Abstract
In 1975, 50 year-old Americans could expect to live slightly longer than most of their Western European counterparts. By 2005, American life expectancy had fallen behind that of most Western European countries. We find that this growing longevity gap is primarily due to real declines in the health of near-elderly Americans, relative to their Western European peers. We use a microsimulation approach to project what US longevity would look like, if US health trends approximated those in Western Europe. The model implies that differences in health can explain most of the growing gap in remaining life expectancy. In addition, we quantify the public finance consequences of this deterioration in health. The model predicts that gradually moving American cohorts to the health status enjoyed by Western Europeans could save up to $1.1 trillion in discounted total health expenditures from 2004 to 2050.
Keywords: disability, mortality, international comparisons, microsimulation, USA, Europe
Introduction
The populations of the United States and Western Europe have experienced large gains in life-expectancy over the last century. U.S. life expectancy at birth increased from 61 years in 1933 to 78 years in 2004. In many other developed countries, age-specific death rates have declined exponentially over this period (Tuljapurkar et al., 2000). During the first half of the 20th century, it was large declines in infectious diseases that drove down these mortality rates, particularly for the young. But in the second half of the 20th century, it was reductions in mortality among the elderly, rather than the young, that propelled increases in life expectancy (Olshansky and Carnes, 2001).
During the first half of the 20th century, when infectious diseases were on the decline, life expectancy across developed countries converged (White, 2002). The second half, however, witnessed divergence, as the US began to fall behind other developed countries in terms of life expectancy (Oeppen and Vaupel, 2002). So far, little is known about the causes and consequences of this widening gap (Lee, 2003).
The U.S. allocates the highest share of national income to health expenditures, yet does not lead the world in life expectancy. This has been used by some to suggest the inefficiency of the U.S. health care system. However, a recent study by Preston and Ho (2009) questions this conclusion by demonstrating that the U.S. ranks high in terms of life expectancy for people already diagnosed with chronic or terminal illness. They conclude that the health care system, at least in terms of curative treatment, is unlikely to be responsible for the deterioration in life expectancy. Instead, these findings point toward poor health behaviors and prevention strategies in the US population.
Indeed, many studies have shown that the health of middle-aged Americans, and health behaviors such as smoking and obesity, are much worse than those of Western Europeans (Banks et al., 2006; Andreyeva et al., 2007; Thorpe et al., 2007). This raises the question as to whether health behaviors have contributed to the divergence in life expectancy, and the question of where in the life-cycle the deterioration in U.S. life expectancy originates. Understanding both the fact and the source of deteriorations in health is a prerequisite for intervening against such trends.
In this paper, we argue that the worsening health of middle-aged Americans relative to their Western European counterparts is responsible for this disparity. Furthermore, we quantify the fiscal consequences of this gap. We use a dynamic microsimulation model calibrated to match historical U.S. health and longevity dynamics over the life course. We use the model to simulate the total longevity, disability, and financial costs to the US population of its poorer health status. We also quantify the gains that could be realized over time by gradually transitioning US cohorts to the health levels enjoyed by their Western European counterparts.
For the balance of the paper, the term “European” refers to the population of a sub-group of Western European countries (Denmark, France, Germany, Greece, Italy, The Netherlands, Spain and Sweden). This group of countries is quite representative of heterogeneity in health and socio-economic conditions within Western Europe, and growth in life expectancy has been higher than in the U.S. both for this group of countries and for an enlarged group of Western European countries (EU-15).
The paper is structured as follows. We first describe the data on mortality and health in the U.S. and Europe. Then, we describe the model that is used to describe the long-term economic consequences of these trends. Next, we use the model to quantify the effect of differences in health on longevity and government expenditures/revenues, and finally we discuss the results.
