Abstract
Diversely substituted 2–pyrrolines have been prepared by a novel multicomponent process involving a reaction of various N–(aryl– and alkylsulfonamido)–acetophenones with aldehydes and malononitrile. While the reaction is highly regioselective, it is not stereoselective, generating a mixture of cis and trans 2–pyrrolines. A number of analogues from both cis and trans 2–pyrroline libraries were found to have antiproliferative activity in human cancer cell lines.
Keywords: Multicomponent synthesis, Antiproliferative, Apoptosis, Heterocycles
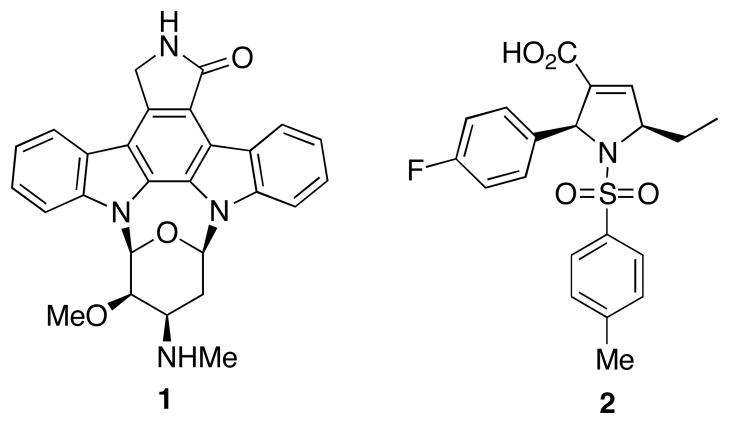
Nitrogen-containing five-membered heterocycles, such as pyrrolines, are common structural scaffolds in natural products and pharmaceutical agents.1,2 They have also received a lot of attention as intermediates in the syntheses of biologically active pyrroles and pyrrolidines.3 Examples of medicinally important pyrroline-based compounds include protein kinase inhibitor staurosporine (1)4 and geranylgeranyltransferase inhibitor 25 (Figure 1).
Figure 1.
Structures of selected biologically active pyrrolines.
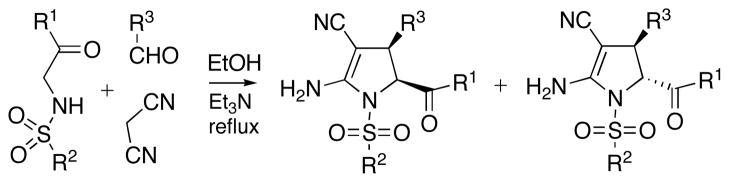
Although many methods to synthesize pyrrolines exist,2,6 the importance of these heterocyclic structures in medicinal and synthetic chemistry provides strong impetus to the development of novel synthetic routes that may lead to pyrroline derivatives inaccessible with the previously developed chemistry.7 As part of an effort aimed at developing multicomponent reactions (MCRs) leading to the preparation of privileged medicinal scaffolds,8 we investigated reactions of N-(arylsulfonamido)-acetophenones with aldehydes and malononitrile. Specifically, we found that refluxing these three components in ethanol in the presence of Et3N leads to the formation of mixtures of densely functionalized cis and trans 2–pyrrolines (Scheme 1).
Scheme 1.
Three-component synthesis of 2–pyrrolines.
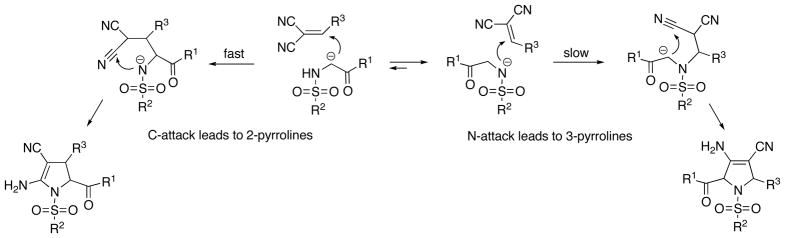
In our proposed mechanistic pathway 2–pyrrolines result from an initial attack of the α-carbon of the acetophenone moiety onto the double bond of the intermediate Knoevenagel adduct, followed by cyclization (Figure 2).
Figure 2.
Proposed mechanistic interpretation of the formation of 2–pyrrolines.
