SUMMARY
Linker of Nucleoskeleton and Cytoskeleton (LINC) complexes span the nuclear envelope and are composed of KASH and SUN proteins residing in the outer and inner nuclear membrane, respectively. LINC formation relies on direct binding of KASH and SUN in the perinuclear space. Thereby, molecular tethers are formed that can transmit forces for chromosome movements, nuclear migration and anchorage. We present crystal structures of the human SUN2-KASH1/2 complex, the core of the LINC complex. The SUN2 domain is rigidly attached to a trimeric coiled-coil that prepositions it to bind three KASH peptides. The peptides bind in three deep and expansive grooves formed between adjacent SUN domains, effectively acting as molecular glue. In addition, a disulfide between conserved cysteines on SUN and KASH covalently links both proteins. The structure provides the basis of LINC complex formation and suggests a model for how LINC complexes might arrange into higher-order clusters to enhance force-coupling.
Keywords: nuclear envelope, SUN, Nesprin, KASH, nuclear migration
INTRODUCTION
The nuclear envelope (NE), a double lipid bilayer, forms the boundary of the nuclear compartment and serves as a physical barrier between chromatin in the nuclear interior and the cytoplasm. The integrity and physical properties of the NE play pivotal roles in cellular homeostasis, signaling and organization of intracellular architecture (Mekhail and Moazed, 2010; Stewart et al., 2007). The exchange of material between nucleus and cytoplasm is guaranteed by passageways through the NE that are formed by nuclear pore complexes (Onischenko and Weis, 2011).
Communication between nucleus and nuclear exterior is not limited to transport of macromolecules across the NE but also involves connections of the NE and the cytoskeleton. These physical linkages are instrumental for plasticity of cellular organization and are required for processes such as nuclear migration and anchorage, meiotic chromosome movements, centrosome positioning, and global organization of the cytoskeleton (reviewed in Burke and Roux, 2009; Starr and Fridolfsson, 2010; Tzur et al., 2006; Worman and Gundersen, 2006). The molecular tether between NE and cytoskeletal elements is built by protein complexes referred to as LINC (Linker of Nucleoskeleton and Cytoskeleton) (Crisp et al., 2006). LINC complexes are conserved from yeast to human. They form bridges across the perinuclear space by pairing of KASH (Klarsicht, ANC-1, and Syne Homology) and SUN (Sad1, UNC-84) proteins that localize to outer (ONM) and inner nuclear membrane (INM), respectively (Razafsky and Hodzic, 2009; Starr and Fischer, 2005).
KASH proteins are tail-anchored membrane proteins. Their large cytoplasmic domains extend into the cytoplasm, where they interact with actin filaments, microtubules, intermediate filaments or centrosomes. At their C termini, KASH proteins expose a short peptide of around 30 residues into the perinuclear space. This luminal peptide together with the preceding transmembrane segment provides the characteristic signature of KASH proteins referred to as KASH domain (Starr and Han, 2002). The luminal KASH peptides of many family members are similar. Their conserved features are the presence of aromatic/hydrophobic residues at defined positions and proline(s) close to their C-terminal ends. Studies in various organisms have shown that KASH domains are necessary and sufficient for localization of KASH proteins to the ONM (reviewed in Starr and Fridolfsson, 2010). This is achieved by a direct interaction of the KASH peptide with members of the SUN protein family residing in the INM (Ostlund et al., 2009; Padmakumar et al., 2005).
Vertebrates possess at least 4 different KASH proteins (SYNE/Nesprin-1 to -4) that contain spectrin repeats in their cytoplasmic domains (Apel et al., 2000; Zhang et al., 2001). Nesprin-1 and -2 (SYNE1, 2) exist in numerous splice isoforms. The giant isoforms of Nesprin-1 and -2 interact with actin via their N-terminal actin-binding domain, whereas other isoforms can associate with microtubule motors (Padmakumar et al., 2004; Yu et al., 2011; Zhang et al., 2009; Zhen et al., 2002). Nesprin-3 and -4 bind to plectin and kinesin-1, respectively, thereby connecting the nucleus to intermediate filaments and microtubules (Roux et al., 2009; Wilhelmsen et al., 2005). Reflecting the importance of NE-cytoskeletal interactions for human health, mutations in Nesprins have been linked to the pathogenesis of Emery-Dreifuss muscular dystrophy (Puckelwartz et al., 2009; Zhang et al., 2007a).
SUN proteins form the inner half of the LINC complex. They are anchored in the INM by at least one transmembrane segment, exposing their N termini to the nucleoplasm. These nucleoplasmic domains harbor signals that aid targeting of SUN proteins to the INM and mediate association with nuclear binding partners such as the nuclear lamina (Fridkin et al., 2004; Haque et al., 2006; Lee et al., 2002). The C-terminal segments of SUN proteins reside in the perinuclear space and consist of coiled-coil regions followed by the conserved SUN domain, which is required for KASH binding. Among the five characterized mammalian SUN proteins, only SUN1 and SUN2 are widely expressed (Crisp et al., 2006; Wang et al., 2006) and can bind to all four Nesprins (Ketema et al., 2007; Roux et al., 2009; Stewart-Hutchinson et al., 2008). A double knockout of SUN1 and SUN2 in mice is lethal (Lei et al., 2009), similar to Nesprin-1/2 deletion (Zhang et al., 2007b). Through their cellular function in chromatin organization and nuclear migration and anchorage, SUN1/2 and Nesprin-1/2 play pivotal roles in a variety of developmental processes in vertebrates, ranging from gametogenesis to the development of muscles, brain and the retina (Ding et al., 2007; Lei et al., 2009; Puckelwartz et al., 2009; Yu et al., 2011; Zhang et al., 2010; Zhang et al., 2009). How SUN and KASH interact to provide mechanical coupling between structural components of the nucleus and the cytoskeleton has remained elusive. Here, we present crystal structures of the SUN domain of human SUN2 in its free and KASH-bound conformations. These structures reveal a trimeric organization of SUN domains intimately bound to three KASH peptides at the SUN protomer interfaces. Biochemical and cell biological studies support and further qualify our structural findings.
RESULTS
Mapping the KASH-binding competent region of human SUN2
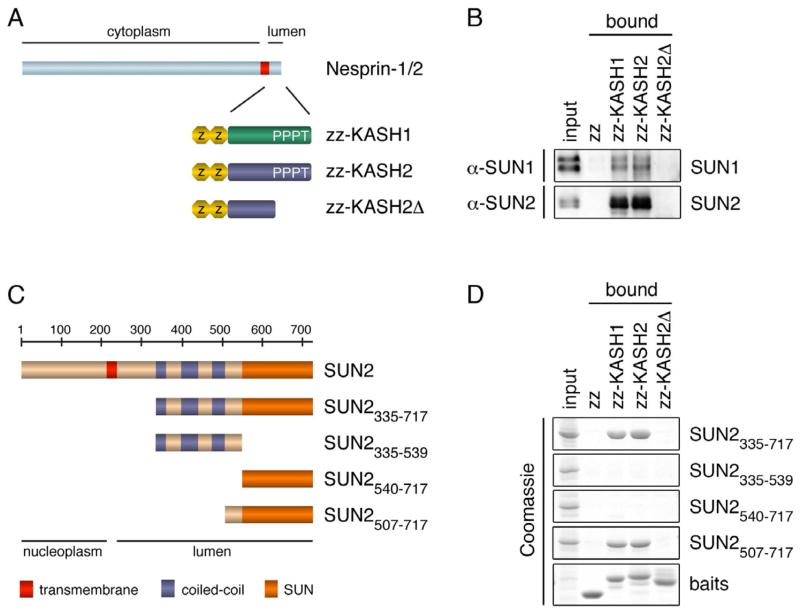
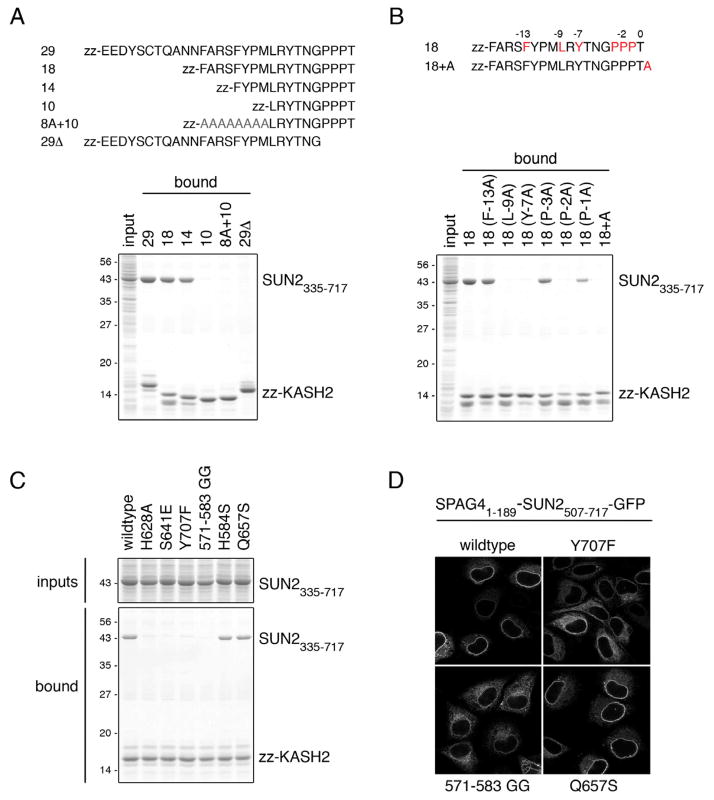
To define the minimal region in human SUN2 required for binding of the luminal part of KASH domains, we used recombinant KASH fusion proteins composed of an N-terminal protein A tag (zz) and the C-terminal 29 residues of human Nesprin-1 or -2 (Figure 1A, zz-KASH1, zz-KASH2) in pulldown experiments. First, we tested whether the KASH fusion proteins could interact with endogenous SUN proteins present in HeLa cell detergent extract. Both KASH1 and KASH2 retrieved SUN1 and SUN2 from the extract as verified by immunoblotting (Figure 1B). Binding of SUN proteins to KASH1/2 was specific, as no binding of SUN proteins was observed to either zz alone or zz-KASH2Δ, in which the four C-terminal amino acids of KASH that are required for interaction with SUN proteins are missing (Padmakumar et al., 2005).