Mortality, Health Behaviors, and Health in the US and Western Europe
Cross-Country Differences in Mortality
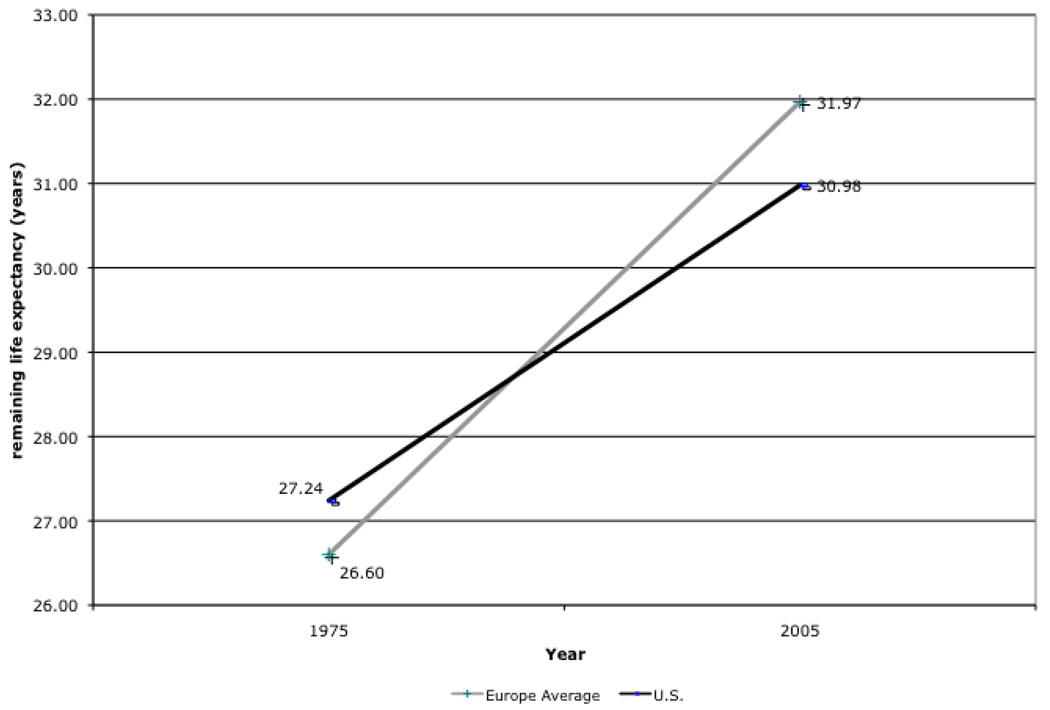
In 1975, 50 year-old Americans could expect to live 0.6 years longer than their counterparts residing in a group of 15 Western European countries. 50 year-old Americans lived on average 27.3 years, compared to 26.6 years for the 15 countries originally forming the European Union (Austria, Belgium, Denmark, Finland, France, Ireland, Italy, The Netherlands, Norway, Portugal, Spain and Sweden, United Kingdom and West Germany). These estimates were obtained from the Human Mortality Database project (www.mortality.org). Over the ensuing decades, however, Western European life expectancy grew more quickly. As Figure 1 shows, a 50 year-old American in 2005 could expect to live for 31 years, compared to 32 years in Europe (32.8 in France and Italy). From 1975 to 2005, life expectancy grew by 5.37 years in Europe compared to just 3.75 years in the U.S. Only Denmark experienced a lower growth in life expectancy over this period (2.9 years).
Figure 1.
Remaining Life Expectancy at Age 50: U.S. – Europe (EU-15) Differences from 1975 to 2006
Source: Human Mortality Database period life tables for 1975 and 2005. EU 15 countries are: Austria, Belgium, Denmark, Finland, France, Ireland, Italy, Luxembourg, The Netherlands, Norway, Portugal, Spain, Sweden, U.K. and West Germany. Weighted average using population size age 50.
The 1.6 year life expectancy gap between the U.S. and the EU-15 countries implies a non-trivial welfare loss. For example, using $100,000 as a lower-bound estimate of the value of a statistical life year (Viscusi and Aldy, 2003), this would represent at least a $700 million dollar disadvantage for the current generation of 50 year-olds. While these differences are not as large as within-country differences in health (across race for example), it is worth noting that these cross-country differences have emerged in spite of similar levels of economic development across countries.
Cross-Country Differences in Health Behaviors
While US life expectancy was deteriorating in relative terms, chronic illnesses associated with more sedentary lifestyles were spreading (Goldman et al., 2005; Lakdawalla et al., 2005). Due to data limitations, it is hard to assess whether trends in chronic disease have spread more rapidly in the US, but historical data do exist on obesity and smoking, two important health behaviors that contribute to chronic disease.