In contrast, the initial attack of the sulfonamidic nitrogen with the subsequent cyclization, which would lead to the formation of 3–pyrrolines, does not occur. It could be speculated that due to the higher acidity of the sulfonamidic nitrogen, the concentration of the N-deprotonated species is higher. However, in accordance with the Curtin-Hammett principle, the faster Michael addition of the C-deprotonated intermediate channels the reaction toward the formation of 2-pyrrolines.
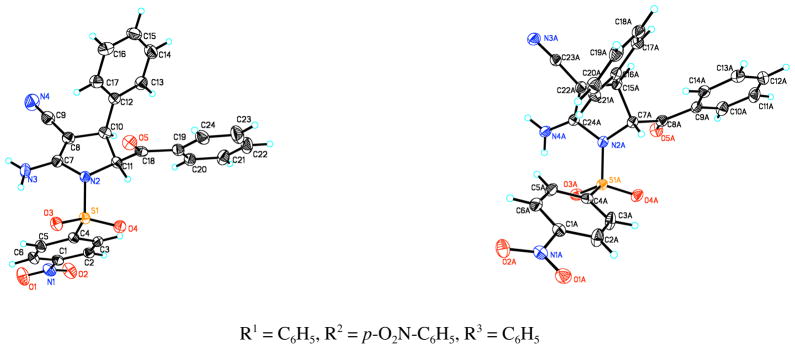
The X-ray crystallographic analysis of the separated cis and trans pyrroline products provided unambiguous proof of our structure assignments (Figure 3).9
Figure 3.
X-ray structures of selected cis and trans pyrroline products.
The yields of the stereoisomeric mixtures generally exceed 90% and, in most cases, the product mixtures can be separated into individual cis and trans pyrroline components on the basis of their dissimilar solubilities. However, this requires extensive experimentation involving crystallization from various combinations of solvents and no general separation method was found. We reasoned that by modulating the steric and electronic nature of the three substituents R1, R2 and R3, it would be possible to significantly enrich the product mixtures in either cis or trans pyrrolines to facilitate separation. To this end, we prepared fourteen N–(sulfonamido)–acetophenones incorporating electron-rich, electron-poor and neutral aromatic as well as aliphatic groups at both sulfonamide and acetophenone parts of the molecule,10 and utilized them in reactions with three aromatic aldehydes also differing in their electronic character.11 The ratios of cis and trans pyrrolines were directly obtained from 1H NMR spectra of the crude reaction mixtures and are shown in Table 1. Although the reaction was successful with all combinations of R1, R2 and R3, including the use of heteroaromatic (ct-16 – ct-18) and aliphatic sulfonamido groups (ct-19 – ct-24), a slight preponderance of the trans product (1:1.5 to 1:2) remained highly conserved irrespective of the differences in the electronic and steric nature of the three substituents.
Table 1.
Ratios of cis to trans pyrrolines for different combinations of substituents R1, R2 and R3 in the starting materials.

| ||||
|---|---|---|---|---|
| pyrroline mixture | R1 | R2 | R3 | cis : trans ratio |
| ct-1 | Ph | p-O2N-C6H5 | Ph | 1 : 2 |
| ct -2 | Ph | p-O2N-C6H5 | p-MeO-C6H5 | 1 : 2 |
| ct -3 | Ph | p-O2N-C6H5 | p-O2N-C6H5 | 1 : 1.4 |
| ct -4 | Ph | p-F-C6H5 | Ph | 1 : 1.6 |
| ct -5 | Ph | p-F-C6H5 | p-MeO-C6H5 | 1 : 1.8 |
| ct -6 | Ph | p-F-C6H5 | p-O2N-C6H5 | 1 : 1.7 |
| ct -7 | Ph | p-Me-C6H5 | Ph | 1 : 1.9 |
| ct -8 | Ph | p-Me-C6H5 | p-MeO-C6H5 | 1 : 1.7 |
| ct -9 | Ph | p-Me-C6H5 | p-O2N-C6H5 | 1 : 1.7 |
| ct -10 | Ph | 2,4,6-i Pr-C6H5 | Ph | 1 : 1.7 |
| ct -11 | Ph | 2,4,6-i Pr-C6H5 | p-MeO-C6H5 | 1 : 2 |