Figure 1. A Minimal Coiled-coil and the SUN Domain are Required for KASH Binding.
(A) Schematic representation of zz-KASH fusion proteins. The luminal peptides of Nesprin-1 and Nesprin-2 (29 aa) were fused to an N-terminal protein A tag (zz-KASH1 and zz-KASH2). zz-KASH2Δ lacks the C-terminal amino acids PPPT.
(B) SUN1 and SUN2 from HeLa cells bind to zz-KASH peptides in vitro. HeLa cells were lysed in RIPA buffer and the extract was incubated with immobilized zz-KASH fusion proteins. Bound proteins were eluted and analyzed by immunoblotting using α-SUN1 and α-SUN2 antibodies. Loads in input and pulldown lanes correspond to 2.5% and 20% of the total, respectively.
(C) Schematic representation of SUN2 constructs used in pulldown experiments in (D). Coiled-coil regions were predicted using Parcoil2 (McDonnell et al., 2006).
(D) KASH binding of recombinant SUN2 fragments. His-tagged SUN2 fragments were expressed in E. coli, purified and added at 0.25 μg/μl to E. coli lysate supplemented with RIPA detergents. Mixtures were incubated with immobilized zz-KASH fusion proteins. Bound proteins were eluted and analyzed by SDS-PAGE and Coomassie staining. Loads in the input and pulldown lanes correspond to 1.25% and 10% of the total, respectively.
See also Figure S1.
Next, we tested direct KASH binding of recombinant SUN2 protein fragments comprising different regions of its luminal domain (Figure 1C,D). In agreement with published data (Ostlund et al., 2009), we observed that a protein fragment comprising both the predicted coiled-coil regions and the C-terminal SUN domain of SUN2 (SUN2335–717) efficiently bound to both KASH peptides. However, neither the isolated coiled-coil region (SUN2335–539) nor the SUN domain alone (SUN2540–717) was sufficient for KASH interaction. Further deletion analysis revealed that the coiled-coil region preceding the SUN domain could be significantly shortened without loosing KASH interaction. A fragment comprising an N-terminally extended SUN domain (SUN2507–717) was found sufficient for efficient binding to both KASH peptides. When we introduced further truncations (Figure S1), we observed that binding to KASH decreased (SUN2514–717) and finally became barely detectable (SUN2521–717). As the protein segment 507–539 is not part of the conserved SUN domain and is just adjacent to the last predicted coiled-coil of SUN2, we reasoned that the contribution of residues 507–539 to KASH binding may be attributed to a potential role in organization of the SUN domain into a higher order structure. To test this assumption, we fused an unrelated, well-characterized coiled-coil region derived from the yeast transcription factor Gcn4 (Ciani et al., 2010) in front of the three extended SUN domain fragments (SUN2521–717, SUN2514–717, and SUN2507–717). Strikingly, addition of this unrelated coiled-coil (ucc) restored the KASH binding capacity of SUN2521–717, demonstrating that residues 521–717 of SUN2 are sufficient for interaction with KASH peptides and suggesting that coiled-coil regions in front of the SUN domain contribute to the higher order organization of SUN domains into a KASH-binding competent state.
Structure determination of apo-SUN2, SUN2-KASH1, and SUN2-KASH2
After determining the minimal SUN domain of human SUN2, we set up crystallization screens for apo-SUN2522–717, SUN2522–717-KASH1 (Nesprin-1, residues 8769–8797) and SUN2522–717-KASH2 (Nesprin-2, residues 6857–6885). We first obtained crystals of apo-SUN2, diffracting to 2.2 Å (Table 1). We used selenomethionine-derivatized protein to solve the structure with the single-anomalous dispersion (SAD) method (for details see Experimental Procedures). The refined apo-SUN2 structure (R/Rfree 18.9%/23.3%) was subsequently used to solve the structures of the SUN2-KASH1 and SUN2-KASH2 complexes by molecular replacement.
Table 1.
Data Collection and Refinement Statistics
| hSUN2 | hSUN2-L546M | hSUN2:KASH1 | hSUN2:KASH2 | |
|---|---|---|---|---|
|
Data collection
| ||||
| Data set | Native | Selenomethionine | Native | Native |
| Space group | R32 | R32 | R32 | R32 |
| a, b, c (Å) | 79.6, 79.6, 199.1 | 79.7, 79.7, 200.2 | 79.6, 79.6, 256.4 | 79.3, 79.3, 260.0 |
| Wavelength (Å) | 0.9795 | 0.9792 | 0.9792 | 0.9792 |
| Resolution range (Å) | 56.7-2.2 (2.3-2.2) | 56.8-2.4 (2.5-2.4) | 85.5-2.3 (2.4-2.3) | 43.3-2.7 (2.8-2.7) |
| Total reflections | 72,988 | 56,714 | 49,146 | 52,267 |
| Unique reflections | 12,305 | 9,510 | 13,944 | 8,912 |
| Completeness (%) | 99.8 (99.9) | 99.6 (99.8) | 99.6 (97.7) | 99.9 (99.8) |
| Redundancy | 5.9 (6.1) | 3.1 (3.1) | 3.5 (3.4) | 5.9 (6.1) |
| Rmerge(%) | 4.8 (57.0) | 4.6 (65.0) | 7.6 (70.7) | 5.5 (69.5) |
| Rr.i.m.(%) | 5.3 (62.3) | 6.6 (78.8) | 9.0 (83.3) | 6.1 (76.1) |
| Rp.i.m.(%) | 2.1 (24.8) | 2.7 (31.8) | 4.7 (43.4) | 2.5 (30.7) |
| I /σ | 20.0 (3.5) | 22.2 (2.5) | 24.9 (1.5) | 21.0 (2.7) |
| Wilson B factor (Å2) | 53.0 | 57.9 | 45.4 | 66.5 |
|
| ||||
|
Refinement
| ||||
| Resolution range (Å) | 56.7-2.2 | 45.0-2.3 | 36.1-2.7 | |
| Rwork (%) | 18.9 | 19.6 | 18.4 | |
| Rfree (%) | 23.3 | 21.1 | 23.9 | |
|
| ||||
|
Number of Reflections
| ||||
| Total | 12,304 | 13,930 | 8,909 | |
| Rfree reflections | 1,250 | 1,393 | 891 | |
|
| ||||
|
Number of Atoms
| ||||
| Protein | 1,585 | 1,762 | 1,751 | |
| Water | 101 | 129 | 41 | |
| n-Decyl-β-maltoside | 25 | |||
|
| ||||
|
B Factors (Å2)
| ||||
| SUN2 | 45.8 | 44.8 | 65.0 | |
| KASH | 59.8 | 93.2 | ||
| Water | 48.2 | 50.4 | 61.6 | |
| n-Decyl-β-maltoside | 71.7 | |||
|
| ||||
|
R.m.s. Deviations
| ||||
| Bond length (Å) | 0.009 | 0.007 | 0.009 | |
| Bond angles (°) | 1.170 | 1.023 | 1.141 | |
|
| ||||
|
Ramachandran Plot
| ||||
| Favored (%) | 94.5 | 94.5 | 93.1 | |
| Allowed (%) | 5.5 | 5.5 | 6.4 | |
| Outliers (%) | 0 | 0 | 0.5 | |
The highest resolution shell is in parenthesis. Rmerge is the merging R factor. Rr.i.m. is the redundancy independent merging R factor.
