Abstract
There are quantitative and/or qualitative mechanisms allowing androgen receptor (AR) growth signaling in androgen ablation refractory prostate cancer cells. Regardless of the mechanism, agents that deplete AR protein expression prevent such AR growth signaling. Thapsigargin (TG) is a highly cell-penetrant sequiterpene-lactone that once inside cells inhibits (IC50, 10 nmol/L) critically important housekeeping SERCA 2b calcium pumps in the endoplasmic reticulum. Using a series of five genetically diverse androgen ablation refractory human prostate cancer lines (LNCaP, LAPC-4, VCaP, MDA-PCa-2b, and CWR22Rv1), TG inhibition of SERCA pumps consistently results in depletion of the endoplasmic reticulum Ca+2 coupled with µmol/L elevation in the intracellular free Ca+2 initiating a molecular cascade that: (a) inhibits Cap-dependent AR protein synthesis resulting in 90% depletion of AR protein by 24 hours of TG exposure, (b) arrests the cells in G0, and (c) induces their apoptotic death. Unfortunately, due to its highly lipophilic nature, TG is not deliverable as a systemic agent without host toxicity. Therefore, TG analogues containing amino acids were developed, which retain ability to deplete AR protein and induce cell death and which can be covalently linked to peptide carriers producing water soluble prodrugs for systemic delivery. Specific amino acid sequences are used to restrict the liberation of cytotoxic amino acid containing TG analogues from the peptide prodrug by prostate-specific proteases, such as prostate-specific antigen and prostate-specific membrane antigen, or cancer-specific proteases, such as fibroblast activation protein, so that toxicity of these prodrugs is selectively targeted to metastatic sites of prostate cancer. Based on these results, these prodrugs are undergoing clinical development.
Introduction
Although androgen ablation has been standard therapy for metastatic prostate cancer for >60 years, eventually, patients progress to androgen-independent form of prostate cancer. Thus, there will be ~30,000 men who will die this year within the United States alone, although these men are given aggressive androgen ablation by combining LHRH analogues plus antiandrogens (1). This therapeutic failure does not mean, however, that androgen receptor (AR) signaling is no longer engaged at the lethal stages of the disease. For example, AR is still strongly expressed in most metastatic prostate cancer tissue sites obtained from autopsy from androgen ablation refractory patients (2). Consistent with these results, the majority of human prostate cancer lines established as permanent cell lines were obtained from androgen-ablation-failing hosts (e.g., LNCaP, LAPC-4, LAPC-9, MDA-PCa-2B, VCap, DuCap, CWR22Rv1, etc.) but still retain high AR protein expression (3). In addition, interference with AR expression within these androgen ablation refractory prostate cancer cell lines results in inhibition of their proliferation and induces their death both in vitro and in vivo (4, 5)
Mechanistic studies have shown that there are both quantitative and/or qualitative malignancy-associated changes that can explain how such AR growth signaling is maintained in androgen ablation refractory prostate cancer cells. These mechanistic explanations are based on the fact that growth of AR-expressing prostate cancer cells is dependent on a critical threshold of Androgen/AR complex signaling to induce their malignant growth (6). Because the level of Androgen/AR complex signaling is enhanced by mass action either by increasing ligand and/or the amount of AR protein, androgen ablation resistance occurs via malignancy-associated increases in either ligand and/or AR protein. There is a growing body of data suggesting that lowering serum testosterone by >90% with standard androgen ablation does not result in sufficient lowering of tissue androgen within sites of prostate cancer to prevent AR growth signaling (7, 8). Such maintenance of tissue androgen after androgen ablation is postulated to involve the acquired ability of the prostate cancer cells to metabolically convert steroidal precursors into androgens (7, 8). Additionally, it has been shown experimentally that if AR protein expression is elevated to a sufficient level, such that even when tissue androgen is significantly decreased after androgen ablation, a sufficient threshold of androgen/AR complexes is generated to stimulate growth signaling in the cancer cells (9). Such an enhanced mass action effect could be due to increased AR protein stabilization via its site-specific phosphorylation induced by “cross-talk” with other growth factor signaling pathways (i.e., mitogen-activated protein kinase kinase cascade, interleukin-6, Stat3, Her2, etc.; refs. 10–14) or enhanced AR mRNA translation and/or transcription (9). Changes in AR-mediated transcriptional control can involve both genetic changes [i.e., amplification of the AR gene is commonly observed in androgen ablation–resistant prostate cancer cells (15–18)] and nongenetic effects.
Besides mass action quantitative effects secondary to elevation in the AR protein expression, there are also qualitative changes in AR that can produce androgen ablation refractory prostate cancer cells. For example, AR can be mutated, producing amino acid variants in its COOH-terminal ligand binding domain (LBD; i.e., T877A, W741C, etc.) such that the mutated AR proteins acquire the malignancy-associated ability to generate growth signaling via binding to alternative steroids or even, paradoxically, antiandrogens (19). However, all of the quantitative and/or qualitative mechanisms discussed still require a degree of ligand binding to the AR protein for the generation of growth signaling in androgen ablation refractory prostate cancer cells. Based on this understanding, a variety of novel therapeutic approaches are being evaluated to more effectively prevent this AR binding. However, a number of observations document that, even using the most efficacious of such therapies, prostate cancer cells will remain refractory to such ligand-targeted approaches. This is based on the demonstration that additional qualitative changes in AR occur via alternative splicing of the AR transcripts producing truncated isoforms of the AR protein that lack the COOH-terminal LBD. Functionally, these LBD-truncated AR isoforms are constitutively active, generating ligand-independent growth signaling of prostate cancer cells and are thus not targetable based on a ligand approach (20–22). Regardless of the mechanism, an agent that depletes AR protein expression would prevent any AR signaling within androgen ablation resistance prostate cancer cells.
By the early 90s, we documented that agents sustaining an increase in intracellular free Ca+2 (Cai) activate apoptosis in both normal and malignant prostate cancer cells (23–26). One of these agents is the natural plant product, thapsigargin (TG). TG is highly cell penetrant and, once inside cells, inhibits a critically important housekeeping calcium pump in the endoplasmic reticulum (ER), known as SERCA 2b (27). Such SERCA pump inhibition results in the depletion of the high (i.e., >500 µmol/L) Ca+2 in their ER, which induces “capacitance entrance” of extracellular Ca+2 via store-operated Ca+2 channels in the plasma membrane (28). This elevates Cai (26, 27) and induces apoptosis of androgen ablation refractory prostate cancer (26). In LNCaP human androgen ablation refractory prostate cancer cells, TG also depletes AR protein (29). In the present studies, we expand upon this observation using a series of five genetically diverse androgen ablation refractory human prostate cancer lines, TG-induced AR depletion is documented as a general phenomenon involving inhibition of AR protein synthesis associated with apoptotic death, validating TG-based approaches for this devastating disease. Unfortunately, due to its highly lipophilic nature, TG is not deliverable as a systemic agent without host toxicity (30). To overcome this limitation, we have developed amino acid–containing TG analogues that retain the ability to deplete AR protein and induce cell death, and can be covalently linked to peptide carriers producing water-soluble prodrugs for systemic delivery. By using specific amino acid sequences to restrict the liberation of the amino acid–containing TG analogues from the peptide prodrug by prostate-specific proteases, such as prostate-specific antigen, prostate-specific membrane antigen (30–35), or cancer-specific proteases such as fibroblast activation protein (36), these prodrugs are selectively targeted to metastatic sites of prostate cancer and are currently undergoing clinical development.