Both the levels of obesity and growth in obesity are higher in the U.S. than in Europe (based on OECD Health Data, at http://www.ecosante.fr). In 1975, 15% of Americans were obese, while obesity rates in European countries such as France, the Netherlands and Spain were less than 8% as recently as the 1980s. By 2005, the obesity rate in the U.S. was well over 30%, while the European average remains close to 12%.
Reductions in the costs of food consumption and technological innovations that led to more sedentary work are two key explanations for the U.S. trend (Lakdawalla and Philipson, 2009). Cutler et al. (2003) argue that these changes may have taken place more slowly in Europe due in particular to stricter food regulation. Obesity elevates the risk of various health conditions such as hypertension, diabetes and heart disease (Colditz, 1995; Willett, 1995). In that sense, it has the potential to explain part of the difference in life expectancy emerging over time.
Tobacco consumption trends are somewhat harder to interpret definitively. On the one hand, tobacco consumption has fallen by more in the U.S. than in Europe. Today, based on data from OECD Health Database (http://www.ecosante.fr), tobacco consumption is higher in Europe (1750 grams per capita vs. 1315 for the U.S.), but in 1975, it was much higher in the US (3506 grams per capita vs. 2540 grams in Europe). This means that the near-elderly Americans are less likely to be smoking now than their European counterparts. On the other hand, these American cohorts are much more likely to have ever smoked, which may have independent effects on health. One plausible explanation for this rapid decrease is that smoking cessation programs have been more effective in the U.S. than in Europe. For example, Cutler and Glaeser (2006) argue that 50% of the gap in current smoking status is due to differences in beliefs about the health effects of smoking.
The health consequences of smoking depend in part on the length of exposure to tobacco, or lifetime consumption, rather than consumption at a point in time. For example, Rogers and Powell-Griner (1991) estimated that for males (females), compared to current smokers, former smokers could expect to live 3.7 (5.2) additional years and those who never smoked an additional 2.4 (1) years. Based on the OECD data, it is not clear whether lifetime exposure to tobacco – in terms of cigarettes smoked -- is greater among Americans or Europeans. In addition, the consequences of smoking for life expectancy will also depend on changes over time in the age composition of smokers; this may vary across countries. In sum, it is unclear whether trends in tobacco use have contributed to worsening or improving health for Americans, compared to Europeans.
Cross-Country Differences in Health
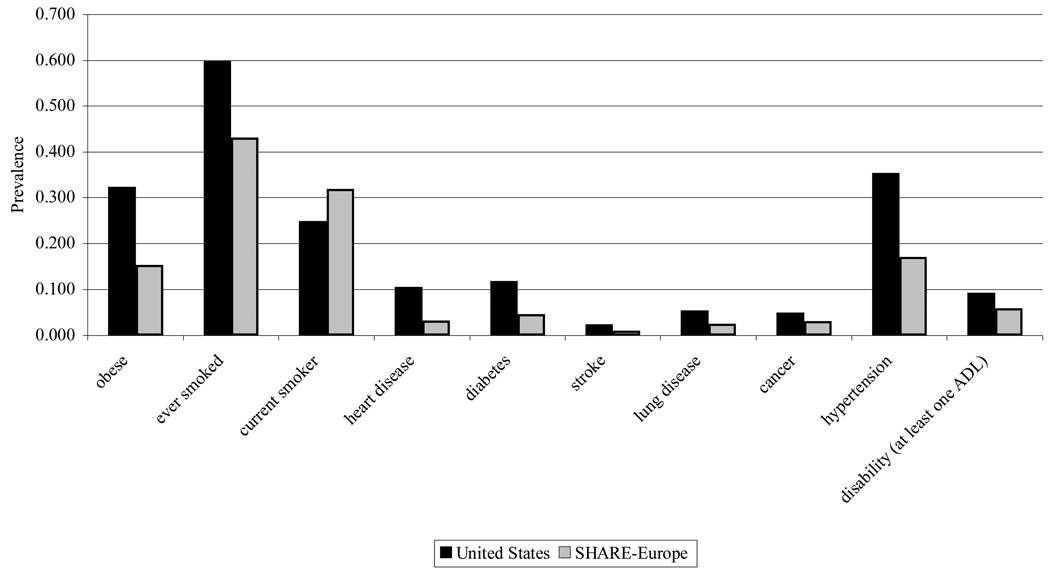
Figure 2 displays the possible health consequences of these divergent trends in health behavior: The US prevalence of different types of chronic disease and risky behavior is much higher than in a selected but representative group of European countries (Banks et al., 2006; Andreyeva et al., 2007). The figure displays these data among the 50–55 year old population using internationally comparable survey micro-data in Denmark, France, Germany, Greece, Italy, the Netherlands, Spain and Sweden. We used the 2004 waves of the Health and Retirement Study in the U.S. and the Survey of Health Ageing and Retirement in Europe (http://www.share-project.org/) to produce these numbers. Properly weighted, both surveys are representative of the age 50+ population in each country. Questions on health conditions are very similar. The text of those questions is reproduced in Table A.1 of the online appendix. Data from Switzerland are not used because of small sample sizes and low response rates (lower than 50%) and data from Austria are not used for lack of sampling weights. Except for the behavioral measure of current smoking status, Americans look worse along all dimensions of health. Americans are about twice as likely to have hypertension, twice as likely to be obese, and twice as likely to have diabetes. As we demonstrate later, these differences are unlikely to be explained by differences in diagnosis or reporting. For example, the prevalence of stroke—a condition that rarely goes undiagnosed—is twice as low in Europe as in the U.S. On the other hand, the prevalence of cancer may be higher in the U.S. because of higher screening rates (Howard et al., 2009).
