Abstract
Blocking efficacy of whole soymilk, non-fat soymilk, SuperBlock™, and non-fat milk was evaluated by performing standard protein immunoblotting procedures on both purified protein and crude nuclear extracts from HEK 293 cells. Non-fat soymilk was found to have superior blocking efficacy compared to other blocking agents in terms of high signal to noise ratio with the shortest blocking times. In addition, the presence of low concentrations of the detergent Tween-20 (0.05–0.1% v/v) in the wash buffer, as well as, antibody incubations significantly lessened the background compared to only including the detergent during wash steps.
Keywords: immunoblots, western blots
Success of immunoblotting (western blotting) depends on multiple factors. A major problem is to block non-specific sites without obscuring the target protein. A good blocking agent should eliminate the background by binding to non-specific sites on the membrane in the shortest time possible and give a high signal to noise ratio and be cost effective. The most commonly used blocking agents, non-fat dry milk, bovine serum albumin (BSA), casein, and several commercially available blocking solutions exhibit variability in their blocking characteristics depending on the antibodies used and target proteins being analyzed resulting in background labeling.
We have found that soymilk is an inexpensive alternative to the current pool of blocking solutions, which also shortens the blocking time, and the use of Tween-20 in more than the washing steps, also improves the efficiency of Western analysis. In the present study, we show that soymilk effectively blocks all non-specific sites on the PVDF membrane, with very short blocking times. The efficacy of blocking was determined by comparing whole soymilk and non-fat soymilk to the commercially available blocking agent SuperBlock™ (Pierce, Thermoscientific) and non-fat dry milk, a widely used blocking agent ever since Johnson et al. published its use in 1984 [1].
To demonstrate the blocking efficiency, western blotting was performed on purified Na,K-ATPase (Fig 1A), crude nuclear extracts from HEK 293 cells, (Fig 2A), and v-5 tagged Na,K-ATPase. Na,K-ATPase (0.5 μg) or HEK 293 nuclear extracts were loaded on a 12% acrylamide gel and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli [2]. Protein samples were mixed with Laemmli sample buffer (1:1:1 v/v 8M urea, 10% v/v SDS, 125 mM Tris-HCl, pH 6.8 with 5% β-Mercaptoethanol) to give a final volume of 50 μL. Separated proteins were stained with Coomassie (5% v/v glacial acetic acid, 5% v/v methanol, 0.1% w/v Coomassie Brilliant Blue R-250) and destained with 10% v/v acetic acid, 10% v/v isopropanol.
Fig 1.
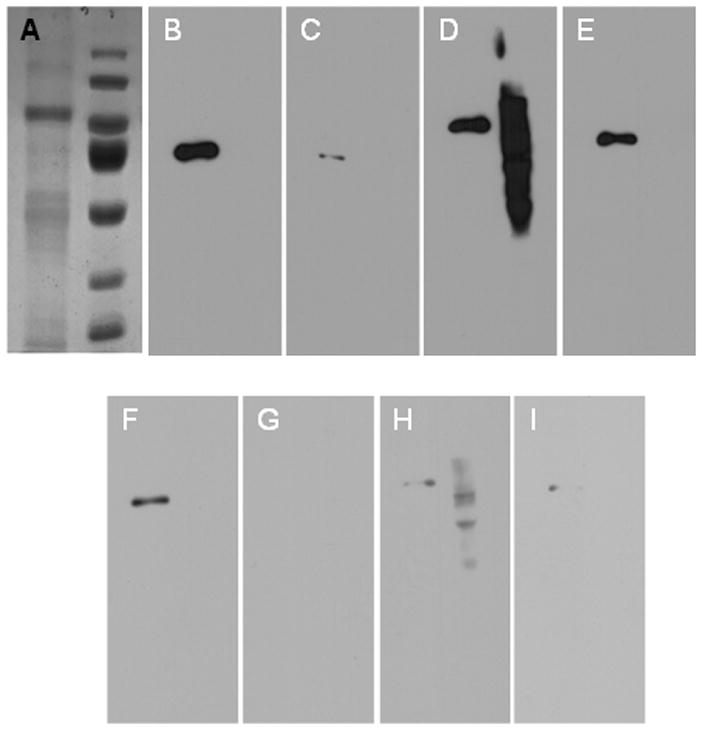