Materials and Methods
Materials
The synthetic androgen R1881 was purchased from Perkin-Elmer. The TG was purified from Thapsia garganica seeds and analogues were synthesized as described in detail previously (30–33).
Cell lines and Cell Culture Assays
The culture conditions and media for all of the androgen ablation refractory human prostate cancer lines used in these studies (i.e., LNCaP, LAPC-4, MDA-PCa-2B, VCaP, and CWR22Rv1) are as described previously (37). Cell viability at various time postexposure to test compounds was determined using trypan blue exclusion as previously described (25). The dose-response ability of test compounds to kill prostate cancer cells was determined using a clonogenic survival assay as described previously (26), and the results are expressed as the nmol/L concentration needed to lose 50% of the clonogenic ability (i.e., LD50) after 48 h of exposure to the test compound.
Biochemical Assays
The dose-response ability of TG and its analogues to inhibit rabbit skeletal muscle SERCA pump activity was done as described previously (31) and the results expressed as the nmol/L concentration of compound needed to inhibit the activity by 50% (i.e., IC50). The ability of the compounds to elevate intracelluar free Ca+2 (Cai) was measured as described previously (32). Baseline Cai was <50 nmol/L in each of the prostate cancer cell lines tested before exposure to TG and its analogues. The Cai response is expressed as the Cai 10 min after exposure to 1 µmol/L of the test agent.
Western Blot Analysis
Western blot analysis was carried out on cell lysates equivalent to 105 cells per lane as described previously (37). Rabbit polyclonal anti-AR (N-20), Cyclin D1 (H-295), GADD34 (S-20), and GRP78 (H-129) antibodies were purchased from Santa Cruz Biotechnology. Mouse monoclonal anti–β-actin and anti-FLAG were purchased from Sigma. Rabbit polyclonal anti–phospho-4E-BP1 (ser 65), phospho-elF4E (ser 209), elF4G, phospho-elF2α (ser 51), and cleaved PARP (asp 214) were purchased from Cell Signaling.
Flow Cytometry and Time-lapse Microscopy
The percentage of cells in cycle was determined using a phycoerythrin-conjugated mouse anti-human Ki67 BD Pharmingen and a LSR flow cytometer with CellQuest Software (BD Biosciences) as described previously (26). To monitor the kinetics of ER stress, LNCaP and CWR22Rv1 cells were transfected with a pcDNA3.1/Zeo expression vector (Invitrogen) containing in its multiple cloning site a FLAG-tagged XBP-1 gene lacking its DNA binding domain fused to venus green fluorescent protein gene (i.e., F-XBP- 1-ΔDBD-venus) driven by a cytomegalovirus enhancer/promoter and then zeomycin-resistant cells isolated. This F-XBP-1-ΔDBD-venus insert was subcloned by Kpu1/BamH1 restriction of the pCAX-F-XBP-1-ΔDBD-venus expression vector whose construction has been described previously (38) and which was generously provided by Masayuki Miura (Tokyo University, Tokyo, Japan; ref. 38). Venus protein fluorescence was monitored using either flow cytometry or time-lapse digital phase contrast and fluorescence microscopy with a TE-2000 Nikon microscope with appropriate filter combinations for the venus protein on a 37°C heated stage in a 5% CO2 chamber (Live Cell NEVE Product Group) to determine the kinetics of ER stress induction in individual cells. Images were captured digitally using a Cool Snap ES CCD camera (Princeton Instruments). The results are expressed as the percentage of ER-stressed cells at indicated times after exposure to TG and its analogues. Additionally, using anti-FLAG antibody in Western blots, the ER stress–induced 45-kDa XBP-1/venus fusion protein can also be used to document activation of IRE-1 as described previously (38).
Measurement of AR mRNA and AR-Specific versus Total Protein Synthesis
Total RNA extraction from cell pellets was conducted using the Qiagen RNeasy Mini kit (Qiagen). Detection of AR transcripts was documented using the SuperScript III One-Step reverse transcription-PCR System with Platinum Taq High Fidelity (Invitrogen Life Technologies) per the manufacturer's instructions. RNA (0.5 µg of total RNA) was used per reaction, and the primers were as follows: AR-Forward (5′-CGGAAGCTGAAGAAACTTGG-3′) and AR-Reverse (5′-ATGGCTTCCAGGACATTCAG-3′); Actin-Forward (5′-CACCAACTGGGACGACATGG-3′) and Actin-Reverse (5′-CCAGGGTACATGGTGGTGCC-3′).
Total protein synthesis was determined using S35-methionine incorporation into Trichloroacetic Acid (TCA) precipitable counts as previously described (26), and the results were expressed as percent of total S35-methionine incorporation per cell in exponentially growing untreated cells. Briefly, cells were treated for 6 or 24 h and incubated with S35-methionine for 30 min before cell lysis. For determination of AR-specific protein synthesis, AR protein was immunoprecipitated from an aliquot of the S35-methionine–incorporated total protein mixture using the N-20 anti-AR antibody from Santa Cruz followed by incubation with Protein-A agarose beads (Sigma). The precipitated protein was Western blotteanti-AR antibody, and the level of radioactivity associated with the AR protein was detected using X-ray film was quantitated on a Kodak Model 440 CF Imaging Station.
Immunocytochemical Staining
Cells grown on Nunc multichamber plastic slides were treated with compounds for indicated times and then fixed with 70% methanol and immunocytochemically stained for apoptosis-inducing factor using a rabbit polyclonal antibody obtained from Santa Cruz as described previously (30).
Results
TG-Induced Depletion of AR Protein Is a General Response in Prostate Cancer Cells Occurring Early before Cell Death
TG, a sequiterpene lactone (Fig. 1) isolated from the Thapsia garganica plant, is highly cell permeable and, once in the cell, is a potent inhibitor (10 nmol/L IC50) of SERCA pumps (Table 1). Sustained exposure of androgen ablation refractory LNCaP human prostate cancer cells to TG induces rapid inhibition of their SERCA pumps, resulting in the depletion of the high (i.e., >500 µmol/L) Ca+2 in their ER, inducing “capacitance entrance” of extracellular Ca+2. This leads to an initial increase in Cai from ~40 to >300 nmol/L within minutes (Table 1) followed by a return to baseline over 9 to 12 hours, which is then followed by a delayed sustained increase in Cai to >1 µmol/L over the next 18 to 36 hours (39–41).