Rp.i.m. is the precision-indicating merging R factor. For definitions, see Weiss (2001).
Structure of the SUN2-KASH2 complex
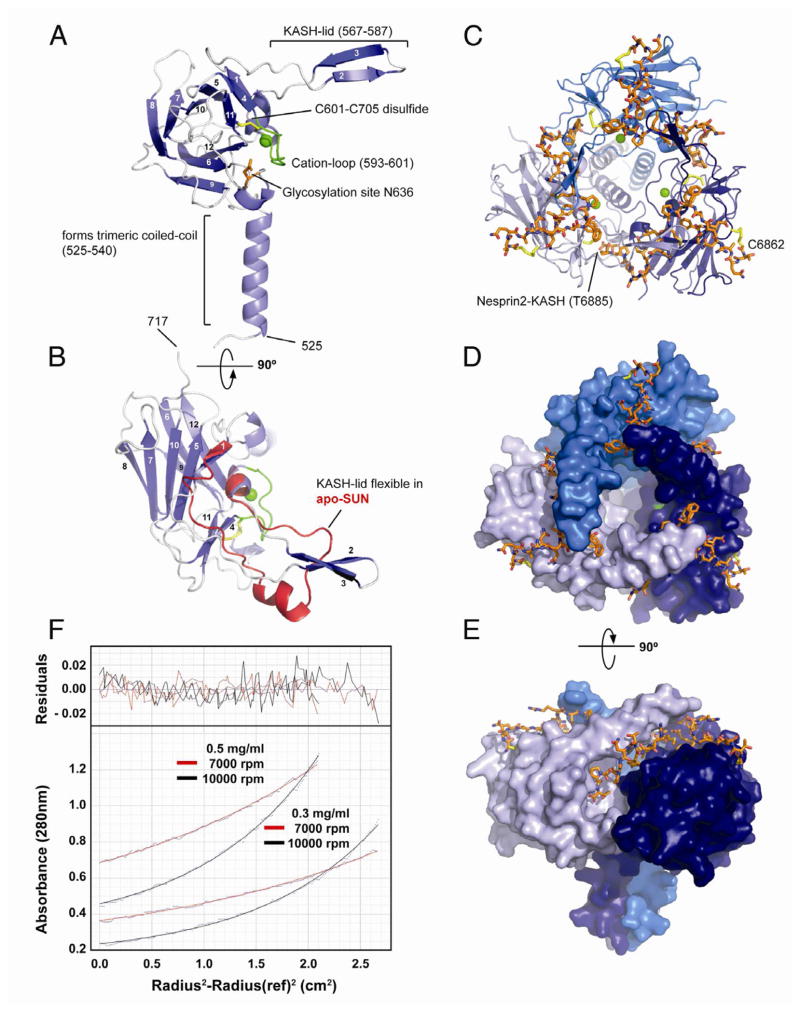
The principal SUN domain of SUN2 folds into a compact β-sandwich (Figure 2A,B). Two sets of antiparallel β-sheets composed of 3 and 5 strands form this basic structure. The β-sandwich is decorated by two main additions, which are of major functional importance. At the N terminus, we find a helical extension (residues 525–540) involved in the formation of a coiled-coil. Between residues 567 and 587, there is a 20-residue extension that folds into a protruding antiparallel β-sheet, which we term the KASH-lid. As a third addition, residues 593–601 form a well-defined loop, which surrounds and coordinates a bound cation via five backbone carbonyls, thus we call this detail the cation-loop. Judged by the coordination sphere, the bond distances (Harding et al., 2006), and the temperature factor, we suggest that the bound cation is a potassium ion in our crystal. However, due to the possible malleability of the loop, the evolutionary relationship to other proteins (see below), and the ionic environment in the perinuclear space, a calcium ion might be coordinated in vivo. The arrangement of the cation-loop is further defined by an intrachain disulfide bond formed between conserved cysteines at positions 601 and 705.
Figure 2. Structural Analysis of the SUN-KASH Interaction.
(A) Overview of a SUN2522–717 protomer isolated from its binding partners in the trimeric SUN-KASH complex. The protein is organized around a compact β-sandwich core, decorated with features important for function (labeled). Bound cation in green.
(B) Top view of the SUN2522–717 protomer (facing the outer nuclear membrane), rotated by 90° around the horizontal axis relative to the view in (A). The apo-protomer of SUN2522–717 is superimposed in red. Only the region with significant change is shown, coinciding largely with the KASH-lid.
(C) Top view of the trimeric SUN2522–717-KASH2 complex. The trimerizing SUN2 domains in cartoon representation and colored in shades of blue, the KASH2 peptide in orange. The peptide is covalently bound via a disulfide bridge between KASH2-C6862 and SUN2-C563.
(D) Same view as (C), but SUN2522–717 in surface representation, illustrating how each bound KASH2 peptide is clamped between two SUN2 protomers.
(E) Side view of the SUN2-KASH2 complex, illustrating the deep binding pocket on SUN2 into which the terminal four residues of the KASH peptide bind.
(F) Sedimentation equilibrium ultracentrifugation analysis of apo-SUN2335–717. The experiment was performed at two protein concentrations and two centrifugation speeds. Data was fitted for a single species. Fitted curves overlaid over primary data (dots) in red and black for the two speeds. Residuals in the upper panel. The mass was determined to be 131.3 ± 6.1 kDa, the calculated mass is 138 kDa for the trimer.
See also Figures S2, S4, S5.
SUN2 is organized around the threefold crystallographic axis to form a stable trimer (Figure 2C,D,E), consistent with a recent crystallographic study of apo-SUN2 (Zhou et al., 2012). The contacts between the SUN2 protomers are substantial. First, the N-terminal helical extension forms an unusual, right-handed supercoiled three-helix bundle around a neatly packed hydrophobic core. Second, each SUN protomer interacts with its two neighbors via two independent binding surfaces. As a result, 1766 Å2 of surface exposed area is buried by SUN-SUN interactions per interface.
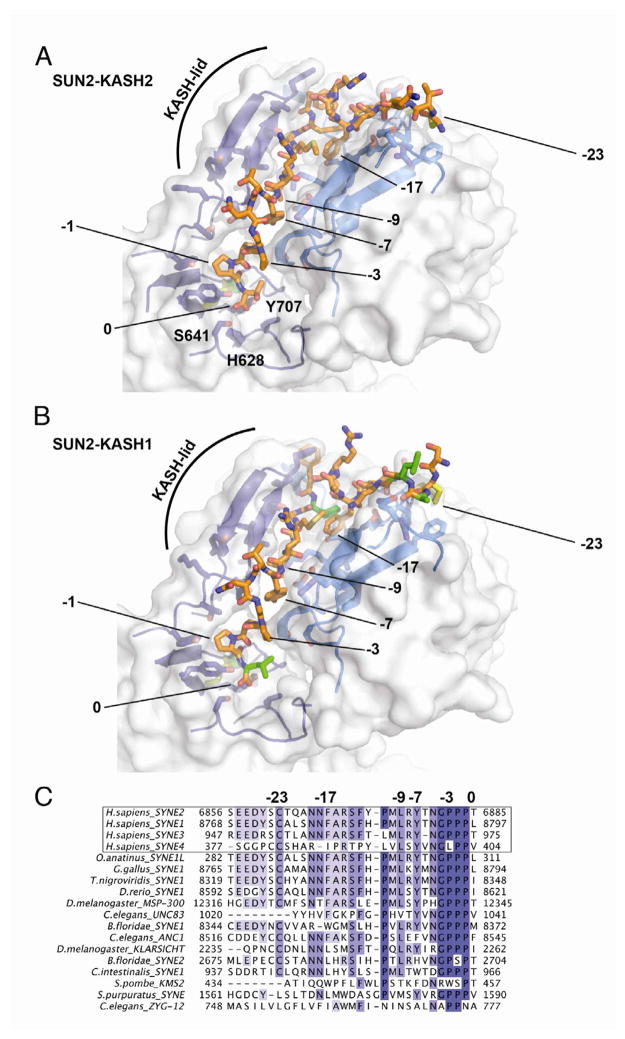
The SUN2 trimer harbors three independent binding sites for KASH2 peptides such that the overall complex is hexameric. The 23 visible residues of an individual KASH2 peptide bind in an extended groove meandering over the surface of the SUN2 trimer. The four C-terminal residues are buried in a deep pocket on SUN2 protomer 1. The next 11 residues are clamped between the KASH-lid of protomer 1 and the β-sandwich core of protomer 2. The eight N-terminal residues are bound by protomer 2 alone. C6862 is the last interacting residue and forms a disulfide bridge with C563 of SUN2. The SUN-KASH interaction is massive and altogether 1520 Å2 are additionally buried on each SUN2 protomer surface upon KASH binding, presumably generating high affinity for KASH and further stabilizing the trimer.
