signal duration and acute daily changes in Mel availability. In contrast, rats are not reproductively photoperiodic (Nelson and Zucker, 1981), and circadian rhythms of adult Syrian hamsters are not phase shifted by Mel (Hastings et al., 1992). We found no differences between Mel-injected and vehicle-injected hamsters in Fos expression assessed over the whole SCN, or more specifically in rostral, mid, or caudal SCN; nor were there differences between vasopressin immunoreactive and nonimmunoreactive areas of the caudal SCN (Butler et al., unpublished data). These results are in keeping with the general pattern for nonphotic zeitgebers (Mikkelsen et al., 1998).
Studies of pars tuberalis function in photorefractory hamsters point to refractoriness as a downstream property of the Mel receptor-containing cells. In common with the SCN, this tissue may independently become refractory (Lincoln and Clarke, 1994). Mel's acute effects on gene expression and cyclic AMP signaling are maintained in the refractory state (Johnston et al., 2003; Weaver et al., 1991). Normal Mel action at the level of the receptor and changes in subsequent pathways may be a general feature of Mel signal processing in photorefractory rodents. Downstream signaling need not be in different cells; the same Mel receptor-containing neurons may remain responsive to acute short-duration Mel and unresponsive to long-duration Mel signals.
The present experiments establish the integrity of Mel's circadian neuroendocrine signaling pathway in photorefractory Siberian hamsters as assessed by a behavioral endpoint. Mel's effects on the seasonal and circadian apparatus are dissociated in the photorefractory state; the photorefractory hamster's nervous system response to brief Mel signals is similar to that of photosensitive hamsters but differs markedly in response to long-duration signals that induce the winter phenotype.
ACKNOWLEDGMENTS
We are grateful to Chris Tuthill and Kim Pelz for technical assistance and to the Office of Laboratory Animal Care for animal husbandry. The manuscript was improved in response to comments of 3 anonymous reviewers. M. P. Butler is a Howard Hughes Medical Institute Predoctoral Fellow. This research was supported by Grant MH-61171 from the National Institute of Mental Health.
REFERENCES
- Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- Armstrong SM. Melatonin and circadian control in mammals. Experientia. 1989;45:932–938. doi: 10.1007/BF01953050. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bittman EL. Hamster refractoriness: The role of insensitivity of pineal target tissues. Science. 1978;202:648–650. doi: 10.1126/science.568311. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Zucker I. Photoperiodic termination of hamster refractoriness: Participation of the pineal gland. Biol Reprod. 1981;24:568–572. doi: 10.1095/biolreprod24.3.568. [DOI] [PubMed] [Google Scholar]
- Butler MP, Turner KW, Park JH, Butler JP, Trumbull JJ, Dunn SP, Villa P, Zucker I. Simulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters. Am J Physiol. 2007;293:R402–R412. doi: 10.1152/ajpregu.00146.2007. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Chesworth MJ, Armstrong SM. Dose-dependent entrainment of rat circadian rhythms by daily injection of melatonin. J Biol Rhythms. 1986;1:219–229. doi: 10.1177/074873048600100304. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Roberts MH, Moore RY. Melatonin inhibits metabolic activity in the rat suprachiasmatic nuclei. Neurosci Lett. 1987;81:29–34. doi: 10.1016/0304-3940(87)90335-1. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Roberts MH, Moore RY. Effects of melatonin on 2-deoxy-[1-14C]glucose uptake within rat suprachiasmatic nucleus. Am J Physiol. 1988;255:R332–337. doi: 10.1152/ajpregu.1988.255.2.R332. [DOI] [PubMed] [Google Scholar]
- Duffield GE, Hastings MH, Ebling FJ. Investigation into the regulation of the circadian system by dopamine and melatonin in the adult Siberian hamster (Phodopus sungorus) J Neuroendocrinol. 1998;10:871–884. doi: 10.1046/j.1365-2826.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage color cycle in the Djungarian hamster, Phodopus sungorus. I. Role of the gonads and pituitary. J Exp Zool. 1984;230:89–95. doi: 10.1002/jez.1402300112. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci U S A. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR. A plastic interval timer synchronizes pubertal development of summer- and fall-born hamsters. Am J Physiol. 2001;281:R1613–R1623. doi: 10.1152/ajpregu.2001.281.5.R1613. [DOI] [PubMed] [Google Scholar]
- Gorman MR. Independence of circadian entrainment state and responses to melatonin in male Siberian hamsters. BMC Physiol. 2003;3:10. doi: 10.1186/1472-6793-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Environmental induction of photononresponsiveness in the Siberian hamster, Phodopus sungorus. Am J Physiol. 1997;272:R887–R895. doi: 10.1152/ajpregu.1997.272.3.R887. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Mead SM, Vindlacheruvu RR, Ebling FJ, Maywood ES, Grosse J. Non-photic phase shifting of the circadian activity rhythm of Syrian hamsters: The relative potency of arousal and melatonin. Brain Res. 1992;591:20–26. doi: 10.1016/0006-8993(92)90973-d. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Ebling FJ, Johnston JD. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr Biol. 2005;15:R449–R450. doi: 10.1016/j.cub.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Influence of photoperiod and melatonin on testis size, body weight, and pelage color in Djungarian hamster (Phodopus sungorus) J Comp Physiol. 1973;85:267–282. [Google Scholar]
