Abstract
The genetic architecture of common epilepsies is largely unknown. HCNs are excellent epilepsy candidate genes because of their fundamental neurophysiological roles. Screening in subjects with febrile seizures and genetic epilepsy with febrile seizures plus revealed that 2.4% carried a common triple proline deletion (delPPP) in HCN2 that was seen in only 0.2% of blood bank controls. Currents generated by mutant HCN2 channels were ~35% larger than those of controls; an effect revealed using automated electrophysiology and an appropriately powered sample size. This is the first association of HCN2 and familial epilepsy, demonstrating gain of function of HCN2 current as a potential contributor to polygenic epilepsy.
Febrile seizures (FS) and idiopathic generalized epilepsy (IGE), including genetic epilepsy with febrile seizures plus (GEFS+), are common epilepsy syndromes that show complex inheritance, where a combination of susceptibility variants is proposed to underlie the etiology in most cases.1-3 A small number of susceptibility genes for IGE have been identified and predominantly include ion channel genes.4
Hyperpolarization-activated cyclic nucleotide-gated ion channels (HCN) conduct Ih important for neuronal pacemaker function. There is growing evidence for a role of HCN in both idiopathic5,6 and acquired epilepsy.7-11 HCN1 and HCN2 variants have been identified, but functional analyses have failed to determine a statistical difference in channel properties.12 The functional changes associated with gene variants that contribute to polygenic disease are, by definition, subtle. Both biological and experimental variability contribute to the variance seen in functional analyses, with the probability of failing to detect a real difference in 2 populations rising dramatically as variation increases. To separate such populations, the sample size needs to increase.13 Here, we used a candidate gene approach to search for HCN1 and HCN2 variants in epilepsy subjects and a medium-throughput automated electrophysiological assay to test channel function with appropriate sample sizes. Currents generated by mutant HCN2 channels were ~35% larger than controls, an increase that could enhance neuronal excitability.8,9
Subjects and Methods
Patient Collection
Diagnostic criteria for IGE and FS followed that of the Commission on Classification and Terminology of the International League Against Epilepsy,14 and for GEFS+ followed that of Scheffer and Berkovic.3 Patients were Australian subjects of Caucasian origin and were screened for variants in a randomized fashion where clinical characteristics were blinded. Controls were randomly drawn anonymous Australian blood donors primarily of Caucasian origin.
DNA Preparation and Mutation Analysis
DNA was extracted from peripheral blood using the QIAamp DNA Blood Maxi Kit (Qiagen, Valencia, Germany). Hexosaminidase-labeled intronic primers flanking each exon were used to polymerase chain reaction (PCR)-amplify products between 250bp to 320bp, and the products were analyzed by single-strand conformation analysis (SSCA) on a real-time gel system using a Gel-Scan 2000 DNA fragment analyzer (Corbett Research, Mortlake, Australia). Products showing band shifts were sequenced using the BigDye Terminator Cycle Sequencing Ready Reaction kit (PE Applied Biosystems, Foster City, CA, v2.0), and the sequences were analyzed on an Applied Biosystems ABI Prism 3700 DNA Analyzer.
The HCN1 open reading frame (accession number NM_021072) contains 8 exons, which were divided into 13 amplicons for SSCA. The human HCN2 open reading frame (accession number NM_001194) contains 8 exons, which were divided into 14 amplicons for SSCA. We used single nucleotide polymorphisms (SNPs) to distinguish the genomic sequence spanning the HCN2 gene on chromosome 19 from the highly related chromosome 15 sequence. HCN1 is located on chromosome 5. Primer sequences used for PCR and SSCA can be found in Supporting Information.
HCN Mutagenesis and In Vitro Transcription
HCN 2-Electrode Voltage Clamp Analysis and Statistical Design
Oocytes from Xenopus laevis were prepared as previously described.15 Thirty-five nanoliters of cRNA-encoding the wild-type (WT) and delPPPHCN2 subunits (65ng/μl; stocks confirmed spectrophotometrically and by gel analysis) was injected into stage 5/6 oocytes using the Roboocyte (Multi Channel Systems, Reutlingen, Germany) and stored for 2 days prior to experimentation. For voltage clamp recordings, oocytes were impaled with 2 glass electrodes containing 1.5M potassium acetate (I) and 0.5M KCl (V) and clamped at a holding potential of −30mV. A current-voltage (I-V) relationship was generated by incrementing voltage in 10mV steps from −140mV to 0mV for 15 seconds with a 2-second test potential of −140mV at the end of the pulse. Oocytes were perfused with a bath solution (mM): 96 KCl, 2 NaCl, 2 MgCl2, and 10 HEPES (pH 7.5 using KOH). To obtain normalized I-V relationships, peak tail current amplitudes were recorded at −140mV and were divided by the largest peak tail current. This normalized current was plotted against voltage and fit with a Boltzmann curve (GraphPad Prism, GraphPad Software, La Jolla, CA [average fits] and AxoGraph X, AxoGraph Scientific, Sydney, Australia [individual fits). Cyclic adenosine monophosphate (cAMP) modulation was investigated by incubating oocytes in 15μM forskolin (Sigma, Castle Hill, Australia) for a period of 7 minutes. Kinetics of activation was determined by measuring the time to half maximal current using a custom analysis program run in MatLab. Power analysis was performed using the G* Power calculator (www.psycho.uni-duesseldorf.de/aap/projects/gpower). Statistical comparisons were made using an unpaired t test (GraphPad Prism).