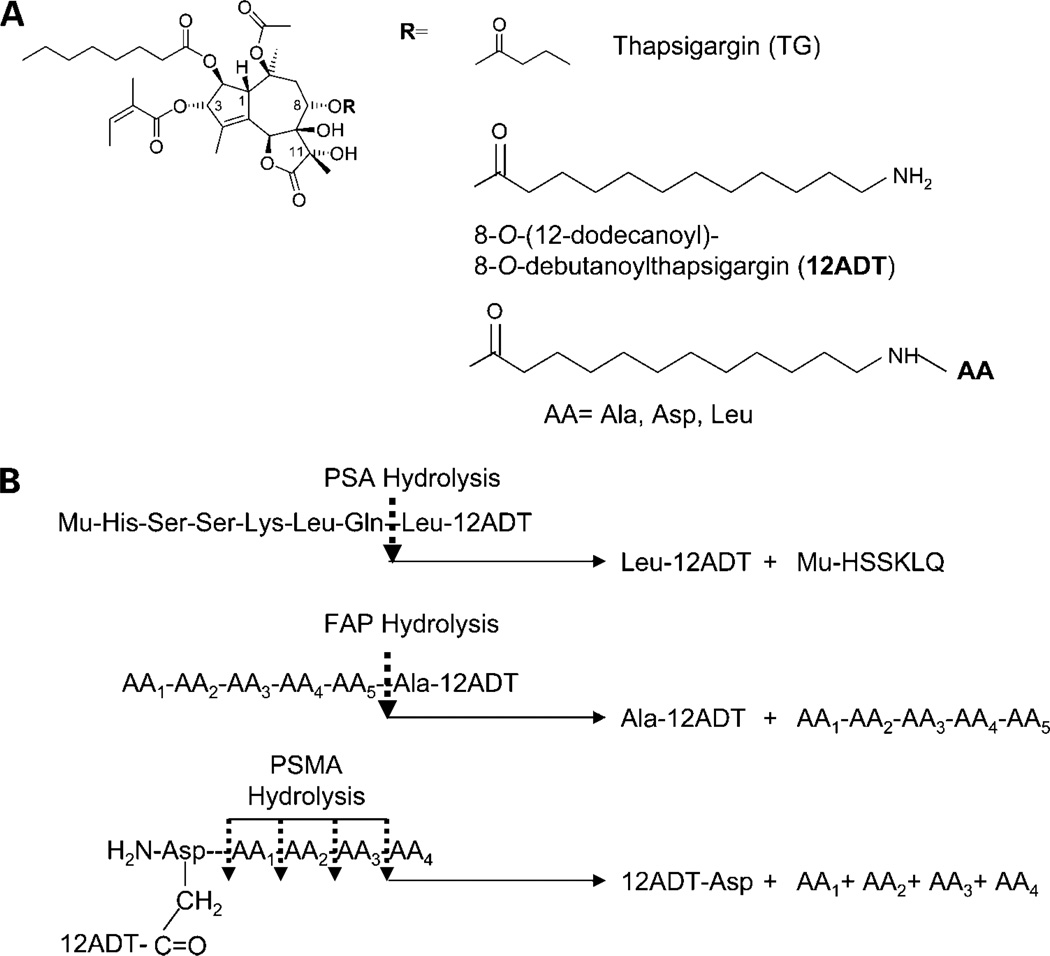
Figure 1.
A, chemical Structure for TG, its amine-containing 8-O-(12-dodecanyl)-8-O-debutanoyl TG analogue (12ADT), and its amino-acid analogues (i.e., Alanyl-12ADT, Aspartyl-12ADT, and Leucyl-12ADT). B, amino acid sequences used to restrict TG analogues from the peptide prodrugto prostate-specific proteases, prostate-specific antigen, fibroblast activation protein, and prostate-specific membrane antigen. Mu, morpholino protection group; AA, amino acid.
Table 1.
Ability of TG and its analogues to inhibit SERCA pump, increase intracellular free calcium, decrease AR protein, and induce cell death of LNCaP cells
| Analog | Amino acid | SEARCA pump inhibition | Intracellular free calcium | AR | Cell death of LNCaP |
|---|---|---|---|---|---|
| IC50 | (nmol/L)* | Protein† | LC50‡ | ||
| (nmol/L) | (% of control) | (nmol/L) | |||
| TG | None | 13 ± 2 | 441 ± 35 | 10 ± 2 | 10 ± 5 |
| 12ADT | None | 35 ± 4 | 369 ± 23 | 14 ± 3 | 55 ± 12 |
| A12ADT | Alanine | 17 ± 2 | 410 ± 45 | 15 ± 4 | 8 ± 4 |
| D12ADT | Aspartate | 18 ± 2 | 385 ± 40 | 11 ± 6 | 40 ± 12 |
| L12ADT | Leucine | 45 ± 3 | 414 ± 44 | 8 ± 4 | 14 ± 8 |
NOTE: Values are the mean of a minimum of three independent experiments ± SE.
Abbreviations: A-12ADT, alanyl-12ADT; D-12ADT, asparty-12ADT; L-12DT, leucyl-12ADT.
After 10 min after exposure to 1 µmol/L TG, starting concentration was ~40 nmol/L.
After 24 h of exposure to 1 µmol/L TG of LNCap cells.
After 48 h of exposure to TG.
TG cannot be given systemically without host toxicity (30). To target this toxicity, an amine-containing TG analogue (i.e., 12ADT; Fig. 1) was synthesized, which maintains the potent ability to inhibit SERCA, elevate Cai, deplete AR protein, and kill androgen ablation refractory prostate cancer cells (Tables 1 and 2). Like TG, 12ADT cannot be given systemically without host toxicity (30). The advantage of 12ADT, however, is that it can be covalently coupled via a peptide bond between its primary amine to either the COOH-terminal or side chain carboxylic acid of 5 to 6 amino acid peptides to form prodrugs (30, 35). The sequences of these peptides are designed so that they solublize the12ADT for systemic delivery while preventing entry of the prodrug into cells, restricting its cytotoxicity until the prodrug is hydrolyzed by prostate-specific (prostate-specific antigen or prostate-specific membrane antigen), or cancer-specific proteases (fibroblast activation protein). This hydrolysis liberates 12ADT coupled to a single amino acid. For the prostate-specific antigen–activated prodrug, the liberated toxin is leucyl-12ADT (30); for the prostate-specific membrane antigen–activated prodrug, it is asparty-12ADT where aspartate is coupled to 12ADT via its β-carboxylic acid in its side chain (35); and for fibroblast activation protein, it is alanyl-12ADT (Fig. 1; ref. 36). These amino acid containing TG analogues maintain potent ability to inhibit SERCA, elevate Cai, deplete AR protein, and kill androgen ablation refractory prostate cancer cells (Tables 1 and 2).