Figure 2.
Health Differences between U.S. and SHARE-Europe Population Aged 50–53
Source: Health and Retirement Study 2004 and Survey of Health Ageing and Retirement in Europe (SHARE) 2004 (Denmark, France, Germany, Greece, Italy, the Netherlands, Spain, Sweden). Data from Austria not included because of lack of appropriate population weights and Switzerland because of low response rate and small sample. Sample weights used. Question text and definition are available in Technical Appendix. Obese status defined as body-mass index greater than 30. ADL are limitations in activities of daily living.
Clearly, the apparent gaps in observed health status will contribute to a gap in longevity, but it is not obvious how big the contribution will be. For instance, there are well-documented longevity gaps across racial and socioeconomic lines within countries. These are not fully explained by differences in observed health status. The question is whether “being American” is an independent mortality risk factor, in the same way that being poor or being black increase risk above and beyond observed health.
Microsimulation Model of Health and Economic Dynamics
Background
Compared to Europe, the US has enjoyed smaller longevity increases, and worsening health along many, but not all dimensions. Understanding the fiscal consequences of these trends requires that we account for the varied nature and magnitudes of health and mortality trends. To do this, we construct a transition model that relates current health to the future risk of mortality. Given our interest in fiscal consequences, we also need a model linking health and economic outcomes. Both the epidemiological and economic literatures contain complex models of each, but few integrate both.
The current social science literature features several well-known and complementary approaches for measuring population health and projecting future disease burden and mortality—including models by Manton and co-authors (Manton, Singer, and Suzman, 1993), Lee (Lee, 2000), and Hayward (Hayward and Warner, 2005). Across these models, there is an underlying trade-off between the complexity of the data required, and the broad applicability of the model.
We use a model that considers the dynamic interplay among a large number of individual outcomes, including health status, and economic behavior. The model is an extension of the Future Elderly Model (FEM) (Goldman et al., 2004). The FEM consists of a transition model across health states that allows for unobserved heterogeneity (frailty) and dynamic population simulations. In that sense, it is well-equipped to analyze the effect of health differences on longevity and public financial liabilities, as it allows for complex interactions between multi-dimensional measures of health and economic outcomes.
Functioning of the Dynamic Model
Overview
The Future Elderly Model (FEM) was developed to examine health and health care costs among the elderly Medicare population (age 65+) (Goldman et al., 2004). The most recent version now projects these outcomes for all Americans aged 50+ using data from the Health and Retirement Study. The defining characteristic of the model is its use of real individuals, rather than synthetic cohorts. This allows for more heterogeneity in behavior than would be allowed by a cell-based approach. The model has three core components:
The initial cohort module predicts the health and socio-economic outcomes of new cohorts of 50 year-olds. This module calibrates the Health and Retirement Study (HRS) to reflect population trends observed in younger populations from the National Health Interview Study (NHIS). It allows us to generate new cohorts as the simulation proceeds, so that we can measure outcomes for the age 50+ population in any given year.