(A) Coomassie-stained SDS PAGE gel of purified Na,K-ATPase enzyme (left) and Pageruler pre-stained protein ladder (Fermentas, MD (right). (B-E) Comparison of blocking solutions in western blot analysis of purified Na,K-ATPase. The blots were blocked for 15 mins in non-fat soymilk (B), whole soymilk (C), SuperBlock™ (D), and non-fat dry milk resuspended in PBS (E). Blocking was followed by incubations with anti-Na,K-ATPase alpha antibody at 1:5000 for 1 hr and HRP conjugated anti-mouse secondary antibody for 1 hr at 1:5000 diluted in 1x PBS. Subsequent to antibody incubations, blots were washed three-times with 1x PBS + 0.1% v/v Tween-20. Detection was done using Pierce ECL Western Blotting Substrate and the exposure time of the film was 2 min. (F-I) Comparison of blocking solutions in relation to their signal sensitivity. Non-fat soymilk blocked blot exhibited higher sensitivity in terms of signal to noise ratio in the shortest exposure times such as 10 sec (F) compared to whole soymilk, SuperBlock™, and milk (G,H, and I respectively).Blocking was done as in B-E; detection was done using Pierce ECL Western Blotting Substrate and the exposure time of the film was 10 sec.
Fig 2.
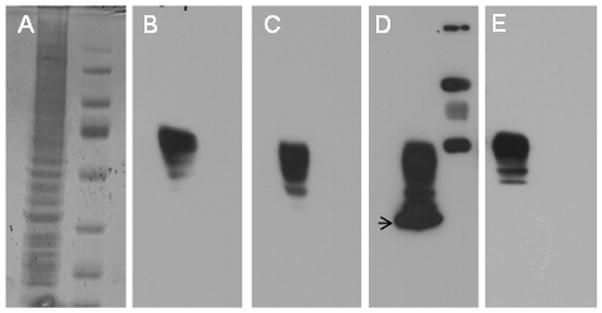
(A) Coomassie-stained SDS PAGE gel of the nuclear extract of HEK 293 cells. (B-E) Western blot analysis of nuclear extracts from HEK 293 cells blocked for 5 min with blocking solutions supplemented with 0.1% v/vTween-20. Non-fat soymilk (B), whole soymilk (C), SuperBlock™ (D), and non-fat milk (E) appeared to have less background in the presence of small amounts of Tween-20 in the blocking solution and antibody incubations and was accomplished in shortest blocking times such as 5 min. Blocking was followed by the western blotting procedure as described in Fig 1. Arrow indicates the degradation products of Na,K-ATPase beta-subunit.
After electrophoresis, proteins were transferred onto PVDF membranes (Millipore) by electrophoretic blotting in 10mM CAPS, 10% v/v MeOH, pH 11.0, for 2 hr at 180 mAmp constant current. The PVDF membrane was blocked with various blocking solutions for 5–15 min. In this study, we used non-fat soymilk, whole soymilk, commercially available Superblock™, and 10% (w/v) non-fat dry milk suspended in Phosphate-Buffered Saline (PBS; 137 mM NaCl, 2.7 mM KCl, 100 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4) as the blocking solutions. Subsequently, membranes were incubated with 1:5000 (diluted in PBS) mouse anti-Na,K-ATPase alpha-subunit, anti-Na,K-ATPase beta-subunit (Affinity Bioreagents Inc.), or anti-v-5 (Invitrogen) for 1h at room temperature (RT). After three washes with PBS/0.1% (v/v) Tween-20 for 10 min each, the PVDF membranes were incubated with HRP-conjugated goat anti-mouse at 1:5000 (Pierce) for 1h at RT. The membrane was then washed three times with PBS/0.1% v/v Tween-20 and the proteins were visualized by chemiluminescent detection of peroxidase activity using the SuperSignal™ substrate kit (Pierce).