Table 2.
Comparative killing ability of TG versus its analogues against a series of human prostate cancer cell lines
| Cell line | Killing ability of compound | ||||
|---|---|---|---|---|---|
| (LD50 nmol/L)* | |||||
| TG | 12ADT | A12ADT | L12ADT | D12ADT | |
| LNCaP | 10 ± 5 | 55 ± 12 | 8 ± 4 | 14 ± 8 | 40 ± 12 |
| LAPC-4 | 25 ± 6 | 80 ± 19 | 28 ± 7 | 32 ± 9 | 55 ± 9 |
| VCaP | 20 ± 7 | 67 ± 25 | 23 ± 9 | 25 ± 11 | 70 ± 18 |
| MDA-PC-2b | 43 ± 12 | 95 ± 35 | 40 ± 6 | 42 ± 5 | 51 ± 12 |
| CWR22Rv1 | 38 ± 9 | 85 ± 27 | 38 ± 11 | 34 ± 16 | 39 ± 7 |
Values are the mean of a minimum of three independent experiments ± SE.
This depletion of the ER Ca+2 coupled with the µmol/L elevation in Cai induces a molecular cascade that results in 90% depletion of AR protein in LNCaP cells by 24 hours of TG exposure (Table 1; Fig. 2A). Death of the LNCaP cells is completed by 72 to 96 hours post-TG exposure determined either via time lapse-digital microscopy, or trypan blue exclusion viability assays. These later results showed that the TG dose needed for 50% of the clonogenic cells to subsequently die after a 48-hour exposure (i.e., LD50 value) is 10 nmol/L, which is nearly identical to the IC50 for SER-CA inhibition (Table 1). This death cascade involves cell cycle arrest in G0 as detected by loss of Ki67 expression (i.e., 91% ± 2% Ki67+ in untreated to 25% ± 8% at 24 hours to >10% at 48 hours) and Cyclin D1 expression coupled with dephosphorylation and degradation of Rb (Fig. 2A). This is caused by a Ca+2-dependent activation of calcineurin resulting in dephoshphorylation of BAD (41), allowing BAD to bind to mitochondria releasing both apoptosis-inducing factor, which is translocated into the nucleus causing DNA fragmentation (30) and cytochrome C release (41), which activates the caspase cascade (30) inducing PARP cleavage (Fig. 2A), and completion of the process of cellular fragmentation into Annexin V–positive apoptotic bodies (30, 41, 42).
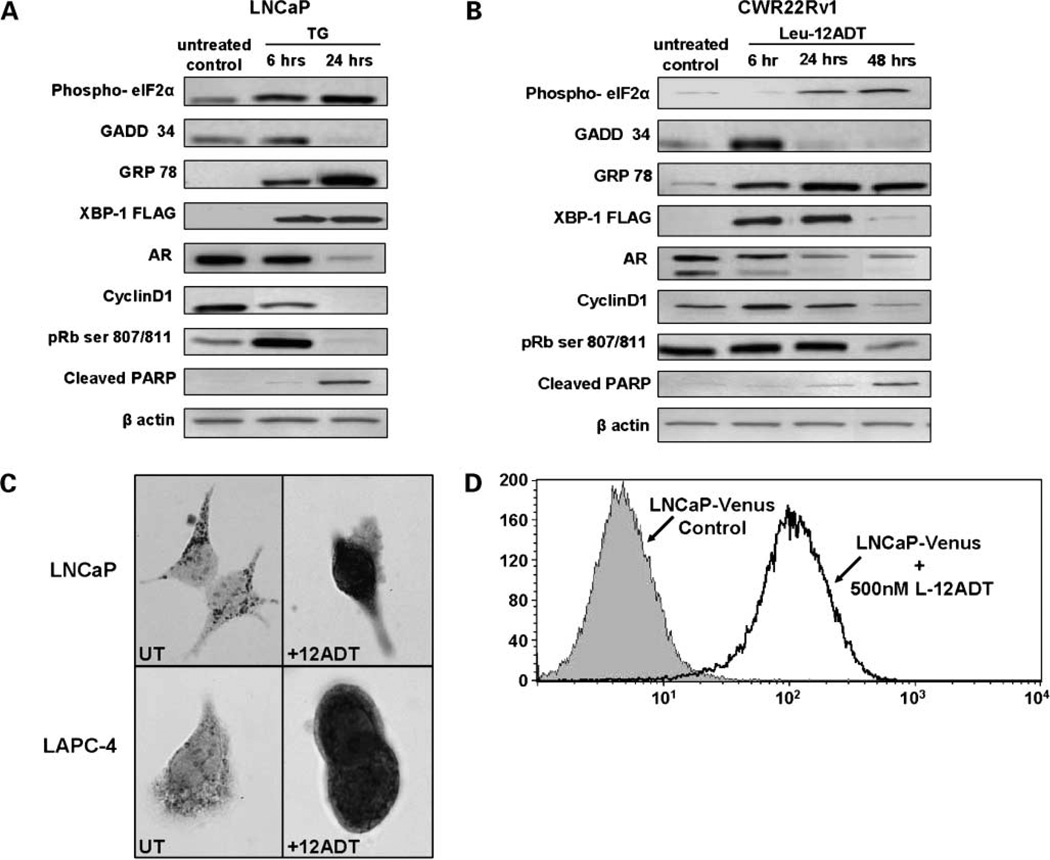
Figure 2.
A, changes in the level of indicated proteins in LNCaP cells during the first 24 h of exposure to 500 nmol/L TG. B, changes in the level of indicated proteins in CWR22Rv1 cells duringthe first 48 h of exposure to 500 nmol/L Leu-12ADT. C, translocation of apoptosis-inducingfactor protein from mitochondria to nucleus of LNCaP and LAPC-4 prostate cancer cells by 48-h exposure to 500 nmol/L TG. Left, untreated cells. Right, 12ADT-treated cell. D, XBP-1 processingin LNCaP prostate cancer cells treated with 500 nmol/L leucyl-12ADT (Leu-12ADT) for 24 h. LNCaP cells were transfected with an expression construct encoding a FLAG-tagged form of the XBP-1 gene fused to venus-GFP so that a fluorescent protein can only be produced when the fusion gene is processed by IRE1. Under non-ER–stressed conditions, the transgenic transcript is not spliced, and therefore, its translation is terminated at the stop codon between FLAG-tagged XBP-1-ΔDBD and the venus gene so that no fluorescent protein is produced. In contrast, during ER stress, the activated IRE-1 slices out a 26-nt intron in the XBP-1 portion, resultingin a frame shift of the fusion mRNA, allowingthe synthesis of a 45-kDa FLAG-tagged XBP-1-ΔDBD/venus fusion fluorescent protein. Thus, flow cytometric analysis of GFP expression documents that IRE1-dependent XBP-1 processing occurs in all cells within 24 h.