It has been suggested previously that SUN proteins form higher order oligomers, assuming a dimeric organization as the basic unit (Lu et al., 2008). Oligomerization was also observed during gelfiltration of our samples (data not shown). To confirm that the trimeric form is the physiologically relevant oligomerization state of human SUN2, we studied its behavior in solution by analytical ultracentrifugation (Figure 2F). We used SUN2335–717 that comprises almost the entire luminal domain of SUN2, including both the predicted coiled-coil regions and the SUN domain. Data obtained by sedimentation equilibrium analysis using two different centrifugal speeds and two protein concentrations was globally fitted to a mass of 131.3 ± 6.1 kDa, in good agreement with the calculated mass of 138 kDa of a SUN2335–717 homotrimer. We conclude that the luminal domain of SUN2 is a homotrimer in solution.
Details of the SUN2-KASH2 interaction
A hallmark feature of all KASH peptides identified to date is their strict location at the very C terminus of a protein. Thus, in the following discussion, we count the KASH peptide residues inversely, starting at the C terminus with 0 for the last residue followed by negative numbers (Figure 3). Residues 0 to -3 line a pocket crafted into the SUN2 surface of protomer 1 that is perfectly complimentary to the shape of the peptide. The terminal carboxyl group points into this pocket, indicating that the positioning of C-terminal end of KASH should be incompatible with extending the peptide by even one residue. The carboxyl group is specifically coordinated by the hydroxyl groups of S641, Y703, and Y707. H628, the C601-C705 disulfide, and a part of the cation-loop form the backside of the pocket. The three prolines in the -1, -2, and -3 positions are all in the trans-conformation and in close van-der-Waals contact with SUN2. Residues in positions -4, -5, and -6 are more solvent exposed and their side chains are not interacting strongly with SUN2. Tyr at -7 is again deeply buried in a slot lined by SUN2 protomer 1 and 2, explaining its strong conservation. The next 3 KASH-residues are sandwiched between the KASH-lid of protomer 1 and the core of protomer 2. They hydrogen-bond to the KASH-lid, in turn forming a third β-strand. Like Tyr at -7, Leu at -9 is also deeply buried in a cleft between SUN2 protomers 1 and 2, and is again highly conserved. Pro at -11 ends the β-strand. Between positions -11 and -12, the peptide kinks by about 90° to continue to the N-terminal Cys at -23. After the kink, the peptide exclusively interacts with SUN2 protomer 2. Other than bridging the distance to Cys at -23, the most noticeable interaction is burial of Phe at -17 into a hydrophobic pocket. Similar to positions -7 and -9, the large hydrophobic residue at -17 is also conserved. Cys at -23 is perfectly positioned to engage in a disulfide bond with SUN2-C563. The conservation of SUN2-C563 (Figure S2), and the presence of the SUN-KASH disulfide bond in living cells (Figure S3) suggest that it is physiologically important. Altogether 24 hydrogen bonds, 11 between SUN2 protomer 1 and KASH2, and 13 between protomer 2 and KASH2, tie the peptide strongly to the SUN2 trimer base. In comparison, merely three hydrogen bonds are formed between the protomer interfaces.
Figure 3. Details of the SUN-KASH Interaction.
(A) Close-up view of the SUN2-KASH2 interaction. Two neighboring SUN protomers are shown in two shades of blue, with the KASH2 peptides in between in orange. Surface of the SUN2 binding area is half-transparent. KASH residues crucial for interaction are numbered. ‘0’ denotes the C-terminal residue of the peptide. Pocket residues that abolish KASH-binding if mutated are labeled.
(B) Same view of the SUN2-KASH1 interaction. Residues that differ between KASH1 and KASH2 are colored in green.
(C) Multiple sequence alignment of the four identified human KASH proteins, followed by a list of KASH peptides from highly diverged eukaryotes. The numbers match residues important for SUN binding.
See also Figure S3.
Notably, SUN2 is glycosylated at N636 (Stewart-Hutchinson et al., 2008). N636 lies on the SUN2 surface (Figure 2A) and does not contact the KASH peptide, consistent with the previous finding that N-glycosylation is dispensable for KASH binding.
Comparison of SUN2-KASH2 with apo-SUN2
Apo-SUN2 forms a structure very similar to KASH-bound SUN2 (Figure 2B). Both structures superpose with an rmsd of 0.8 Å over 180 Cα positions. The one major difference between apo-SUN2 and KASH-bound SUN2 is the conformation of the KASH-lid. While ordered in the bound form, the KASH-lid is poorly ordered and lacks the β-hairpin in the apo-form. SUN2 trimers tightly pack sideways into a two-dimensional array in the crystal, with the KASH-lids of neighboring trimers caking the 2D-arrays into a 3D-lattice. Under physiological conditions, the KASH-lid most likely adopts a random conformation that becomes ordered upon KASH-binding. In the apo-form published recently (Zhou et al., 2012), the KASH-lid adopts yet another varied conformation, further supporting the flexibility of this region in the absence of KASH peptide.
Comparison of SUN2-KASH2 with SUN2-KASH1
We also solved the structure of the SUN2-KASH1 complex. Since the KASH peptide is not involved in crystal-packing contacts, we anticipated that both complexes would crystallize in the same crystal form, which they did. Overall, both complexes look very similar, and the KASH peptides are bound in the same binding groove. Compared to KASH2, KASH1 differs in 5 positions within the 24 SUN2-binding KASH residues (Figure 3B). For position 0, the Thr to Leu substitution is subtle enough to not change the interaction with the SUN2 binding pocket. The four other changes in positions -12, -20, -21, and -22, respectively, can be accommodated easily by SUN2. There are hardly restrictions on size and charge of the KASH side chains in these positions, well reflected by their poor conservation (Figure 4).
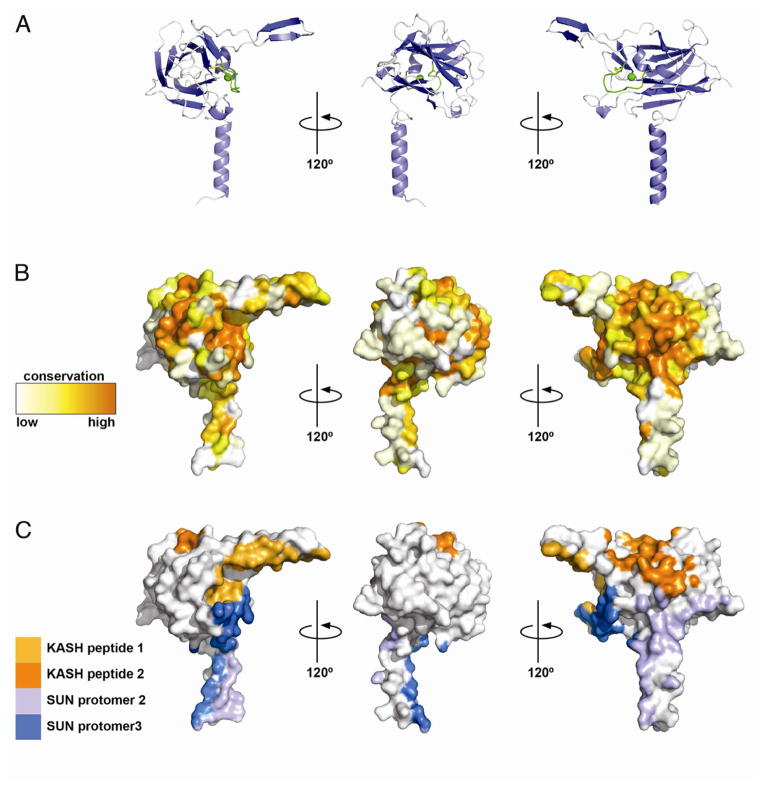
Figure 4. Surface Analysis of the SUN protomer.
The SUN protomer in three different orientations, related to one another by 120° rotations around the vertical axis.
(A) Cartoon representation, coloring as in Figure 2A.