- Hoffmann K. Effects of short photoperiods on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus) J Reprod Fertil. 1978;54:29–35. doi: 10.1530/jrf.0.0540029. [DOI] [PubMed] [Google Scholar]
- Jacob N, Vuillez P, Pevet P. Photoperiod does not act on the suprachiasmatic nucleus photosensitive phase through the endogenous melatonin, in the Syrian hamster. Neurosci Lett. 1997;229:117–120. doi: 10.1016/s0304-3940(97)00428-x. [DOI] [PubMed] [Google Scholar]
- Johnston JD, Cagampang FR, Stirland JA, Carr AJ, White MR, Davis JR, Loudon AS. Evidence for an endogenous per1- and ICER-independent seasonal timer in the hamster pituitary gland. FASEB J. 2003;17:810–815. doi: 10.1096/fj.02-0837com. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Freeman DA, Zucker I. Termination of neuroendocrine refractoriness to melatonin in Siberian hamsters (Phodopus sungorus) J Neuroendocrinol. 2003;15:191–196. doi: 10.1046/j.1365-2826.2003.00966.x. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Landel HB, Nagy GS, Sutin EL, Dement WC, Heller HC. Melatonin influences Fos expression in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1992;16:47–56. doi: 10.1016/0169-328x(92)90192-e. [DOI] [PubMed] [Google Scholar]
- Kumar V, Goguen DM, Guido ME, Rusak B. Melatonin does not influence the expression of c-fos in the suprachiasmatic nucleus of rats and hamsters. Brain Res Mol Brain Res. 1997;52:242–248. doi: 10.1016/s0169-328x(97)00260-x. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Clarke IJ. Photoperiodically-induced cycles in the secretion of prolactin in hypothalamo-pituitary disconnected rams: Evidence for translation of the melatonin signal in the pituitary gland. J Neuroendocrinol. 1994;6:251–260. doi: 10.1111/j.1365-2826.1994.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Johnston JD, Andersson H, Wagner G, Hazlerigg DG. Photorefractoriness in mammals: Dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology. 2005;146:3782–3790. doi: 10.1210/en.2005-0132. [DOI] [PubMed] [Google Scholar]
- Margraf RR, Lynch GR. An in vitro circadian rhythm of melatonin sensitivity in the suprachiasmatic nucleus of the Djungarian hamster, Phodopus sungorus. Brain Res. 1993;609:45–50. doi: 10.1016/0006-8993(93)90853-f. [DOI] [PubMed] [Google Scholar]
- Mason R, Rusak B. Neurophysiological responses to melatonin in the SCN of short-day sensitive and refractory hamsters. Brain Res. 1990;533:15–19. doi: 10.1016/0006-8993(90)91789-j. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- Messager S, Garabette ML, Hastings MH, Hazlerigg DG. Tissue-specific abolition of Per1 expression in the pars tuberalis by pinealectomy in the Syrian hamster. Neuroreport. 2001;12:579–582. doi: 10.1097/00001756-200103050-00029. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Vrang N, Mrosovsky N. Expression of Fos in the circadian system following nonphotic stimulation. Brain Res Bull. 1998;47:367–376. doi: 10.1016/s0361-9230(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Mrugala M, Zlomanczuk P, Jagota A, Schwartz WJ. Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. Am J Physiol. 2000;278:R987–994. doi: 10.1152/ajpregu.2000.278.4.R987. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Zucker I. Photoperiodic control of reproduction in olfactory-bulbectomized rats. Neuroendocrinology. 1981;32:266–271. doi: 10.1159/000123171. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Park JH, Horton TH, Alvarez MI, Burke MK, Place NJ, Zucker I. Photoperiodic regulation of compensatory testicular hypertrophy in hamsters. Biol Reprod. 2006;75:261–269. doi: 10.1095/biolreprod.106.050781. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Volume 2. Elsevier Science; New York: 2002. pp. 93–156. [Google Scholar]
- Puchalski W, Lynch GR. Characterization of circadian function in Djungarian hamsters insensitive to short day photoperiod. J Comp Physiol [A] 1988a;162:309–316. doi: 10.1007/BF00606119. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Daily melatonin injections affect the expression of circadian rhythmicity in Djungarian hamsters kept under a long-day photoperiod. Neuroendocrinology. 1988b;48:280–286. doi: 10.1159/000125023. [DOI] [PubMed] [Google Scholar]
- Purvis CC, Duncan MJ. Discrete thalamic lesions attenuate winter adaptations and increase body weight. Am J Physiol. 1997;273:R226–235. doi: 10.1152/ajpregu.1997.273.1.R226. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Panke ES, Reiter RJ. Pineal melatonin content in male hamsters throughout the seasonal reproductive cycle. Proc Soc Exp Biol Med. 1980;165:330–334. doi: 10.3181/00379727-165-40981. [DOI] [PubMed] [Google Scholar]
- Song CK, Bartness TJ. The effects of anterior hypothalamic lesions on short-day responses in Siberian hamsters given timed melatonin infusions. J Biol Rhythms. 1996;11:14–26. doi: 10.1177/074873049601100102. [DOI] [PubMed] [Google Scholar]
- VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Liu C, Reppert SM. Nature's knockout: The Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol. 1996;10:1478–1487. doi: 10.1210/mend.10.11.8923472. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Provencio I, Carlson LL, Reppert SM. Melatonin receptors and signal transduction in photorefractory Siberian hamsters (Phodopus sungorus) Endocrinology. 1991;128:1086–1092. doi: 10.1210/endo-128-2-1086. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J Neurosci. 1989;9:2581–2590. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]