| ct -12 | Ph | 2,4,6-i Pr-C6H5 | p-O2N-C6H5 | 1 : 1.9 |
| ct -13 | Ph | p-MeO-C6H5 | Ph | 1 : 1.9 |
| ct -14 | Ph | p-MeO-C6H5 | p-MeO-C6H5 | 1 : 1.6 |
| ct -15 | Ph | p-MeO-C6H5 | p-O2N-C6H5 | 1 : 1.7 |
| ct -16 | Ph |
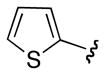
|
Ph | 1 : 1.2 |
| ct -17 | Ph |
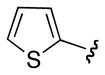
|
p-MeO-C6H5 | 1 : 1.7 |
| ct -18 | Ph |
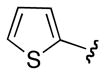
|
p-O2N-C6H5 | 1 : 1.4 |
| ct -19 | Ph | Bu | Ph | 1 : 1.7 |
| ct -20 | Ph | Bu | p-MeO-C6H5 | 1 : 2 |
| ct -21 | Ph | Bu | p-O2N-C6H5 | 1 : 1.4 |
| ct -22 | Ph | Me | Ph | 1 : 1.4 |
| ct -23 | Ph | Me | p-MeO-C6H5 | 1 : 1.5 |
| ct -24 | Ph | Me | p-O2N-C6H5 | 1 : 1.3 |
| ct -25 | p-Br-C6H5 | p-Me-C6H5 | Ph | 1 : 1.6 |
| ct -26 | p-Br-C6H5 | p-MeO-C6H5 | Ph | 1 : 1.5 |
| ct -27 | p-Br-C6H5 | p-O2N-C6H5 | Ph | 1 : 1.5 |
| ct -28 | p-MeO-C6H5 | p-Me-C6H5 | Ph | 1 : 1.6 |
| ct -29 | p-MeO-C6H5 | p-MeO-C6H5 | Ph | 1 : 1.4 |
| ct -30 | p-MeO-C6H5 | p-O2N-C6H5 | Ph | 1 : 1.6 |
The synthesized pyrroline library was biologically tested for its effect on the proliferation of two cancer cell lines, HeLa and MCF7/AZ, as models for human cervical and breast adenocarcinomas respectively. Because of the labor-intensive separation of the stereoisomeric pyrroline mixtures, the initial tests were performed with these mixtures. The corresponding cells were treated with the test pyrrolines at final concentrations of 100 μM for 48 h and cell viability was assessed through measurements of mitochondrial dehydrogenase activities using the MTT method.12 The percent of remaining cell viability for each pyrroline mixture is shown in Table 2.
Table 2.
Antiproliferative effect of pyrroline mixtures.
| mixture | % cell viabilitya
|
|
|---|---|---|
| HeLa | MCF-7AZ | |
| ct -1 | 81 ± 4 | 72 ± 2 |
| ct -2 | 97 ± 1 | 80 ± 4 |
| ct -3 | 70 ± 2 | 45 ± 4 |
| ct -4 | 72 ± 2 | 50 ± 4 |
| ct -5 | 86 ± 3 | 61 ± 3 |
| ct -6 | 103 ± 3 | 93 ± 2 |
| ct -7 | 65 ± 1 | 58 ± 4 |
| ct -8 | 91 ± 4 | 72 ±3 |
| ct -9 | 108 ± 4 | 97 ± 4 |
| ct -10 | 69 ± 1 | 45 ± 1 |
| ct -11 | 65 ± 2 | 54 ± 2 |
| ct -12 | 105 ± 1 | 72 ± 3 |
| ct -13 | 36 ± 1 | 50 ± 2 |
| ct -14 | 98 ± 2 | 69 ± 4 |
| ct -15 | 115 ± 2 | 90 ± 4 |
| ct -16 | 79 ± 1 | 45 ± 3 |
| ct -17 | 84 ± 2 | 57 ± 4 |
| ct -18 | 104 ± 2 | 78 ± 2 |
| ct -19 | 54 ± 1 | 38 ± 2 |
| ct -20 | 46 ± 1 | 27 ± 1 |
| ct -21 | 107 ± 2 | 90 ± 4 |
| ct -22 | 102 ± 3 | 81 ± 2 |
| ct -23 | 49 ± 3 | 58 ± 2 |
| ct -24 | 102 ± 4 | 72 ± 2 |
| ct -25 | 45 ± 3 | 35 ± 3 |
| ct -26 | 36 ± 1 | 54 ± 2 |
| ct -27 | 45 ± 3 | 31 ± 2 |
| ct -28 | 82 ± 4 | 73 ± 2 |
| ct -29 | 98 ± 1 | 80 ± 4 |
| ct -30 | 82 ± 4 | 73 ± 2 |
% Remaining cell viability after 48 h of treatment with indicated pyrroline mixtures at the final concentrations of 100 μM relative to DMSO control. The data are mean ± SD of two independent experiments, each performed in 4 replicates, determined by MTT assay.