(B) Surface representation, gradient-colored to illustrate the conservation.
(C) Surface representation, colored to show the different binding interfaces to the two neighboring SUN protomers (shades of blue), and the two KASH peptides (shades of orange).
See also Figure S2.
SUN2 Conservation
We generated an alignment of maximally diverged eukaryotic SUN domains to analyze their sequence conservation (Figure S2). The SUN domain is relatively well conserved, despite that the basic β-sandwich fold itself can be adopted by highly divergent sequences. The conservation can be explained by the extensive surface areas that are involved in protein-protein interactions (Figure 4). Examining the conservation profile of surface residues of a SUN2 protomer, one can appreciate that conserved regions match with both KASH binding and homotrimerization interfaces. This analysis strongly supports that the binding mode observed in the crystal is physiologically relevant.
Comparison of SUN2 with F-lectins
A VAST search for structural homologs of SUN2 reveals similarity to a number of functionally diverse β-sandwich proteins implicated e.g. in DNA repair, intraflagellar transport, and various catalytic activities. Most intriguing among the close homologs are F-lectins involved in fucose binding. A comparison of the KASH-bound SUN domain to eel agglutinin bound to fucose (Bianchet et al., 2002) demonstrates that not only is the basic β-sandwich fold present, but also the additional cation-loop and the stabilizing disulfide bridge are maintained (Figure S4). In both proteins, these elements are involved in substrate binding. In the agglutinin structure, fucose is bound close to the position where the pocket in SUN2 is found, which binds the terminal PPPX motif of KASH. This similarity suggests that both proteins are ancestrally connected and diverged into today’s function by crafting the peptide/sugar interacting moiety (Mans et al., 2004).
Mutational Analysis of SUN-KASH interaction
To support the conclusions derived from the structural model of SUN-KASH, and to determine the contribution of features in SUN and KASH required for complex formation, we performed mutational analysis of the KASH peptide and the SUN domain.
First, we analyzed which region of KASH2 is required for interaction with SUN in pulldown experiments. Truncation of the N-terminal 11 or 15 residues of the KASH2 29-mer yielding a KASH 18-mer and a 14-mer, respectively, did only slightly diminish interaction of the peptides with the luminal domain of SUN2 (SUN2335–717) (Figure 5A,B). As these deletions removed the conserved cysteine at position -23, the disulfide bond generated between this residue and C563 of SUN2 appears dispensable for binding. Still, it is possible that the disulfide might influence functionality of the LINC complex in another way (see discussion). When the KASH peptide was shortened by additional 4 residues, the resulting 10-mer was incompetent for interaction with SUN2 in our assay, indicating that a short PPPX containing peptide is not sufficient to promote binding. Clearly, this effect is not explained solely by reducing the length of the peptide, as an 18-mer in which residues between positions -9 and -17 were substituted for alanines also failed to recruit SUN2335–717.
Figure 5. KASH Interaction Depends on Both the Terminal Binding Pocket and the β-Hairpin of the SUN Domain.
(A) The C-terminal 14 aa of KASH2 are sufficient for interaction with the SUN domain. The depicted zz-KASH2 derivatives were bound to beads and incubated with E. coli lysate of cells expressing His-SUN2335–717. Bound proteins were eluted and analyzed by SDS-PAGE and Coomassie staining. Loads in the input and pulldown lanes correspond to 1.25% and 10% of the total, respectively.
(B) Contribution of conserved residues within the C-terminal 14 aa of KASH2 to SUN binding. Binding of His-SUN2335–717 to the depicted zz-KASH2 derivatives was analyzed as in (A). Note that the extension of the KASH peptide by one residue abolishes SUN interaction.
(C) KASH binding of SUN domain mutants. Wildtype His-SUN2335–717 or mutant derivatives were added to E. coli lysate (1.6 μM of trimer) and incubated with immobilized zz-KASH2 as in Figure 1D. Bound proteins were eluted and analyzed as in (A).
(D) SUN domain mutants deficient in KASH interaction fail to mediate NE targeting of a SUN domain-dependent reporter construct in vivo. The localization of SPAG41–189-SUN2507–717-GFP wildtype or indicated SUN domain mutants was analyzed after transfection of HeLa cells. Cells were analyzed by confocal fluorescence microscopy.
To define the contribution of specific residues in KASH for SUN binding, we mutated conserved amino acids residing within the last 14 residues to alanines in the context of the KASH2 18-mer (Figure 5B). Residues at -7 and -9 both fit snuggly in deep hydrophobic pockets that are created between the KASH-lid of SUN protomer 1 and core of protomer 2. Consistently, mutating either position into alanine abolishes SUN-KASH interaction. Ala substitution of Phe at -13, however, only slightly reduces KASH binding. This suggests that the groove formed upon KASH-binding between two SUN protomers, which accommodates the KASH β-strand comprising residues -11 to -7, is essential for KASH interaction.
The crystal structure suggests that the three C-terminal prolines at -1 to -3 of KASH are important for positioning the C terminus of the peptide into the surface binding pocket. Pro at -2 plays a central role in this, as its substitution to Ala abolished SUN binding. Mutation of Pro at either -1 or -3 reduced SUN interaction but did not prevent it, suggesting that the central proline might be key to orient the KASH2 C terminus to dip into its binding pocket on SUN.
If the correct positioning of KASH’s C terminus into its binding pocket on SUN is required for SUN-KASH interaction, then extending KASH at the C terminus should not be tolerable. We tested this prediction by adding one alanine to the C terminus of KASH2 and observed that binding of this KASH mutant (18+A) was indeed prevented. Consistently, it was previously shown that extending KASH C-terminally by about 10 residues abolishes SUN binding (Stewart-Hutchinson et al., 2008). Taken together, the mutational analysis suggests that key determinants for SUN-KASH binding reside in the last 14 residues of KASH, including both contributions of the KASH β-strand and the terminal PPPX motif.
The structural data and mutational analysis of KASH indicated that both the KASH binding pocket and the KASH-lid on SUN are expected to be pivotal for complex formation. To test the significance of both elements on KASH binding, we introduced mutations into the SUN domain (using SUN2335–717). Mutation of H628 at the back site of the KASH binding pocket (H628A) as well as mutations of residues involved in coordination of KASH’s C terminus (S641E, Y707F) impaired KASH interaction (Figure 5C). Likewise, deletion of the KASH-lid, which was achieved by replacing residues 571 to 583 by two glycines (571–583 GG), strongly diminished binding to KASH. Two unrelated mutations on the surface of the SUN domain, H584S and Q657S, had no effect on SUN-KASH complex formation. Thus, both KASH binding pocket and KASH-lid are instrumental for SUN-KASH interaction.
Next, we tested the importance of SUN2 trimerization and of the cation loop for KASH interaction. We disrupted the relative orientation of the three SUN domains by distorting the short trimeric coiled-coil segment 525–539, which is not in direct contact with KASH. L536D interferes with the hydrophobic core of the coiled-coil, while ΔR538 changes the phase of the helix. Both mutations expectedly disrupt SUN-KASH interaction (Figure S5), strongly indicating that the specific trimeric orientation of the SUN domains is necessary for KASH binding. We further examined a D542N mutation that was previously shown to exert a nuclear migration defect in C. elegans (Malone et al., 1999). D542 forms a salt bridge with R707 to anchor the coiled-coil to the SUN domain. D542N also disrupts KASH binding, but the protein fold seems to be compromised since protein solubility was significantly reduced. The importance of the cation-loop was probed using a ΔN600 mutant, which shortens the loop. As expected, the mutant does not bind KASH (Figure S5).
Finally, we verified that the identified features of SUN2 are important for functionality of the SUN domain in living cells. Based on our previous report that the SUN domain serves as one of several determinants for localization of SUN2 to the NE, we employed NE targeting of a SUN domain-dependent reporter protein as readout (Turgay et al., 2010). The reporter consists of the N-terminal, membrane-bound domain of the SUN family member SPAG4 (SPAG41–189), followed by SUN2507–717 comprising the SUN domain and part of its preceding trimeric coiled-coil, and a C-terminal GFP (Figure 5D). During biosynthesis, the reporter protein is inserted into the ER membrane by help of the SPAG4 transmembrane domain and then targeted to the INM using the SUN domain as localization signal (Turgay et al., 2010). Consistent with our in vitro analysis (Figure 5C), mutation of either the KASH binding pocket on SUN2 (Y707F) or deletion of the KASH-lid (571–583 GG) compromised NE targeting, resulting in an accumulation of the SPAG41–189-SUN2507–717-GFP reporter in the ER. The same effect was observed for mutations L536D and ΔR538, which were designed to change the relative orientation of the three SUN domains (Figure S5). Notably, also deletion of the coiled-coil region in front of the SUN domain (SPAG41–189-SUN2540–717-GFP) impaired NE accumulation, consistent with the inability of this SUN2 fragment, which is monomeric in solution, to bind KASH in vitro (Figures 1, S5). Collectively, these data demonstrate that the respective mutations interfere with the functionality of the SUN domain in its physiological environment, verifying the conclusions drawn from both the structure and the biochemical analysis, and further indicating that the function of the SUN domain in NE targeting relies on KASH interaction.