The transition module calculates the probabilities of entering and exiting various health states, and the likelihood of various financial outcomes. The module takes as inputs risk factors such as smoking, weight, age, and education, along with lagged health and financial states. This allows for a great deal of heterogeneity and fairly general feedback effects. The transition probabilities are estimated from the longitudinal data in the Health and Retirement Study (HRS). These probabilities are then used to simulate the path of individuals in the simulation.
The policy outcomes module aggregates projections about individual-level outcomes into policy outcomes such as taxes, medical care costs, pension benefits paid, and disability benefits. This component takes account of public and private program rules to the extent allowed by the available outcomes. Because we have access to HRS-linked restricted data from Social Security records and employer pension plans, we are able to realistically model receipt of retirement benefits.
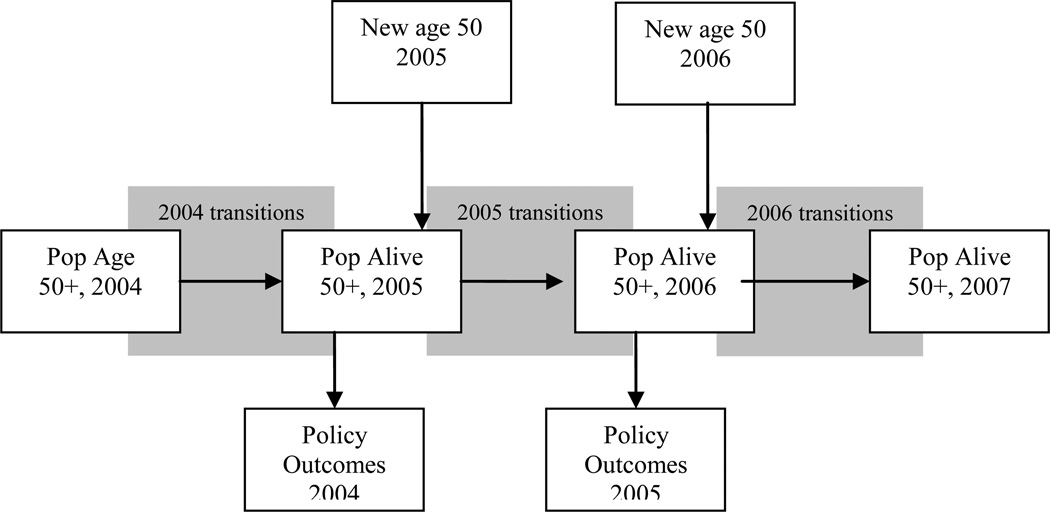
Figure 3 provides a schematic overview of the model. We start in 2004 with an initial population aged 50+ taken from the HRS. We then predict outcomes using our estimated transition probabilities. Those who survive make it to the end of that year, at which point we calculate policy outcomes for the year. We then move to the following year, when a new cohort of 50 year-olds enters (with a different health profile). These entrants, along with the survivors from the last period, constitute the new age 50+ population, which then proceeds through the transition model as before. This process is repeated until we reach the final year of the simulation. In what follows, we give an overview of each component of the model. The online technical appendix accompanying this paper contains more details on the implementation.
Figure 3.
Overview of the Future Elderly Model
Initial Cohort Module
We need to characterize outcomes for the age 50+ population. Hence, we need to predict the characteristics of the current and future 50 year-old population, in terms of health, demographics, and economic outcomes. Unfortunately, the HRS does not include respondents younger than age 50; therefore, the characteristics of tomorrow‘s 50 year-olds must be modeled using data on people who are younger than age 50 today.
We estimate trends in the health of 50 year-olds using two methods. First, we use the method described in Goldman, Hurd et al. (2004) to calculate trends in disease prevalence from the National Health Interview Surveys (NHIS). This method adopts a synthetic cohort approach and uses historical age-year prevalence estimates to “age-forward” prevalence rates, taking account of cures and mortality. The trends we estimate from this procedure are relatively close to other independent estimates, as documented in the online appendix. For outcomes other than disease prevalence, we use existing estimates, all of which are documented in the appendix.