It is critical to evaluate whether the ability of TG to deplete AR protein is a general phenomenon observed in a wide spectra of phenotypic and genotypic diverse prostate cancer cell types or is a restricted response only observed with LNCaP cells, and whether AR depletion precedes cell death. Therefore, in addition to LNCaP cells, four other androgen ablation refractory human prostate cancer cell lines were tested for the ability of TG to deplete their AR protein. Each of these five lines was independently derived from a different androgen ablation refractory human prostate cancer and each grows when xenografted into castrated male nude mice documenting their androgen ablation resistance and each expresses wild-type or mutated AR protein (3). The lines include the following: LAPC-4, which is derived from a lymph node metastasis and expresses wild-type AR; VCap, which is derived from a vertebral bone metastasis and expresses gene-amplified wild-type AR (43); MDA-PCa-2b, which is derived from a bone metastasis and expresses an AR with two mutations in its LBD; CWR22Rv1 is derived from a primary human prostate cancer, which relapsed after castration during its serial transplantation as a xenograft in a nude male mouse and expresses multiple isoforms of AR including a constitutively activate exon 2–truncated AR lacking LBD as well as an exon 3–duplicated AR with a mutation in its LBD (22); and LNCaP, which is derived from a lymph node and expresses an AR with a single mutation in its LBD. By using this series of human prostate cancers, a representative spectra of androgen ablation refractory human prostate cancer cell types from primary to soft tissue to bone metastases including a range of AR isoforms from wild-type, gene amplified, mutated, and truncated can be evaluated for the generality of AR depletion by TG treatment.
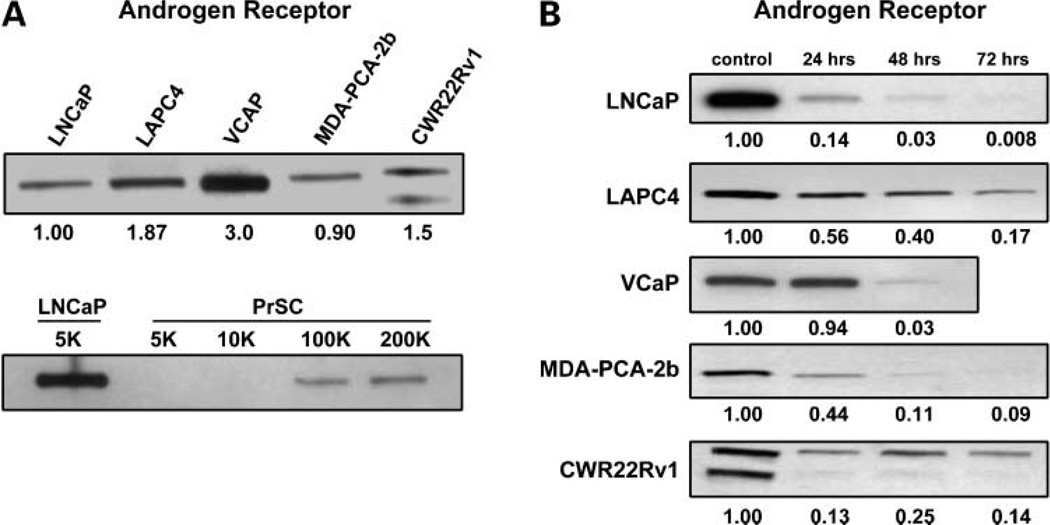
Regardless of whether the androgen ablation refractory prostate cancer lines have wild-type, mutated, or truncated isoforms, each expresses AR protein within 1- to 3-fold of each other when untreated and growing exponentially but at levels that are more ~100 times higher than wild-type AR protein expressed by normal prostate stromal cells in culture (Fig. 3A). The time course of AR protein response to 500 nmol/L TG in each of the cancer cell lines was evaluated over a 72-hour exposure period (Fig. 3B). This dose and time frame is chosen because after 48 hours, there is a dose-dependent increase in cell death for each line evaluated either via time lapse-digital microscopy, trypan blue exclusion viability assays, or using clonogenic survival assays (i.e., LD50, <100 nmol/L for each of the lines; Table 2). These results document that TG-induced AR protein depletion is a robust early response (i.e., within the first 24 hours) characteristically preceding cell death of androgen ablation refractory human prostate cancer cells, which occurs after 48 hours.
Figure 3.
Relative level of AR protein in the various androgen ablation refractory human prostate cancer cell lines compared with each other versus normal human prostate stromal cells (PrSCs). Values below the upper gel are the relative expression normalized to LNCaP cells. In the second blot, the level of AR in 5,000 LNCaP cells is compared with 5,000 to 200,000 prostate stromal cells. Kinetics of the depletion of AR protein in the various human prostate cancer lines after exposure to 500 nmol/L TG. Values below the each gel are the relative expression normalized to control (unexposed) cells for each line.
TG-Induced Depletion of AR Is via ER Stress-Induced Inhibition of Protein Synthesis
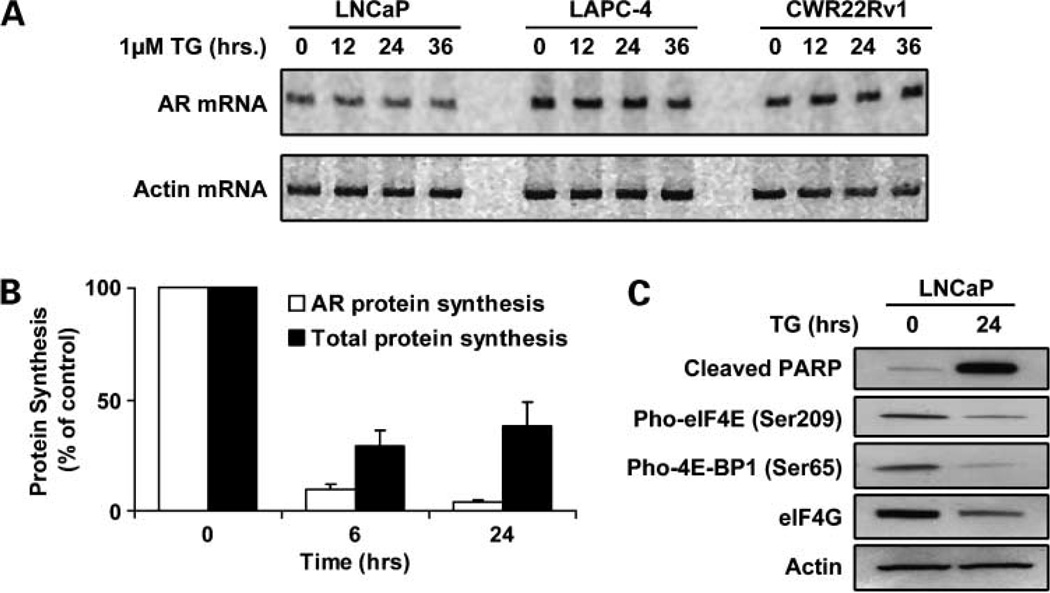
The depletion of AR protein by TG in androgen ablation refractory prostate cancers could occur via transcriptional, translational, and/or posttranslational affects. To evaluate the role of transcription, the level of AR mRNA was evaluated after TG exposure of LNCaP, LAPC-4, and CWR22Rv1 cells because these lines express mutated, wild-type, and truncated AR, respectively. Contrary to the kinetics of the depletion of AR protein, there is no change in AR mRNA in any of the lines tested after TG exposure (Fig. 4A).