DISCUSSION
One of the key findings of this study is that three KASH peptides bind to a trimeric arrangement of SUN domains. We hypothesize that the trimeric arrangement is universally conserved for all SUN domains, since it is the prerequisite for the mode of KASH binding as we observe it in our structures. All known SUN homologs from divergent species contain predicted coiled-coil segments N-terminal to the SUN domain, supporting our hypothesis. It is curious how well the position of the trimeric coiled-coil, relative to the SUN domain, is conserved. The coiled-coil rigidly emanates from the β-sandwich domain and is held in position by a highly conserved salt bridge between D542 at the base of the coil and the R708/710 pair in strand 12 (Figure S2). Why is the SUN domain not a stable trimer itself, without the coiled-coil? One possibility is that in the observed configuration, it might be possible to regulate the SUN-KASH bridge formation by pivoting the SUN domain away from the coiled-coil thereby efficiently inhibiting interaction.
The SUN2-KASH1 and SUN2-KASH2 complexes excellently explain conservation of the KASH peptide as defined in the Pfam database. KASH peptide positions -1 to -11 are generally the most conserved, with a particularly high level of identity at positions -1, -2, -3, -4, -7, -9, and -11. Further upstream, the hydrophobic residue at -17 and Cys at -23 are strongly conserved (Figure 3C). Accordingly, the minimal KASH peptide displaying SUN interaction in our assay contains 14 residues (Figure 5). Thus, we expect the largest variations within the part of the KASH peptide that exclusively interacts with SUN protomer 2, positions -12 to -23, which shows the strongest sequence variations and is less ordered in our structures. Some of the characterized metazoan KASH peptides are shorter than others, for example the luminal domains of C. elegans UNC83 and ZYG-12 (Figure 3C). It will be interesting to see structural details of how those sequences are recognized. We hypothesize that the principal interaction mode will be the same, i.e. peptide-in-pocket interaction of the four C-terminal residues, and sandwiching of the preceding extended stretch, positions -5 to -11, between two SUN protomers.
In a recently published apo-SUN structure, the authors attempted to model the KASH-binding site and proposed that each SUN protomer would bind a single KASH peptide (Zhou et al., 2012). It was argued that binding of KASH2 to SUN2 would resemble the interaction of a CD40 peptide with tumor necrosis receptor associated factor 2 (TRAF2). However, although TRAF2 is a trimerized β-sandwich like SUN2, the surface area in question is not conserved in SUN2 proteins and remote from the three KASH binding sites revealed by our structure. Consistent with our data, Zhou et al. find that modifying their proposed surface area through A647E and F610A mutations on SUN2 does in fact not significantly affect KASH binding. In contrast, changing the well conserved Gly608 to Asp, disrupts KASH binding, however, Gly608 is a buried residue and its mutation expected to affect the β-sandwich fold rather than the local surface area. Finally, the model proposed by Zhou et al. does not explain why SUN trimerization is necessary for KASH binding, which we show is critical for LINC complex formation.
In contrast to metazoa, putative SUN-KASH interactions in yeasts are more difficult to predict. The Sad1-Kms2 interaction in S. pombe might still follow the same scheme, although the difference in the KASH-lid of Sad1 might explain the substantial sequence variation in the KASH peptide of Kms2 (Figure S2). The candidate SUN-KASH bridge in S. cerevisiae between Mps3 and Mps2 or Csm4 appears to be very different. In Mps3, the intramolecular disulfide as well as the salt bridge stabilizing the coiled-coil position are missing, and the KASH-lid is hardly recognizable by sequence.
One major function of LINC complexes is to provide mechanical coupling of the nucleus to the cytoskeleton, allowing for force transmission across the NE (Lombardi and Lammerding, 2011). The large KASH-peptide binding groove between neighboring SUN domains appears to be the foundation for strong SUN-KASH interactions. Yet, our structural model bears several additional implications for how the structural organization of LINC complexes contributes to the establishment of a force-resistant coupling device. First, the presence of three KASH peptide binding sites on each SUN trimer entails a contribution of binding avidity to the strength of SUN-KASH interaction. Second, a disulfide bond covalently links SUN and KASH, thus explaining how this bridge can resist the substantial forces that nuclear migration and chromosome rearrangements might require. And third, the trimeric arrangement of SUN proteins is ideally suited to allow for organization of higher-order SUN-KASH complexes, which would obviously potentiate possible forces that LINC complexes can transmit.
The disulfide bond between SUN and KASH raises the intriguing question of how the LINC complex can get separated after it has been formed. The perinuclear space, as an extension of the ER, is rife with protein disulfide isomerases (PDIs) that help remodel disulfide bonds. Therefore PDIs are prime candidates for breaking the SUN-KASH disulfide bond. Almost all of the 20 PDI families detected in humans are ER-resident proteins, but their specific substrates are poorly defined (Appenzeller-Herzog and Ellgaard, 2008). It is an interesting possibility to speculate whether one of these enzymes has a regulatory role in LINC complex remodeling.
It is presently unclear how KASH proteins are organized and whether each SUN trimer interacts with KASH peptides originating from individual KASH protein monomers or from KASH proteins present in form of dimers, trimers or even higher-order organizational units. For some KASH proteins, there already exists evidence that they are organized by multimerization (Ketema et al., 2007; Mans et al., 2004; Mislow et al., 2002). Using Cys at -23 as marker, the three KASH peptides are ~50 Å apart on the ONM-facing, bottom surface of the SUN-trimer in our structures. The distance between the N-terminal end of the KASH peptide and the transmembrane helix is only ~7 residues, suggesting that the TM helices of an oligomeric KASH protein cannot interact with each other in the membrane if all peptides of a KASH oligomer are bound to one SUN trimer. However, it is striking that also the TM helices of KASH proteins are quite well conserved in sequence, suggesting that they might engage in protein-protein interactions. It remains to be seen how these transmembrane domains are organized and whether their conservation is explained by self-association or by interaction with other partners. If they self-associate, we predict that they cannot interact with the same SUN trimer and thus enable higher-order complexes. The association of KASH peptides from oligomeric Nesprins with neighboring SUN trimers would trigger two-dimensional clusters of SUN-KASH bridges in the NE. Alternatively, clustering of SUN proteins has recently been discussed to originate from the perinuclear coiled-coil domains forming a hybrid of N-terminal dimeric and C-terminal trimeric coils (Zhou et al., 2012). In this hypothesis, one of the three hypothetical N-terminal coils would have to interact with a neighboring SUN trimer to form a coiled-coil, thus would trigger clustering. We note that this hypothesis is in conflict with our solution data that shows that SUN335–717, which includes the predicted dimeric coiled-coil segment, is trimeric and does not from higher order assemblies (Figure 2).
Arrays of LINC complexes have been described in at least two cases. First, TAN lines, linear, actin-associated arrays of SUN2 and Nesprin-2G, reorient the nucleus in wounded cell monolayers (Luxton et al., 2010). Second, SUN-domain clustering is also observed at the attachment sites of meiotic telomeres in diverse organisms (Chikashige et al., 2006; Ding et al., 2007; Penkner et al., 2007; Schmitt et al., 2007). Clearly, a next step in illuminating LINC complex formation must unravel the organizational principle of KASH proteins.
It has been previously observed that depletion of SUN proteins from HeLa cells leads to loss of the regular, 40 - 50 nm spacing between the nuclear membranes, accompanied by irregular expansions of the perinuclear space (Crisp et al., 2006). This suggests that SUN proteins function to maintain the defined proximity of ONM and INM. In fact, the luminal coiled-coil domains of both SUN2 and SUN1 are ideally suited to define the distance of both membranes. If one assumes that the luminal part of SUN2 preceding the SUN domain is composed of a trimeric coiled-coil with the potential of forming a rigid rod, it would extend over a length of ~ 45 nm when compared to experimentally determined trimeric coiled-coils (Testa et al., 2009). Together with the extension of the SUN domain trimer along its longitudinal axis, this results in a predicted length of the entire luminal domain of SUN2 of ~ 48 nm (Figure 6). Notably, the luminal domains of SUN1 and SUN2 share the same organizational principles and are of similar length (477 and 482 aa). Thus, the predicted length of these luminal domains correlates well with the observed spacing of both nuclear membranes. Similarly, it has been suggested that the coiled-coil protein CLIMP63 serves a luminal spacer defining the width of ER sheets (Shibata et al., 2010).