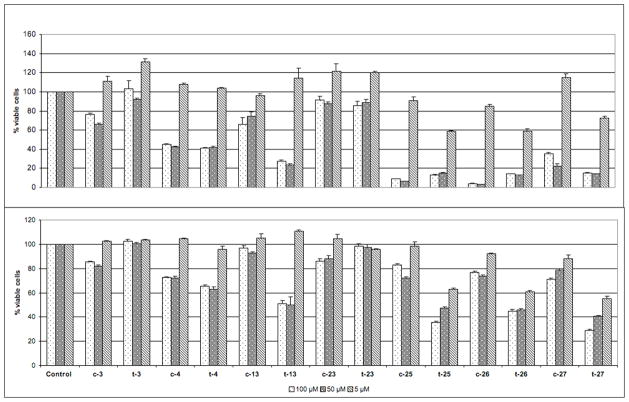
We were pleased to find that many pyrroline mixtures exerted an antiproliferative effect in both cell lines with some of them decreasing cell viability below 50%. These mixtures were then separated into the individual stereoisomerically pure cis and trans pyrroline components (to give c and t series), which were further evaluated in these cell lines at three concentrations (Figure 4). Although the activity profiles are similar for the two cell lines, HeLa cells are more responsive. The GI50 values of the most active pyrrolines fall in the range between 50 and 5 μM in this cell line. Another interesting observation is that in most of the active mixtures both cis and trans pyrroline products showed antiproliferative effect, although it is more pronounced in the t series (e.g. t-25 vs c-25, t-26 vs c-26, t-26 vs c-26 in both cell lines).
Figure 4.
Antiproliferative effect of the individual cis and trans pyrrolines.
% Remaining cell viability of HeLa (top) and MCF7/AZ (bottom) cells after 48 h of treatment with indicated pyrrolines at the final concentrations of 100, 50 and 5 μM relative to DMSO control. The data are mean ± SD of two independent experiments, each performed in 4 replicates, determined by MTT assay.
Our preliminary investigation of the biological mechanism underlying the antiproliferative properties of the pyrroline library points to an exciting possibility of dissimilar modes of action for the cis and trans pyrroline series. The library members c-25 and t-25 were evaluated for their ability to induce apoptosis in Jurkat cells (a model for human T-cell leukemia) using a flow cytometric annexin-V/propidium iodide assay. In contrast to compound t-25, which shows little effect in this assay, treatment of Jurkat cells with pyrroline c-25 produces large populations of dead cells as well as cells manifesting apoptotic phenotype (Table 3).
Table 3.
Apoptosis inducing and cell killing properties of pyrroline c-25.
| compound | % apoptosisa | % live cellsb |
|---|---|---|
| c-25 (50 μM) | 30 ± 2 | 56 ± 4 |
| t-25 (50 μM) | 3 ± 1 | 96 ± 3 |
| podophyllotoxin (5 μM) | 63 ± 2 | 22 ± 1 |
% Apoptotic Jurkat cells after 48 h of treatment with indicated compounds relative to DMSO control.
% Live Jurkat cells after 48 h of treatment with indicated compounds relative to DMSO control. The data are mean ± SD of two independent experiments, each performed in 3 replicates, determined by flow cytometric Annexin-V/propidium iodide assay.
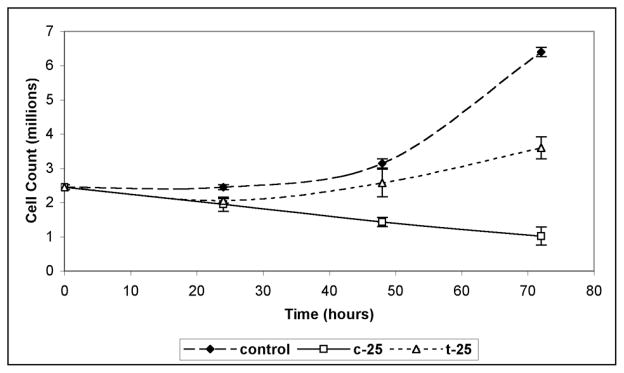
It appears that c-25 exerts its antiproliferative effect through induction of apoptosis in cancer cells, while t-25 is strictly growth inhibitory and lacks cell killing properties. This conclusion is consistent with the results of a cell counting assay, which revealed that the number of Jurkat cells decreased after 48 h as compared with the original quantity, when the cells were treated with c-25. In contrast, treatment with t-25 only blocked the cell growth (Figure 5).13
Figure 5.