Figure 4.
AR mRNA expression by indicated cell lines at various times after exposure to 1 µmol/L TG. Kinetics of the decrease in the rate of AR-specific protein synthesis versus total protein synthesis induced in LNCaP cells exposure to 1 µmol/L TG. Change in the level of cleaved PARP, phosphorylated elF4E, phosphorylated 4E-BP1, and elF4G protein in LNCaP cells exposed for 24 h to 1 µmol/L TG.
To evaluate whether translation is involved in AR protein depletion, the level of total protein synthesis per cell versus AR-specific protein synthesis per cell was determined using S35 incorporation into total versus immunoprecipitated AR protein in LNCaP cells after TG exposure. These studies documented that the rate of total protein synthesis per cell decreases by 70% to 80% after exposure to TG and that the rate of AR-specific protein synthesis decreases even more quickly and to a greater extent (i.e., >90%) than total protein synthesis in LNCaP cells (Fig. 4B).
These results raise the issue of the mechanism for such inhibition of AR and total protein synthesis. Previous studies have documented that translation of AR mRNA is a Cap-dependent translational process (44) and that such Cap-dependent protein translation involves formation of a phosphorylated elF4E/elF4G complex that is inhibited during apoptosis due to caspase-mediated cleavage of the translation scaffolding protein eIF4G (45). PARP cleavage is a hallmark of caspase activation during apoptosis and results in the fragmentation of the 116-kDa full-length PARP protein into an 89- and 24-kDa fragment (46). Figure 4C documents that the level of elF4G decreases while the level of the 89-kDa cleaved PARP increases after TG treatment. Likewise, both phospho-elF4E as well as phospho-4E-BP1 decrease, which allows 4E-BP1 to bind to and inhibit the binding of elF4E to elF4G (Fig. 4C), consistent with inhibition of Cap dependent translation of AR.
Besides Cap dependence, translation requires proper folding of nascent proteins within the ER. Such protein maturation requires high Ca+2, and when Ca+2 is depleted in the ER, this results in “ER stress” triggering the unfolded protein response (UPR; ref. 35). Three transmembrane proteins in the ER are sensors for ER stress and initiation of UPR. These include the PRK-like ER kinase, the activating transcription factor 6, and the kinase endoribonuclease, IRE1 (35). Each of these sensors is negatively regulated by binding to the chaperone protein GRP78. When misfolded proteins accumulate in the ER after Ca+2 depletion, they bind GRP78, liberating the three sensors. Once liberated, PRK-like ER kinase becomes activated and phosphorylates elF2α inhibiting its ability to initiate general protein synthesis, although it enhances translation of activating transcription factor 4, which translocates into the nucleus and induces the transcription of GADD34 (34). This leads to enhanced GADD34 protein, which is an activator for a phosphatase that dephosphorylate elF2α. Whether protein synthesis remains inhibited is determined by whether the ER pool of Ca+2 is restored or whether it remains depleted maintaining sufficient PRK-like ER kinase–dependent elF2α phosphorylation to overcome its dephosphorylation via GADD34-activated phosphatase. Therefore, the steady-state level of elF2α phosphorylation versus the level of GADD34 protein is a read out of the extent of UPR-induced PRK-like ER kinase activation. The steady-state level of phosphorylated elF2α protein increases and remains elevated even at 24 hours, unlike GADD34 that initially increases at 6 hours and then becomes undetectable in LNCaP cells exposure to 500 nmol/L TG (Fig. 2B). This is consistent with the >60% inhibition of general protein synthesis and >90% AR-specific protein synthesis detected at 24 hours post-TG exposure (Fig. 4B).
Activating transcription factor 6 is the second sensor of UPR that is liberated by its release from GRP78 in the presence of unfolded ER proteins. Once released, it is transported to the Golgi where it is processed, thereby liberating its NH2-terminal portion; this portion translocates to the nucleus where it activates the transcription of genes including GRP78 and the X-box binding protein-1 (XBP-1). Therefore, the steady-state level of GRP78 is a read out for UPR-induced activating transcription factor 6 activation. The steady-state level of GRP78 protein increases and remains elevated even at 24 hours of exposure of LNCaP cells to 500 nmol/L TG (Fig. 2A).
IRE1 is the third sensor of UPR that when liberated from GRP78 binding by unfolded ER proteins, dimerizes and auto-phosphorylates activating its RNase activity, allowing it to process XBP-1 m-RNA up-regulated by AT6 to produce a sliced transcript removing a stop codon so that it now codes for the synthesis of a larger (i.e., mature) form of XBP-1 protein that is a transcription factor in the UPR induction (35). To detect the production of this UPR-induced IRE1-dependent XBP-1 processing, LNCaP and CWR22Rv1 cells were transfected with an expression construct encoding a FLAG-tagged form of the XBP-1 gene fused to venus green fluorescent protein so that fluorescent protein can only be produced when the fusion gene is processed by IRE1 (38). This allows determination of the kinetics of induction of UPR on an individual cell basis using time-lapse fluorescence digital microscopy to follow induced IRE1-dependent XBP-1 processing (38). Using this method, an increase in the percent of fluorescent cells from both lines is detectable as early as 6 hours postexposure to 500 µmol/L TG and >80% all of the cells being positive by 24 hours of treatment (Fig. 2D). This construct also allows the use of anti-FLAG antibody to detect the IRE1-processed mature (i.e., larger size) XBP-1 protein. The steady-state level of FLAG-tagged XBP-1 protein increases by 6 hours and remains elevated even at 24 hours of exposure of LNCaP cells to 500 nmol/L TG (Fig. 2A and B).