Figure 6. Model for the LINC-Complex Bridging the Nuclear Envelope.
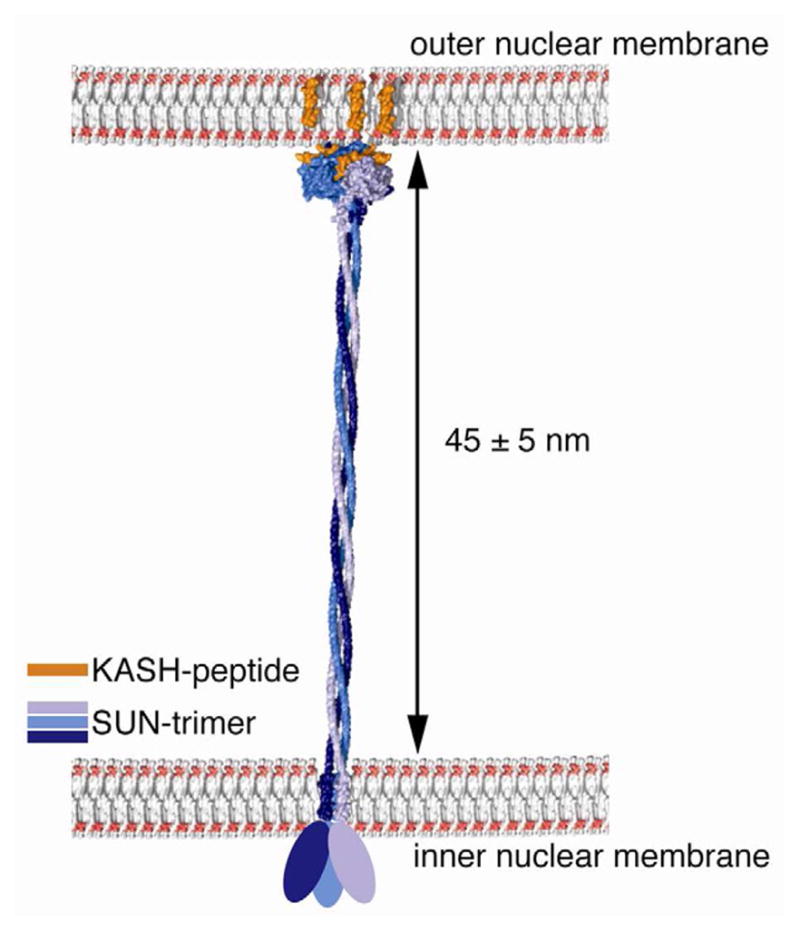
The LINC complex is displayed to scale within the perinuclear space between ONM and INM. The trimeric SUN2 is modeled based on our experimental structure, with a theoretically calculated trimeric coiled-coil extending N-terminally to the INM (http://arteni.cs.dartmouth.edu/cccp/). The N-terminal, nucleoplasmic domain is indicated as an oval sphere of approximate size assuming a globular shape. Atomic coordinates for the lipid bilayers and the transmembrane segments are from public sources.
The tight association of SUN and KASH raises the issue of whether and how these linkages can be taken apart. One attractive possibility is that the putative AAA+ ATPase torsinA could be involved in remodeling or disassembly of SUN-KASH bridges. This hypothesis is strengthened by the observation that torsinA harbors a redox sensor motif (Zhu et al., 2010), which, we speculate, might be necessary for breaking disulfide-bonded SUN-KASH pairs. TorsinA is found in the ER lumen, and, interestingly, a torsinA mutant bearing a single glutamate deletion accumulates in the perinuclear space (Goodchild and Dauer, 2004) and has reported affinity for KASH peptides (Nery et al., 2008). The same mutation in torsinA causes the majority of torsinA-associated cases of early-onset dystonia, a severe movement disorder (Ozelius et al., 1997). Although there is no data yet that the development of dystonia can be attributed to a potential function of torsinA in remodeling SUN-KASH bridges, there is increasing evidence that functionality of the LINC complex is important for human health. It might not be a coincidence that mutations in Nesprins have recently been linked to another movement disorder, Emery-Dreifuss muscular dystrophy (EDMD) (Puckelwartz et al., 2009; Zhang et al., 2007a). Most commonly, EDMD is caused by mutations in the emerin (EMD) gene, and less frequently by mutations in LMNA, the gene encoding for A-type lamins. Notably, lamins and the INM protein emerin are associated with LINC complexes (Fridkin et al., 2009). In addition, mutations in LUMA (TMEM43), another SUN2 interacting protein, have recently been identified in EDMD-related myopathy patients (Liang et al., 2011). Thus, the LINC complex and associated proteins emerge at the root of several inherited diseases associated with impaired locomotion.
In summary, our structural and biochemical characterization of the SUN-KASH complex has revealed first insights into the organization of this important link. The structure can now help to formulate specific, testable hypotheses, which will undoubtedly enrich this field in the short and long term.
EXPERIMENTAL PROCEDURES
Plasmids, Protein Expression and Purification
Recombinant proteins were expressed in E. coli. The C-terminal KASH peptides comprising the last 29 aa of Nesprin-1/Nesprin-2 (KASH1/KASH2), a KASH23 deletion variant lacking the C-terminal 4 aa, and all other KASH2 variants were expressed from pQE60-2z as fusions with an N-terminal protein A-tag (zz-tag). SUN2 fragments were expressed from pETDuet-1 (Novagen) as N-terminally 6xHis-tagged fusion proteins. For crystallographic purposes, SUN2522–717 was expressed as a fusion with a short, coiled-coil fragment of GCN4 and a cleavage site for human rhinovirus 3C protease, inserted between the His-tag and the SUN fragment. For co-expression of SUN2522–717 and KASH1 or KASH2, the second cassette of pETDuet-1 SUN2522–717 was used to produce 3C-cleavable MBP-KASH fusions. All SUN2 constructs were purified over a Ni-affinity resin. For crystallography, Apo-SUN2522–717, SUN2-KASH1 and SUN2-KASH2 were subsequently purified by size exclusion chromatography. Affinity tags were removed with 3C protease, followed by gelfiltration. SPAG41–189-SUN2507–717-GFP has been described (Turgay et al., 2010). Mutations were introduced by site-directed mutagenesis.
Antibodies
α-SUN1 was raised in rabbits against a recombinant fragment (SUN1520–626) of human SUN1. α-SUN2 has been described (Turgay et al., 2010).
In Vitro Binding Experiments
IgG sepharose beads were saturated with zz-KASH derivatives. For Figure 1B, HeLa cells were lysed in RIPA buffer. For binding of recombinant SUN2 constructs (Figure 1D; Figure 5C), purified proteins were added to E. coli lysate supplemented with RIPA detergents. For Figures 5A and B, cleared lysate of E. coli expressing recombinant SUN2335–717 was used. In all cases, samples were incubated for 4 hr at 4°C, beads washed and bound proteins retrieved in SDS sample buffer.
Protein Crystallization and Structure Determination
Proteins were concentrated and crystallized by hanging drop vapor diffusion. Apo-SUN2522–717 crystallized in 16% (w/v) PEG-3350, 200 mM KSCN; SUN2-KASH1 in 100 mM HEPES pH 7.5, 200 mM NH4OAc, 25% 2-propanol and 0.3% n-decyl-β-D-maltoside (DM).; SUN2-KASH2 in 100 mM HEPES pH 7.4, 7% PEG-4000, 10% 1,6-hexandediol and 0.25% DM. Data were collected at beamlines 24ID-C/-E at Argonne National Laboratory. The structure of apo-SUN2 was solved using single anomalous dispersion data from a SeMet derivatized SUN2(L546M) mutant. The SUN2-KASH1 and SUN2-KASH2 structures were solved subsequently by molecular replacement. Note that for KASH1 and KASH2, the N-terminal 3 and 4 residues, respectively, could not be positioned.
Analytical Ultracentrifugation
SUN2335–717 was analyzed by analytical ultracentrifugation using an An60Ti rotor in an Optima XL-A centrifuge. Samples were loaded into Epon-charcoal 6 channel centerpieces, fit with quartz windows, and spun at 7,000 and 10,000 rpm, respectively. Sedimentation to equilibrium was monitored with Winmatch and equilibrium data fitted with Ultrascan II (http://ultrascan.uthscsa.edu) using a single ideal species model.