Cell counting assay performed with c-25 and t-25.
Effect of c-25 (50 μM) and t-25 (50 μM) on the growth of Jurkat cells estimated by Trypan Blue dye exclusion method. The error bars represent data from multiple counts.
In conclusion, a novel three-component reaction, leading to the synthesis of diversely substituted cis and trans 2–pyrrolines, was discovered and a library of these compounds was prepared. Both cis and trans pyrrolines were found to exhibit growth inhibitory properties in a number of human cancer cell lines. The preliminary investigation of the biological mechanism of action revealed that the modes of action of the two series of stereoisomeric pyrrolines may be different. Further work is underway to make the reaction stereoselective and study the biology of these compounds in more detail, as it appears that multiple biological activities reside in these novel pyrroline libraries. The results of these studies will be reported in a full article elsewhere.
Acknowledgments
US National Institutes of Health (grants CA-99957 and RR-16480) are gratefully acknowledged for financial support of this work. G. L. thanks Artem S. Kireev for his invaluable assistance with experimental techniques and general guidance in the laboratory. P. T. and M. Yu. A. are grateful to NSF/DMR (grant 0420863) for the acquisition of X-ray single crystal diffractometer and to the Distributed Nanomaterials Characterization Network in the framework of New Mexico NSF EPSCoR Nanoscience initiative. We thank Professors Tatiana V. Timofeeva and Snezna Rogelj for their kind assistance with X-ray crystallography and flow cytometry respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Butler MS. J Nat Prod. 2004;67:2141. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 2.Bellina F, Rossi R. Tetrahedron. 2006;62:7213. [Google Scholar]
- 3.For some examples, see: Green MP, Prodger JC, Hayes CJ. Tetrahedron Lett. 2002;43:6609.Batey RA, Simonic PD, Lin D, Smyj RP, Lough AJ. Chem Commun. 1999:651.Ulbrich H, Fiebich B, Dannhardt G. Eur J Med Chem. 2002;37:953. doi: 10.1016/s0223-5234(02)01418-6.
- 4.Rüegg UT, Burgess GM. Trends Pharmacol Sci. 1989;10:218. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 5.Castellano S, Fiji HDG, Kinderman SS, Watanabe M, de Leon P, Tamanoi F, Kwon O. J Am Chem Soc. 2007;129:5843. doi: 10.1021/ja070274n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shvekhgeimer MGA. Khim Geterotsiklicheskikh Soedin. 2003:583. [Google Scholar]
- 7.For some recently developed methodologies for the synthesis of pyrrolines and pyrrolidines, including MCR approaches, see: Melhado AD, Luparia M, Toste FD. J Am Chem Soc. 2007;129:12638. doi: 10.1021/ja074824t.Blyumin EV, Gallon HJ, Yudin AK. Org Lett. 2007;9:4677. doi: 10.1021/ol7015302.Zhu X-F, Henry CE, Kwon O. Tetrahedron. 2005;61:6276.Huang P-Q. Synlett. 2006:1133.Hourcade S, Ferdenzi A, Retailleau P, Mons S, Marazano C. Eur J Org Chem. 2005:1302.Garner P, Kaniskan HU. J Org Chem. 2005;70:10868. doi: 10.1021/jo051926y.
- 8.Evdokimov NM, Magedov IV, Kireev AS, Kornienko A. Org Lett. 2006;8:899. doi: 10.1021/ol052994+. [DOI] [PubMed] [Google Scholar]; Evdokimov NM, Kireev AS, Yakovenko AA, Antipin MY, Magedov IV, Kornienko A. Tetrahedron Lett. 2006;47:9309. doi: 10.1016/j.tetlet.2006.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evdokimov NM, Kireev AS, Yakovenko AA, Antipin MY, Magedov IV, Kornienko A. J Org Chem. 2007;72:3443. doi: 10.1021/jo070114u. [DOI] [PubMed] [Google Scholar]; Magedov IV, Manpadi M, Evdokimov NM, Elias EM, Rozhkova E, Ogasawara MA, Bettale JD, Przeval’skii NM, Rogelj S, Kornienko A. Bioorg Med Chem Lett. 2007;17:3872. doi: 10.1016/j.bmcl.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Manpadi M, Uglinskii PY, Rastogi SK, Cotter KM, Wong Y-SC, Anderson LA, Ortega AJ, Van slambrouck S, Steelant WFA, Rogelj S, Tongwa P, Antipin MY, Magedov IV, Kornienko A. Org Biomol Chem. 2007;5:3865. doi: 10.1039/b713820b. [DOI] [PubMed] [Google Scholar]
- 9.The crystal structures of these cis and trans pyrrolines have been deposited at the Cambridge Crystallographic Data Centre and the allocated deposition numbers are CCDC 669040 and 669041 respectively. The detailed structure descriptions will be published elsewhere.