Results
Variation of HCN1 and HCN2 in Epilepsy Patients
No major-effect sequence variation was detected in human HCN1 (Table 1). Analysis of HCN2 revealed a number of synonymous SNPs (Table 1) and a variant, c.2156-2164delCGCCGCCGC, p.719-721PPP, predicted to lead to the deletion of 3 consecutive proline residues (delPPP) in the HCN2 protein. delPPP was found to be heterozygous in 3/65 unrelated patients (allele frequency = 2.3%) with GEFS+ (OMIM #604233), 3/61 unrelated patients (allele frequency = 2.5%) with FS, 3/772 blood bank controls (allele frequency = 0.2%), and 0/72 patients with classical IGE. The 3 FS patients had simple FS with a mean onset of 2 years. Frequency varied in each case, with 1, 3, or multiple events. The 3 GEFS+ patients had myoclonic-astatic epilepsy, FS+ or FS (this patient also carried the SCN1B[C121W] mutation16). Clinical characteristics of patients who were negative for delPPP are described in Supporting Information.
TABLE. Summary of the DNA Sequence Variants Found in the Open Reading Frame and Flanking Intronic Sequence of the Human HCN1 and HCN2 Genes in Subjects with Epilepsy.
| Amplicon | DNA Sequence Variant | Amino Acid Change |
Allele Frequency (%) | ||||
|---|---|---|---|---|---|---|---|
| Intronic | Coding | GEFS+ | IGE | FS | Controls | ||
| HCN1 | |||||||
| 1-2 | c.199-207delGGTGGCGGC | p.67delGGG | ~5.0 | ~5.0 | ~5.0 | ~5.0 | |
| 1-2 | c.214-222delGGCGGCGGC | p.72delGGG | ~5.0 | ~5.0 | ~5.0 | ~5.0 | |
| 1-2 | c.217-222delGGCGGC | p.73delGG | ~5.0 | ~5.0 | ~5.0 | ~5.0 | |
| 7 | IVS7+8insT | — | 1.6 | 0 | 0.8 | 0 | |
| 8-1 | c.1797A>G | — | 0.8 | 0.7 | 0.8 | 0 | |
| HCN2 | |||||||
| 2-2 | c.858T>C | — | 17.7 | 22.9 | 19.8 | 20.3 | |
| c.915C>T | — | ||||||
| c.963C>T | — | ||||||
| 2-2 | c.921C>T | — | 1.5 | 0 | 0 | 0 | |
| 4 | c.1239G>C | — | 4.0 | 2.8 | 4.0 | 5.1 | |
| 5 | IVS5+7C>T | 1.6 | 1.5 | 0 | 3.8 | ||
| c.1452G>A | 2.4 | 2.9 | 4.2 | 1.1 | |||
| 8-1 | c.2156-2164delCGCCGCCGC | p.719-721 delPPP | 2.3 | 0 | 2.5 | 0.2 | |
The allele frequency of the least common allele is indicated.
GEFS+ = genetic epilepsy with febrile seizures plus; IGE = idiopathic generalized epilepsy; FS = febrile seizures; delPPP = triple proline deletion.
Functional Analysis of delPPPHCN2 Channels
For current magnitude analysis, the inherent variance meant that sample sizes of 180 per group were required to detect differences of 25% with a power of 0.95 to reduce type 2 errors. Although all available data were used, smaller sample sizes were sufficient for all other parameters, because current magnitude had the highest standard deviation.