Transfection and Microscopy
HeLa cells were transiently transfected with plasmid DNA encoding for SPAG41–189-SUN2507–717-GFP using X-tremeGENE (Roche). After 24 hr, cells were fixed and images acquired by confocal microscopy.
Supplementary Material
HIGHLIGHTS.
Crystal structures of SUN domain-KASH complexes are presented
Three KASH peptide binding grooves are built at the subunit interfaces of the SUN domain trimer
KASH engages in an intricate network of contacts with two neighboring SUN subunits
Structures provide the molecular basis for mechano-transduction across the nuclear envelope
Acknowledgments
We thank the staff at NE-CAT beamlines 24-ID-C/-E at the Advanced Photon Source (APS), especially K. Rajashankar for technical help with data collection. We thank C. Ashiono for excellent assistance, W. Antonin, A. Keating and A. Helenius for discussion, and K. Knockenhauer and G. Kabachinski for critical reading of the manuscript. Imaging was performed on instruments of the ETH Light Microscopy Center. This work was supported by NIH Grant NS075883 to TUS, a Boehringer Ingelheim PhD fellowship to AR and an SNSF grant (310030–313135) to UK.
Footnotes
ACCESSION NUMBERS
The coordinate file and structure factors for the SUN2-KASH1, SUN2-KASH2, and apo-SUN2 crystal structure were deposited in the Protein Data Bank under accession code 4DXR, 4DXS, and 4DXT, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem. 2000;275:31986–31995. doi: 10.1074/jbc.M004775200. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Bianchet MA, Odom EW, Vasta GR, Amzel LM. A novel fucose recognition fold involved in innate immunity. Nat Struct Biol. 2002;9:628–634. doi: 10.1038/nsb817. [DOI] [PubMed] [Google Scholar]
- Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell. 2009;17:587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Ciani B, Bjelic S, Honnappa S, Jawhari H, Jaussi R, Payapilly A, Jowitt T, Steinmetz MO, Kammerer RA. Molecular basis of coiled-coil oligomerization-state specificity. PNAS. 2010;107:19850–19855. doi: 10.1073/pnas.1008502107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Fridkin A, Mills E, Margalit A, Neufeld E, Lee KK, Feinstein N, Cohen M, Wilson KL, Gruenbaum Y. Matefin, a C. elegans germ line-specific SUN-domain nuclear membrane protein, is essential for early embryonic and germ cell development. PNAS. 2004;101:6987–6992. doi: 10.1073/pnas.0307880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin A, Penkner A, Jantsch V, Gruenbaum Y. SUN-domain and KASH-domain proteins during development, meiosis and disease. CMLS. 2009;66:1518–1533. doi: 10.1007/s00018-008-8713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation. PNAS. 2004;101:847–852. doi: 10.1073/pnas.0304375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding MM. Small revisions to predicted distances around metal sites in proteins. Acta Cryst D. 2006;62:678–682. doi: 10.1107/S0907444906014594. [DOI] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A. Requirements for the localization of nesprin-3 at the NE and its interaction with plectin. J Cell Sci. 2007;120:3384–3394. doi: 10.1242/jcs.014191. [DOI] [PubMed] [Google Scholar]
- Lee KK, Starr D, Cohen M, Liu J, Han M, Wilson KL, Gruenbaum Y. Lamin-dependent localization of UNC-84, a protein required for nuclear migration in C. elegans. Mol Biol Cell. 2002;13:892–901. doi: 10.1091/mbc.01-06-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. PNAS. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WC, Mitsuhashi H, Keduka E, Nonaka I, Noguchi S, Nishino I, Hayashi YK. TMEM43 mutations in Emery-Dreifuss muscular dystrophy-related myopathy. Ann Neurol. 2011;69:1005–1013. doi: 10.1002/ana.22338. [DOI] [PubMed] [Google Scholar]
- Lombardi ML, Lammerding J. Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem Soc Trans. 2011;39:1729–1734. doi: 10.1042/BST20110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Gotzmann J, Sironi L, Jaeger VM, Schneider M, Luke Y, Uhlen M, Szigyarto CA, Brachner A, Ellenberg J, et al. Sun1 forms immobile macromolecular assemblies at the NE. Biochim Biophys Acta. 2008;1783:2415–2426. doi: 10.1016/j.bbamcr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans evelopment. Development. 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Anantharaman V, Aravind L, Koonin EV. Comparative genomics, evolution and origins of the NE and NPC. Cell Cycle. 2004;3:1612–1637. doi: 10.4161/cc.3.12.1316. [DOI] [PubMed] [Google Scholar]
- McDonnell AV, Jiang T, Keating AE, Berger B. Paircoil2: improved prediction of coiled coils from sequence. Bioinformatics. 2006;22:356–358. doi: 10.1093/bioinformatics/bti797. [DOI] [PubMed] [Google Scholar]
- Mekhail K, Moazed D. The nuclear envelope in genome organization, expression and stability. Nat Rev Mol Cell Biol. 2010;11:317–328. doi: 10.1038/nrm2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, McNally EM. Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- Nery FC, Zeng J, Niland BP, Hewett J, Farley J, Irimia D, Li Y, Wiche G, Sonnenberg A, Breakefield XO. TorsinA binds the KASH domain of nesprins and participtes in linkage between nuclear envelope and cytoskeleton. J Cell Sci. 2008;121:3476–3486. doi: 10.1242/jcs.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onischenko E, Weis K. Nuclear pore complex-a coat specifically tailored for the nuclear envelope. Curr Opin Cell Biol. 2011;23:293–301. doi: 10.1016/j.ceb.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122:4099–4108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295:330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The INM protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- Penkner A, Tang L, Novatchkova M, Ladurner M, Fridkin A, Gruenbaum Y, Schweizer D, Loidl J, Jantsch V. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev Cell. 2007;12:873–885. doi: 10.1016/j.devcel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, Earley JU, Hadhazy M, Holaska JM, Mewborn SK, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D, Hodzic D. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol. 2009;186:461–472. doi: 10.1083/jcb.200906068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an ONM protein that can induce kinesin-mediated cell polarization. PNAS. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the NE. PNAS. 2007;104:7426–7431. doi: 10.1073/pnas.0609198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA. Mechanisms determining the morphology of the peripheral ER. Cell. 2010;143:774–788. doi: 10.1016/j.cell.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Fischer JA. KASH ‘n Karry: the KASH domain family of cargo-specific cytoskeletal adaptor proteins. BioEssays. 2005;27:1136–1146. doi: 10.1002/bies.20312. [DOI] [PubMed] [Google Scholar]
- Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–1412. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008;314:1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa OD, Moutevelis E, Woolfson DN. CC+: a relational database of coiled-coil structures. Nucleic Acids Res. 2009;37:D315–322. doi: 10.1093/nar/gkn675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgay Y, Ungricht R, Rothballer A, Kiss A, Csucs G, Horvath P, Kutay U. A classical NLS and the SUN domain contribute to the targeting of SUN2 to the INM. EMBO J. 2010;29:2262–2275. doi: 10.1038/emboj.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur YB, Wilson KL, Gruenbaum Y. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol. 2006;7:782–788. doi: 10.1038/nrm2003. [DOI] [PubMed] [Google Scholar]
- Wang Q, Du X, Cai Z, Greene MI. Characterization of the structures involved in localization of the SUN proteins to the NE and the centrosome. DNA Cell Biol. 2006;25:554–562. doi: 10.1089/dna.2006.25.554. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel ONM protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ, Gundersen GG. Here come the SUNs: a nucleocytoskeletal missing link. Trends Cell Biol. 2006;16:67–69. doi: 10.1016/j.tcb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Felder A, Liu Y, Guo LT, Lange S, Dalton ND, Gu Y, Peterson KL, Mizisin AP, Shelton GD, et al. Nesprin 1 is critical for nuclear positioning and anchorage. Hum Mol Genet. 2010;19:329–341. doi: 10.1093/hmg/ddp499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for NE integrity. Hum Mol Genet. 2007a;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007b;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Du X, Cai Z, Song X, Zhang H, Mizuno T, Suzuki E, Yee MR, Berezov A, Murali R, Wu SL, Karger BL, Greene MI, Wang Q. Structure of the SUN domain defines features of a molecular bridge in the nuclear envelope. J Biol Chem. 2012;287:5317–5326. doi: 10.1074/jbc.M111.304543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Millen L, Mendoza JL, Thomas PJ. A unique redox-sensing sensor motif in torsinA plays a critical role in nucleotide and partner binding. J Biol Chem. 2010;285:37271–37280. doi: 10.1074/jbc.M110.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.