Amino Acid Containing TG Analogues Retain the Ability to Deplete AR via ER Stress–Induced Inhibition of Protein Synthesis
The ability to deplete AR protein and induce cell death by each of the three amino acid containing TG analogues involves an induction of ER stress in each of the five prostate cancer lines. This is documented by an essentially equal kinetic of increase in the % fluorescent XBP-1/venus–transfected cells after exposure to 500 nmol/LTG (i.e., by 24-hour exposure to each of the amino acid analogues, >80% of the cells are fluorescent). Also such as the situation for TG, ER stress induced by each of the 3 amino acid–containing TG analogues results in phosphorylation of elF2α, increase in GADD34 and XBP-1 proteins (Fig. 2B), as well as a decrease in phosphorylated elF-4E and phosphorylated E4-BP1, resulting in the inhibition of protein synthesis (i.e., each of the amino acids analogues lowers protein synthesis by >50% at 24 hours of exposure), coupled with nuclear translocation of apoptosis-inducing factor (Fig. 2C), and PARP cleavage and depletion of AR, Cyclin D1, and phospho-Rb proteins (Fig. 2A and B).
Discussion
In normal prostate epithelial cells and androgen-dependent prostate cancer cells, androgens act to both stimulate growth and sustain survival through activation of AR signaling pathways. The absence of androgens activates cell death pathways in these androgen-dependent cell types, leading to regression of the normal prostate and produces significant clinical benefit in patients with prostate cancer. More recently, it has been shown that AR signaling is also maintained in androgen ablation refractory prostate cancer cells (5). Although these cells do not undergo apoptosis in the absence of androgens, they do activate cell death if the AR protein level is reduced below a critical level (5). These observations suggest that, regardless of the mechanism, an agent that depletes AR protein expression would prevent any AR signaling within both androgen-dependent and androgen ablation resistance prostate cancer cells. In the present studies using a series of five genetically diverse androgen ablation refractory human prostate cancer lines, TG-induced AR depletion is documented as a general phenomenon involving inhibition of Cap-dependent AR protein synthesis associated with apoptotic death, validating TG-based approaches for this devastating disease.
Although these studies document that TG can induce AR depletion as one means of activating death pathways in AR-expressing cells, the toxic effect of TG is not restricted to AR-expressing cell types (25, 26, 32). TG inhibition of the SERCA pump leads to a sustained elevation of intracellular calcium that can, in turn, activate ER stress response pathways, release apoptotic factors from the mitochondria, and directly activate calcium-dependent endonucleases within the nucleus (35). As expected, based on the critical importance of the SERCA pump for the maintenance of cell viability, TG potently kills all cell types, including AR-negative prostate cancer cells (32).
However, the recognition that TG can deplete AR in androgen ablation refractory prostate cancer cells suggests that TG-based therapy may be particularly suited for this tumor type. Besides its physiologic effects on growth and survival, androgens also interfere with the induction of cell death induced by chemotherapeutic agents (47). Androgen ablation is also now used to improve the efficacy of radiation therapy (48). The mechanisms underlying protective effect from the AR axis are not entirely clear but most likely involve regulation of the expression of survival factors such as fibroblast growth factor and vascular endothelial growth factor, and attenuation of the expression or activity of key cell death regulators such as BAX, BCL-xL, and the Caspases (47, 49, 50). These findings suggest that the beneficial effect of standard chemotherapies and radiation on prostate cancer cells could be greatly enhanced through combination with a TG-based treatment.
Any TG-based treatment strategy, however, requires a method to target TG selectively to prostate cancer cells because TG is a potent proliferation-independent killer of all cell types producing significant host toxicity when administered systemically (29). In addition, TG is a highly lipophilic molecule that is difficult to formulate for clinical use. To overcome these obstacles, we have developed an approach that both solubilizes TG and targets its potent activity to prostate cancer cells by coupling amine containing TG analogues to water-soluble peptides that are recognized as substrates by prostate-specific proteases, such as prostate-specific antigen and prostate-specific membrane antigen, or cancer-specific proteases, such as fibroblast activation protein (32, 35, 36). Because TG has no functional groups that would allow for coupling to peptides, we needed to identify a primary amine containing TG analogue that could be easily coupled to amino acids. We developed a computer model of TG bound to the SERCA pump (33). This model identified the O-8 side chain of TG as the position of the molecule that could be most readily modified without affecting the ability of TG to inhibit the SERCA pump (33). More recently, this modeling approach was validated by crystal structural analysis of 12ADT bound to the SERCA pump, which showed binding interactions that correlated very closely with our computer model (34). The model identified a hydrophobic channel within the SERCA pump that could interact with a hydrophobic linker substituted in the O-8 position. Using the model and an iterative synthetic approach, we document that if the linker were of sufficient length (i.e., 12 carbons), charged amino acids attached to the linker would be outside of the hydrophobic transmembrane channel and in the cytoplasm (31, 33). The model predicted that, with this linker length, any amino acids could be introduced making this approach adaptable to the creation of substrates for a variety of proteases (33).
On this basis, the 12ADT molecule was generated and coupled to a variety of amino acids (31). As predicted by the model and shown here, these amino acid–containing analogues retained the ability to deplete AR protein and induce cell death. These amino acid analogues can be covalently linked to peptide carriers producing water-soluble prodrugs that are selectively targeted to metastatic sites of prostate cancer (32). These prodrugs can be administered as doses that produce regression of human prostate cancers growing as xenografts in mice without significant toxicity to the host (32). On the basis of the preclinical results demonstrating efficacy in prostate cancer models, these prodrugs have entered clinical development as therapy for men with metastatic prostate cancer.
Acknowledgments
This work is dedicated to the memory of Leslie Meszler. We thank Leslie Meszler and Lillian Dasko-Vincent (Johns Hopkins Cell Imaging Core Facility) for assistance with the image analysis and Dr. Masayuki Miura, University of Tokyo, for the pCAX-F-XBP-1-ΔDBD-venus expression vector.
Grant support: NIH grant 5RO1CA124764 (S.R. Denmeade), NIH grant CA091409 (J.T. Isaacs), NIH Urology training grant T32DK07552 (D.J. Vander Griend), and Danish Cancer Society (S. Christensen).