Two important trend assumptions are made in the status-quo for obesity and smoking. The trend for obesity comes from Ruhm (2007), who predicts prevalence in different classes of obesity up to 2030. In particular, using his predictions, obesity prevalence in 2050 would near 50%. Although this is likely a “doomsday” prediction, it reveals a worst-case scenario which serves as a useful benchmark. Similarly, Levy (2006) uses a simulation model to predict smoking prevalence. We extended those projections up to 2050. The prevalence of current smokers is predicted to decrease among age 50–54 individuals from 25% to less than 10% by 2050.
Second, we use the 50 year-old HRS respondents from 2004 as a template for future cohorts of 50 year-olds. Due to sample size consideration, we consider individuals aged 50–53 as our initial cohort of “50 year-olds.” We adjust their health to match the levels of health predicted for future 50 year-olds, according to the methods discussed earlier. For example, if obesity is projected to rise in 2020, we increase the rate of obesity within the cohort of 50 year-olds, by reassigning enough non-obese individuals to obesity status. Since obesity is correlated with other outcomes such as hypertension and diabetes, we reassign obesity status so that those at greatest risk are more likely to be designated as being obese.
The reassignment is governed by a latent health model with correlated unobservables. An individual‘s disease status is a function of the mean population probability of the disease, along with a random error term. For an individual, the error terms are correlated across diseases. This builds on the possibility that, for instance, the occurrence of diabetes and hypertension are correlated. The bottom panel of Table 1 lists all the outcomes that we consider in this latent health model. There are seven binary outcomes: hypertension, heart disease, diabetes, fair or poor self-reported health, labor force participation, insurance status and positive wealth. There are three ordered outcomes: BMI status, smoking status and functional status in the transition model. Finally, there are five continuous outcomes measuring pension eligibility and savings.
Table 1.
Outcomes in the Future Elderly Model
| Initial Conditions Outcomes | |
|---|---|
| Economic Outcomes | Health Outcomes |
| Employment | Hypertension |
| Earnings | Heart Disease |
| Wealth | Self Reported Health |
| Defined Contribution | |
| Pension Wealth | BMI Status |
| Pension Plan Type | Smoking Status |
| AIME (average indexed monthly earnings) | Functional Status |
| Social Security Quarters of Coverage | |
| Health Insurance | |
| Transition Outcomes | |
| Economic Outcomes | Health Outcomes |
| Employment | Death |
| Earnings | Heart |
| Wealth | Stroke |
| Demographics | Cancer |
| Health Insurance | Hyper-tension |
| Disability Insurance Claim | Diabetes |
| Defined Benefit Claim | Lung Disease |
| SSI Claiming | Nursing Home |
| Social Security Claiming | BMI Status |
| Smoking Status | |
| Functional Status | |
Notes: More detail on each outcome in online technical appendix.
Each of these outcomes depends on fixed characteristics such as race, education, gender and marital status. We also consider cancer, lung disease and stroke as fixed covariates, because their prevalence is very low in this population (age 50–53). Estimates are presented in the online appendix.
Finally, the size of the entering cohort is adjusted to reflect population projections from Census by gender and race. We also adjust the size of the initial new cohort in 2004 to Census estimates by gender, race, and ethnicity.
Transition Model
The transition model tracks movement among states as a function of risk and demographic factors. The online technical appendix provides details on the parametric structure, estimation, and validation of the model. These consist of first-order Markovian limited-dependent variable models (probits, ordered probits, multinomial logits, censored regressions, etc). We enumerate and discuss all the key inputs and outputs of the model, and how they are measured.
The data come from the 1992 to 2004 biennial waves of the HRS. We consider both health and economic outcomes, all of which are listed in the top panel of Table 1. The table lists several groups of variables: diseases, risk factors, functional status, labor force and benefit status, financial resources, nursing home residence, and death. At a particular point in an individual‘s life, the model takes as inputs risk factors, along with the individual‘s lagged disease status, functional status, labor force and benefit status, financial resources, and nursing home status. The outputs are current disease status, functional status, labor force and benefit status, financial resources, and nursing home status. More detail on variable measurement is presented below.