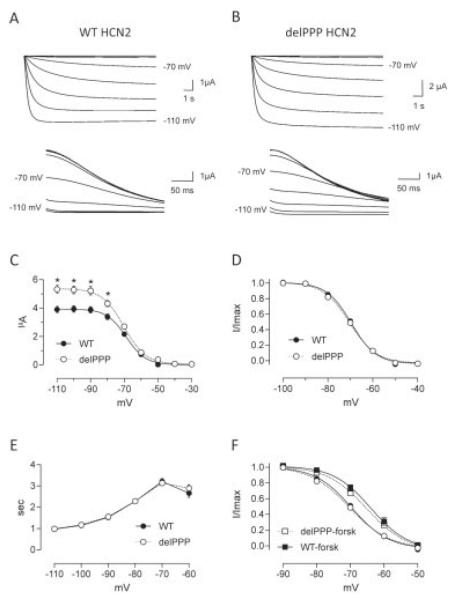
To achieve these high sample sizes, a medium-throughput electrophysiological assay was used. WT and delPPPHCN2 currents were elicited at hyperpolarizing potentials (Fig). A significant ~35% increase in tail current was observed for the delPPPHCN2 channel compared with WT (5.32 ± 0.29μA vs 3.89 ± 0.23μA; n = 189 and 181, p = 0.001). Western blot analysis showed that the total level of WT and delPPPHCN2 channel expression were the same (Supporting Information). Normalized current-voltage relationships were also constructed from tail currents. Half maximal activation voltage (V1/2), calculated by fitting a Boltzmann equation to individual activation curves, was identical in the WT and delPPPHCN2 channels (−69.9 ± 0.4mV vs −70.4 ± 0.4mV; p = 0.4). However, the slopes of the Boltzmann curve were significantly different for WT and delPPPHCN2 channels (4.70 ± 0.07 vs 5.18 ± 0.11, p = 0.0003). Activation kinetics were not significantly different across a range of activating voltages (p > 0.35).
FIGURE.
Electrophysiological characterization of triple proline deletion (delPPP) HCN2 channels. (A) Steady state (top) and tail (bottom) currents from oocytes expressing wild-type (WT) and (B) delPPPHCN2 channels. (C) Average tail current-voltage relationship of WT and delPPPHCN2. *p < 0.05 (D) Activation curve constructed from average normalized current relationships constructed from tail currents fit with the Boltzmann equation. (E) Time to half maximal activation for WT and delPPPHCN2 channels across a range of voltages. (F) Modulation of the voltage dependence of activation of WT and delPPPHCN2 channels by forskolin (forsk), data from D replotted for comparison.
cAMP Gating in delPPPHCN2 Channels
Because the delPPP variant is close to the cyclic nucleotide-binding domain, we investigated whether cAMP gating was altered by comparing channel sensitivity to forskolin, an activator of adenylate cyclase. V1/2 standard deviation was approximately 11% of the mean, with power calculation suggesting that a minimum 10% difference could be detected at power 0.95 with a sample size of 50 per group. This sampling regime would be sensitive to a minimum 2.5% change in slope factor. There was no difference in V1/2 in forskolin for the WT and delPPPHCN2 channels (−64.2 ± 1.1mV vs −64.4 ± 1.0mV, n = 40 and 55, p = 0.6, see Fig 1F). The slopes of the activation curve in forskolin were also not significantly different (4.05 ± 0.08 vs 4.23 ± 0.13, p = 0.28).
Discussion
Our comparison of HCN1 and HCN2 variation in epilepsy patients and controls revealed little genetic heterogeneity overall, suggesting an intolerance of sequence changes. However, a deletion of 3 consecutive prolines (delPPP) in HCN2 was identified in patients with GEFS+ and FS. The delPPP occurs in a 7-proline repeat in humans and is conserved as a 6–7 repeat in cows, chimpanzees, dogs, and mice, suggesting functional importance. The occurrence of the delPPP allele in controls is not unexpected, because susceptibility alleles are predicted to be present at low frequency in the general population.2 We were able to detect the difference in the current between WT and delPPP channels by taking advantage of an automated electrophysiology system to record large numbers of oocytes. This enabled statistical detection of a current magnitude increase for the mutant channel. The need for large sample sizes is likely to become more common as an ever-increasing number of putative susceptibility alleles are identified.
The HCN2 delPPP variant occurs with highest frequency in patients with GEFS+ and FS, which both have febrile seizures at presentation. Increases in Ih occur following induced febrile seizures in animal models and are proposed to contribute to hippocampal hyperexcitability.8,9 As we did not observe HCN2 delPPP in patients with IGE, which do not present with FS, this suggests that the variant may be a specific susceptibility allele for FS. The impact of changes in HCN function on neuronal excitability is multifaceted, with Ih contributing to both resting membrane potential and input resistance.17 Increases in Ih, as predicted here, will depolarize membrane potential, taking the neuron closer to the firing potential, and in this way be considered proexcitatory. A recent study that included computer simulation modeling supports this view.9 It is important to note that reduced HCN channel function is also thought to increase neuronal network excitability.5,6 Our kinetic analysis also isolated a very subtle difference in the slope of activation of the delPPPHCN2 channel in comparison to WT, the functional significance of which is unclear.