Footnotes
Disclosure of Potential Conflicts of Interest
J.T. Isaacs and S.R. Denmeade: financial interest, Genspera, Inc., and Protox Therapeutics, Inc. J.T. Isaacs: grant support, Active Biotech. No other potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Shah RB, Mehra R, Chinnaiyan AM, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy programd. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 3.van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Fung KM, Day WV, Kropp BP, Lin HK. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int. 2005;5:8. doi: 10.1186/1475-2867-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 6.Ellis WJ, Isaacs JT. Effectiveness of complete versus partial androgen withdrawal therapy for the treatment of prostatic cancer as studied in the Dunning R-3327 system of rat prostatic adenocarcinomas. Cancer Res. 1985;45:6041–6050. [PubMed] [Google Scholar]
- 7.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 10.Hobisch A, Eder IE, Putz T, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 11.Chen T, WangLH, Farrar WL. Interleukin 6 activates androgen receptor- mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60:2132–2135. [PubMed] [Google Scholar]
- 12.Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- 13.Guo Z, Dai B, Jiang T, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 15.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 16.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 17.Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford OH, III, Gregory CW, Kim D, Smitherman AB, Mohler JL. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol. 2003;170:1817–1821. doi: 10.1097/01.ju.0000091873.09677.f4. [DOI] [PubMed] [Google Scholar]
- 19.Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4:236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 20.Jagla M, Feve M, Kessler P, et al. A splicing variant of the androgen receptor detected in a metastatic prostate cancer exhibits exclusively cytoplasmic actions. Endocrinology. 2007;148:4334–4343. doi: 10.1210/en.2007-0446. [DOI] [PubMed] [Google Scholar]
- 21.Lapouge G, Marcias G, Erdmann E, et al. Specific properties of a C-terminal truncated androgen receptor detected in hormone refractory prostate cancer. Adv Exp Med Biol. 2008;617:529–534. doi: 10.1007/978-0-387-69080-3_53. [DOI] [PubMed] [Google Scholar]
- 22.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyprianou N, English HF, Isaacs JT. Activation of a Ca2+-Mg2+-dependent endonuclease as an early event in castration-induced prostatic cell death. Prostate. 1988;13:103–117. doi: 10.1002/pros.2990130203. [DOI] [PubMed] [Google Scholar]
- 24.Martikainen P, Isaacs J. Role of calcium in the programmed death of rat prostatic glandular cells. Prostate. 1990;17:175–187. doi: 10.1002/pros.2990170302. [DOI] [PubMed] [Google Scholar]
- 25.Martikainen P, Kyprianou N, Tucker RW, Isaacs JT. Programmed death of nonproliferating androgen-independent prostatic cancer cells. Cancer Res. 1991;51:4693–4700. [PubMed] [Google Scholar]
- 26.Furuya Y, Lundmo P, Short AD, Gill DL, Isaacs JT. The role of calcium, pH, cell proliferation in the programmed (apoptotic) death of androgen-independent prostatic cancer cells induced by TG. Cancer Res. 1994;54:6167–6175. [PubMed] [Google Scholar]
- 27.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tombal B, Denmeade SR, Isaacs JT. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium. 1999;25:19–28. doi: 10.1054/ceca.1998.0005. [DOI] [PubMed] [Google Scholar]
- 29.Gong Y, Blok LJ, Perry JE, Lindzey JK, Tindall DJ. Calcium regulation of androgen receptor expression in the human prostate cancer cell line LNCaP. Endocrinology. 1995;136:2172–2178. doi: 10.1210/endo.136.5.7720667. [DOI] [PubMed] [Google Scholar]
- 30.Christensen SB, Andersen A, Kromann H, et al. Thapsigargin analogues for targeting programmed death of androgen-independent prostate cancer cells. Bioorg Med Chem. 1999;7:1273–1280. doi: 10.1016/s0968-0896(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 31.Jakobsen CM, Denmeade SR, Isaacs JT, et al. Design, synthesis, and pharmacological evaluation of thapsigargin analogues for targeting apoptosis to prostatic cancer cells. J Med Chem. 2001;44:4696–4703. doi: 10.1021/jm010985a. [DOI] [PubMed] [Google Scholar]
- 32.Denmeade SR, Jakobsen CM, Janssen S, et al. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Mhaka AM, Christensen SB, et al. Applying linear interaction energy method for rational design of noncompetitive allosteric inhibitors of the sarco- and endoplasmic reticulum calcium-ATPase. J Med Chem. 2005;48:3005–3014. doi: 10.1021/jm049319a. [DOI] [PubMed] [Google Scholar]
- 34.Sohoel H, Jensen AM, Moller JV, et al. Natural products as starting materials for development of second-generation SERCA inhibitors targeted towards prostate cancer cells. Bioorg Med Chem. 2006;14:2810–2815. doi: 10.1016/j.bmc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Denmeade SR, Isaacs JT. The SERCA pump as a therapeutic target: making a "smart bomb" for prostate cancer. Cancer Biol Ther. 2005;4:14–22. doi: 10.4161/cbt.4.1.1505. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal S, Brennen WN, Kole TP, et al. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry. 2008;47:1076–1086. doi: 10.1021/bi701921b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalrymple S, Antony L, Xu Y, et al. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res. 2005;65:9269–9279. doi: 10.1158/0008-5472.CAN-04-3989. [DOI] [PubMed] [Google Scholar]
- 38.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 39.Tombal B, Denmeade SR, Gillis JM, Isaacs JT. A supramicromolar elevation of intracellular free calcium ([Ca(2+)](i)) is consistently required to induce the execution phase of apoptosis. Cell Death Differ. 2002;9:561–573. doi: 10.1038/sj.cdd.4400999. [DOI] [PubMed] [Google Scholar]
- 40.Lin XS, Denmeade SR, Cisek L, Isaacs JT. Mechanism and role of growth arrest in programmed (apoptotic) death of prostatic cancer cells induced by thapsigargin. Prostate. 1997;33:201–207. doi: 10.1002/(sici)1097-0045(19971101)33:3<201::aid-pros9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 41.Denmeade SR, Lin XS, Tombal B, Isaacs JT. Inhibition of caspase activity does not prevent the signaling phase of apoptosis in prostate cancer cells. Prostate. 1999;39:269–279. doi: 10.1002/(sici)1097-0045(19990601)39:4<269::aid-pros7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Tombal B, Weeraratna AT, Denmeade SR, Isaacs JT. Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells. Prostate. 2000;43:303–317. doi: 10.1002/1097-0045(20000601)43:4<303::aid-pros10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 43.Korenchuk S, Lehr JE, L MC, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15:163–168. [PubMed] [Google Scholar]
- 44.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by Mammalian target of rapamycin. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 45.Marissen WE, Lloyd RE. Eukaryotic translation initiation factor 4G is targeted for proteolytic cleavage by caspase 3 during inhibition of translation in apoptotic cells. Mol Cell Biol. 1998;18:7565–7574. doi: 10.1128/mcb.18.12.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 47.Kimura K, Markowski M, Bowen C, Gelmann EP. Androgen blocks apoptosis of hormone-dependent prostate cancer cells. Cancer Res. 2001;61:5611–5618. [PubMed] [Google Scholar]
- 48.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 49.Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4:505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 50.Rosini P, Bonaccorsi L, Baldi E, et al. Androgen receptor expression induces FGF2, FGF-binding protein production, and FGF2 release in prostate carcinoma cells: role of FGF2 in growth, survival, and androgen receptor down-modulation. Prostate. 2002;53:310–321. doi: 10.1002/pros.10164. [DOI] [PubMed] [Google Scholar]