We observed that non-fat soymilk consistently outperformed other blocking solutions in terms of high signal to noise ratio in the shortest exposure times (even as little as 10 sec) (Fig 1B-I). Although whole soymilk blocked non-specific sites equally well, it tended to obscure the target protein to some extent resulting in less sensitivity (Fig 1). However, in blots exposed to film for longer times (~10 min), whole soy appeared to have blocked unspecific sites better than the other blocking solutions (data not shown). We also observed that a quick rinse (1x PBS, 10 sec) after blocking in soymilk increases the signal intensity, likely due to removing loosely associated blocking proteins from the target protein and increasing its accessibility to primary antibody. We found that soymilk was the best blocking agent for 3 different sets of antibodies, anti-Na,K-ATPase alpha-subunit, anti-Na,K-ATPase beta-subunit, and anti-v5. Although 10% nonfat dry milk blocked well in these experiments, its blocking efficiency has been much more variable over the past year in our hands (unpublished observations). For example, it performed especially poorly on blots that were stripped for reuse and subsequently blocked with 10% w/v milk (data not shown). Moreover, casein (i.e. the primary blocking protein in nonfat dry milk) is a phosphoprotein. Thus, nonfat dry milk is not suitable as a blocking solution for phospho-specific antibodies (e.g. anti-phosphoserine and anti-phosphothreonine). Indeed, this is an instance where SuperBlock™ serves as an appropriate alternative to nonfat dry milk.
In addition, we observed that using small amounts (0.05–0.1% v/v) of the detergent Tween-20 within the blocking solutions, as well as, within the antibody-containing solutions eliminated background to a greater extent without any effect on immunoreactivity (data not shown). This observation was ubiquitous regardless of blocking agent or antibody being used. The addition of Tween-20 to all the solutions gave much more consistent results; it cut down dramatically on blot to blot variability. For example: Fig 1 represents block solution without Tween-20 (15 min block time) and Fig 2 represents block solution with Tween-20 (5 min block time). The presence of Tween-20 gave comparable or better results in shorter blocking times (5 min). The improvement was only for Superblock™ and sometimes non-fat dry milk.
In this report, we present evidence that supports nonfat soymilk as an inexpensive alternative to SuperBlock™. In some cases, our western blot results indicate that soymilk may be a better blocking agent than SuperBlock™. For example, we observed that SuperBlock™ does not sufficiently mask the molecular weight standard proteins (Figs 1 and 2) and degradation products of beta subunit of Na,K-ATPase (see Fig 2). In many instances, longer film exposure times are necessary to resolve targeted proteins within sample lanes. Once again soymilk seems more desirable as the blocking solution in these cases. In addition, given that soymilk is casein-free [3], biotin-free, and devoid of animal proteins altogether it serves as viable alternative compared to nonfat dry milk, and an inexpensive alternative to SuperBlock™, roughly $0.25 versus $5.00 per immunoblot. We presume that the applicability of soymilk as the blocking agent may not be limited to immunoblotting but can be extended to other techniques such as ELISA [4]. Preliminary findings from this study have been reported by Milanick et al. [5].
Soymilk is an inexpensive, and in some cases more efficient, blocking agent than Superblock™ and might be better than non-fat dry milk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson DA, Gautsch JW, Sportsman JR, Elder JH. Improved technique utilizing nonfat dry milk for analysis of proteins and nucleic acids transferred to nitrocellulose. Gene Anal Tech. 1984;1:3–8. [Google Scholar]
- 2.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 3.AutismWeb.com: The GFCF (Gluten-Free, Casein-Free) Diet for Autism Spectrum Disorders.
- 4.Santa-Marta M, da Silva FA, Fonseca AM, Goncalves J. HIV-1 Vif can directly inhibit apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G-mediated cytidine deamination by using a single amino acid interaction and without protein degradation. J Biol Chem. 2005;280:8765–8775. doi: 10.1074/jbc.M409309200. [DOI] [PubMed] [Google Scholar]
- 5.Milanick M, Virgin GK, Gatto C. Soymilk is an excellent and economical blocking agent for immunoblots. Bioscence Letter. 2009;35:49–50. [Google Scholar]