Transition rates are allowed to differ across demographic and economic groups. In particular, we allow differences by gender, race and ethnicity, education, and marital status. Transition equations are estimated using 7 waves of HRS data. We assess the fit of the model by simulating 2004 outcomes for the 1992 HRS respondents; these are then compared with actual outcomes. We use half the sample for estimation and the other half for simulation. In general, the model fits the data quite well, with a close correspondence between predicted and actual outcomes in most areas. Complete results can be found in Table A.9 of the online technical appendix.
Model Restrictions
We make several restrictions on the transition risks permitted in the model. First, we only allow feedback from diseases where clinical research supports such a link, based on consultation with several physicians from the Southern California Evidence-Based Practice Center. For example, we allow hypertensive patients to have higher risk of heart disease, but we do not allow hypertensive patients to have higher risk of cancer. These clinical restrictions are documented in the online technical appendix and elsewhere (Goldman et al., 2004) and generally do not affect the results of the simulations done here (many of the restrictions are in fact valid using statistical tests).
Another important restriction we impose is that economic outcomes do not feed back into health status. Although controversial across disciplines, this is consistent with the findings from recent studies looking at the elderly population (Adams et al., 2003). SES innovations (shocks) do not appear to have a causal effect on health outcomes in this age range. The correlation between SES and health appears to be generated by feedback effects from health to economic status, most notably through the effect of health shocks on labor supply and medical spending. Also playing a role are predetermined (earlier) events or common factors (genetics, etc) that induce a non-causal correlation between SES and health. Both these factors are accounted for in the estimation.
This assumption of no feedback from SES to health does not qualitatively affect our thought-experiment. Because we keep economic outcomes constant at U.S. levels when changing the health of Americans at baseline, failure of this assumption would lead us to underestimate the effects of improving US health. In earlier estimation stages of the transition equations, we found that very few of the economic variables were statistically significant. Hence, this assumption tends toward parsimony and tractability.
Policy Outcomes
The model simulates a number of relevant health and economic outcomes for individuals. First, we consider a set of health outcomes such as life expectancy, healthy life expectancy (no ADL limitations), and medical expenditures. Average medical expenditures by disease and demographic group are calculated from two sources. For those younger than age 65, we use the Medical Expenditure Panel Survey (MEPS) and include in medical expenditures the respondents‘ medical care costs and the cost of drugs. For those above age 65, we use the Medicare Current Beneficiary Survey (MCBS). Some adjustments are made such that aggregate expenditures match National Health Accounts estimates (see online appendix).
In addition to the individual outcomes, the model predicts tax revenues and medical expenditures by the Federal Government for the age 50+ population. As part of the predicted medical expenditures, we also predict expenditures by source, including those by Medicare and Medicaid. Next, we compute Social Security retirement benefits for those predicted to receive such benefits. We account for spouse and survivor benefits. We also compute disability insurance (DI) benefits and Supplemental Security Income (SSI). As Goldman et al. (2010) report, the simulation is able to replicate basic fiscal aggregates for 2004. Where deviations exist, we adjust our predictions from the model so that we match national aggregates in 2004.
Simulation Methods
For population scenarios, the simulation starts with the existing age 50+ population in the 2004 wave of the HRS. The microsimulation is stochastic, meaning that transitions are randomly drawn from the joint distribution of state variables, which is estimated from the HRS. This process is repeated a number of times to ensure independence from any particular sequence of random numbers. We average more than 100 replications.
These simulations are bound to be imprecise due to two sources of uncertainty. First, there is simulation noise due to the use of pseudo-random draws in drawing state-variables. However, since we average over many simulated individuals and replications, this type of noise tends to be minimal. A more important source of noise comes from the sampling error in estimating the parameters of the transition and initial condition model. We have not incorporated this type of uncertainty in these calculations. Hence, our estimates should be interpreted as point estimates which illustrate magnitudes rather than precise estimates of the effect of the scenarios we consider.