- 10.The substituted N-(sulfonamido)-acetophenones were prepared in the following way. To the solution of the hydrochloride of a required aminomethylaryl ketone (30 mmol) in H2O (30 mL) was added a corresponding sulfonyl chloride (30 mmol) in acetone (70 mL) dropwise with stirring at 10 °C. Et3N (65 mmol) was then added with vigorous stirring over 1 h. The reaction mixture was further stirred for additional 1.5 h at the same temperature and the precipitate was collected by vacuum filtration. The filtrate was reduced in volume to one half of the original and additional precipitate was collected by filtration. The precipitates were combined and recrystallized from ethanol. The yields were in the range of 86–92%.
- 11.A representative procedure for the synthesis of cis and trans pyrrolines c-1 and t-1: To a solution of benzaldehyde (95 mg, 0.9 mmol), malononitrile (59 mg, 0.9 mmol) in EtOH (5 mL) were added Et3N (0.05 mL) and N-(p-nitrobenzenesufonamido)-acetophenone (290 mg, 0.9 mmol). The mixture was refluxed for 1.5 h and cooled to room temperature. The formed precipitate was isolated by filtration and washed with cold ethanol (2 × 3 mL) to yield a mixture of pyrrolines c-1 and t-1. The mixture was suspended in MeCN (25 mL) and refluxed for 30 min. After cooling the suspension to room temperature the solid was isolated by filtration and washed with methanol (3 × 2 mL) to yield pure cis pyrroline c-1 as a yellow solid (124 mg, 29%). The filtrate was evaporated to dryness to give pure trans pyrroline t-1 as a yellow solid (265 mg, 62%). c-1: m.p. 201 °C (MeCN); 1H NMR (DMSO-d6) δ 8.53 (d, J = 8.8 Hz, 2H), 8.38 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 7.7 Hz, 2H), 7.49 (t, J = 7.4 Hz, 1H), 7.32 (d, J = 7.4 Hz, 1H), 7.17 (s, 2H), 6.97 (s, 2H), 6.91 (s, 1H), 6.38 (d, J = 11.0 Hz, 1H), 4.77 (d, J = 11.0 Hz, 1H); 13C NMR (DMSO-d6) δ 194.2, 155.0, 151.3, 143.0, 136.4, 135.5, 134.0, 129.9, 128.8, 128.3, 128.0, 125.5, 118.2, 67.5, 62.7, 47.1. HRMS m/z (ESI) calcd for C24H19N4O5S (M+H+) 475.1076, found 475.1058. t-1: m.p. 194 °C (MeCN); 1H NMR (DMSO-d6) δ 8.47 (d, J = 8.3 Hz, 2H), 8.34 (d, J = 8.3 Hz, 2H), 7.90 (d, J = 7.4 Hz, 2H), 7.74 (t, J = 6.6 Hz, 1H), 7.59 (d, J = 7.4 Hz, 2H), 7.29 (s, 2H), 7.22 (t, J = 7.2 Hz, 1H), 7.11 (d, J = 7.2 Hz, 2H), 6.51 (d, J = 7.2 Hz, 2H), 5.56 (s, 1H), 3.83 (s, 1H); 13C NMR (DMSO-d6) δ 193.3, 154.4, 151.4, 142.5, 141.6, 135.0, 133.5, 129.9, 129.6, 128.3, 127.2, 125.6, 118.1, 71.0, 62.0, 47.0. HRMS m/z (ESI) calcd for C24H19N4O5S (M+H+) 475.1076, found 475.1079.
- 12.Mosmann T. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.These observations also argue against the oxidation of the pyrrolines in the cell culture to the corresponding pyrroles, because in this case the biological effects of trans and cis pyrrolines would be expected to be similar.