Simple expression systems lack sufficient complexity to reveal changes that may be neuron specific (eg, subcellular expression). Further, investigations of changes inchaperone protein interactions (eg, TRIP8b18) and a host of other second messengers known to alter HCN function (eg, p38 mitogen-activated protein kinase19) need to be considered. Ultimately, in vivo modeling that allows replication of true complex genetics with the introduction of multiple susceptibility alleles will determine the behavioral impact of the HCN2 delPPP variant.20
Supplementary Material
Acknowledgment
This study was supported by the National Health and Medical Research Council of Australia (grants 400121 and 454655 to C.A.R. and S.P.) and an R. D. Wright Fellowship, University of Melbourne (C.A.R.).
We thank Dr N. Poolos for helpful discussions; K. S. Tan, C. Trager, and N. Taylor for technical assistance; and the patients and their families for participating in this study. Human HCN2 cDNA was kindly provided by Dr M. Biel.
Footnotes
Authorship
L.M.D. and C.A.R. contributed equally to this work.
Potential Conflicts of Interest
S.P., I.E.S., and S.F.B. were paid consultants of Bionomics Limited.
References
- 1.Baulac S, Gourfinkel-An I, Nabbout R, et al. Fever, genes, and epilepsy. Lancet Neurol. 2004;3:421–430. doi: 10.1016/S1474-4422(04)00808-7. [DOI] [PubMed] [Google Scholar]
- 2.Dibbens LM, Heron SE, Mulley JC. A polygenic heterogeneity model for common epilepsies with complex genetics. Genes Brain Behav. 2007;6:593–597. doi: 10.1111/j.1601-183X.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 3.Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus. A genetic disorder with heterogeneous clinical phenotypes. Brain. 1997;120(pt 3):479–490. doi: 10.1093/brain/120.3.479. [DOI] [PubMed] [Google Scholar]
- 4.Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol. 2009;87:41–57. doi: 10.1016/j.pneurobio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig A, Budde T, Stieber J, et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss U, Kole MH, Brauer AU, et al. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. doi: 10.1111/j.0953-816X.2004.03392.x. [DOI] [PubMed] [Google Scholar]
- 7.Brewster A, Bender RA, Chen Y, et al. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22:4591–4599. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen K, Aradi I, Thon N, et al. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nat Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender RA, Soleymani SV, Brewster AL, Nguyen ST, Beck H, Mathern GW, Baram TZ. Enhanced expression of a specific hyperpolarizatioin-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus. J Neurosci. 2003;17:6826–6836. doi: 10.1523/JNEUROSCI.23-17-06826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung S, Jones TD, Lugo JN, Jr, et al. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell KL, Ng C, O’Brien TJ, et al. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia. 2008;49:1686–1695. doi: 10.1111/j.1528-1167.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 12.Tang B, Sander T, Craven KB, et al. Mutation analysis of the hyperpolarization-activated cyclic nucleotide-gated channels HCN1 and HCN2 in idiopathic generalized epilepsy. Neurobiol Dis. 2008;29:59–70. doi: 10.1016/j.nbd.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas EA, Reid CA, Berkovic SF, Petrou S. Prediction by modeling that epilepsy may be caused by very small functional changes in ion channels. Arch Neurol. 2009;66:1225–1232. doi: 10.1001/archneurol.2009.219. [DOI] [PubMed] [Google Scholar]
- 14.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Zheng T, Morris MJ, et al. The mechanism of carbamazepine aggravation of absence seizures. J Pharmacol Exp Ther. 2006;319:790–798. doi: 10.1124/jpet.106.104968. [DOI] [PubMed] [Google Scholar]
- 16.Wallace RH, Wang DW, Singh R, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 17.Dyhrfjeld-Johnsen J, Morgan RJ, Soltesz I. Double trouble? Potential for hyperexcitability following both channelopathic up- and downregulation of I(h) in epilepsy. Front Neurosci. 2009;3:25–33. doi: 10.3389/neuro.01.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J Neurosci. 2006;26:7995–8003. doi: 10.1523/JNEUROSCI.2069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan HO, Reid CA, Single FN, et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc Natl Acad Sci U S A. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.