Results
As noted earlier, our objectives are: (1) to assess the extent to which health differences explain the longevity gap; and (2) to quantify the fiscal consequences of gradually closing the health gap. To meet the first objective, we examine the 2004 cohort of 50 year-olds, and consider the counterfactual in which Americans have the same health as Europeans. We compare the resulting longevity estimate to longevity predicted using the baseline health status of Americans. We also assess the total differences in public spending generated by these underlying differences in health. The second objective requires that we analyze the consequences of gradually moving American health levels to those enjoyed by Europeans.
Explaining Differences in Longevity
We use the prevalence rates presented in Figure 2 to construct the first counterfactual cohort. We simulate the baseline outcomes of the cohort using the adjusted prevalence rates from European data. Using the methodology outlined, we preserve the correlation between health and other outcomes in the model. We keep other socio-economic characteristics constant at American levels, while varying the health status of the cohort. We then simulate transitions until everyone dies in the simulation. We compare this counterfactual with the status quo case where American health is unchanged.
Our baseline projection of remaining life-expectancy at age 50 is 31 years. This is very close to the 30.98 years estimated from life tables, as shown in Figure 1. Assigning European health status levels to Americans increases healthy life expectancy (years without ADLs) by 1.3 years, and decreases unhealthy life expectancy (years with 1+ ADLs) by a tenth of a year (virtually zero). The overall effect is to increase life expectancy by 1.2 years, which is 92% of the difference in life expectancy reported in World Health Organization data. In other words, differences in health status explain nearly all the longevity difference across the US and Europe. Moreover, these findings indicate that worse health in the U.S. is associated with a loss of healthy life expectancy, rather than an increase in unhealthy life expectancy.
The Fiscal Consequences of Differences in Health
The differences in health across the US and Europe have important fiscal consequences. Table 2 computes the overall fiscal effects on a per capita basis. Revenue would increase by $2,425 per capita, partly due to the increase in life expectancy and increase in earnings/labor force participation. On the expenditure side, there are two effects. First, old age pension benefits would increase by a substantial amount ($6,593 per capita). This is roughly the size of the average annual Social Security benefit payment. But, there would be a larger decrease in Medicare, Medicaid, and Disability insurance (DI) benefit payments: Total lifetime health-care expenditures would decrease by a stunning $17,791. This represents an 8.5% reduction in lifetime medical expenditures. The average reduction in lifetime payments is $4,717 for Medicare and $3,687 for Medicaid. Adding the reduction in DI costs, the net effect on government expenditures would be $2,477 per capita. Overall, the net fiscal impact of this scenario is an increase in per capita net revenue of $4,902 per capita for the government.
Table 2.
Per Capita Lifetime Fiscal Effects of European Health Scenario for Cohort in 2004
| Expenditure Category | Status Quo |
European Scenario |
Difference |
|---|---|---|---|
| Government Revenues | |||
| Federal Tax | 46,289 | 47,637 | 1,348 |
| State Tax | 16,035 | 16,535 | 500 |
| Social security payroll taxes | 16,566 | 17,031 | 465 |
| Medicare payroll taxes | 4,020 | 4,132 | 112 |
| Total | 82,910 | 85,335 | 2,425 |
| Government Expenditures | |||
| Old Age and Survivors Insurance benefits (OASI) | 138,123 | 144,716 | 6,593 |
| Supplementary Security Income (SSI) | 3,454 | 3,471 | 17 |
| Disability Insurance benefits (DI) | 6,356 | 5,673 | −683 |
| Medicare costs | 73,391 | 68,674 | −4,717 |
| Medicaid costs | 21,745 | 18,058 | −3,687 |
| Total | 243,069 | 240,592 | −2,477 |
| Net Fiscal Effect | 4,902 | ||
| Total Health Care Expenditures | 210,993 | 193,202 | −17,791 |
Source: authors' calculations using the microsimulation model. Amounts reported in $2004 USD. Present discounted values computed using a real discount rate of 3%. Total Health Care Expenditures include private health insurance and other expenditures not covered by Medicare and Medicaid.
Long-Term Fiscal Consequences of US Health Improvements
The experiment we describe above computes the contribution of health differences between the US and Europe to differences in longevity and public spending. But we are also interested in the more practical question concerning the consequences of gradually moving cohorts of near-elderly Americans towards the health status of their European counterparts.
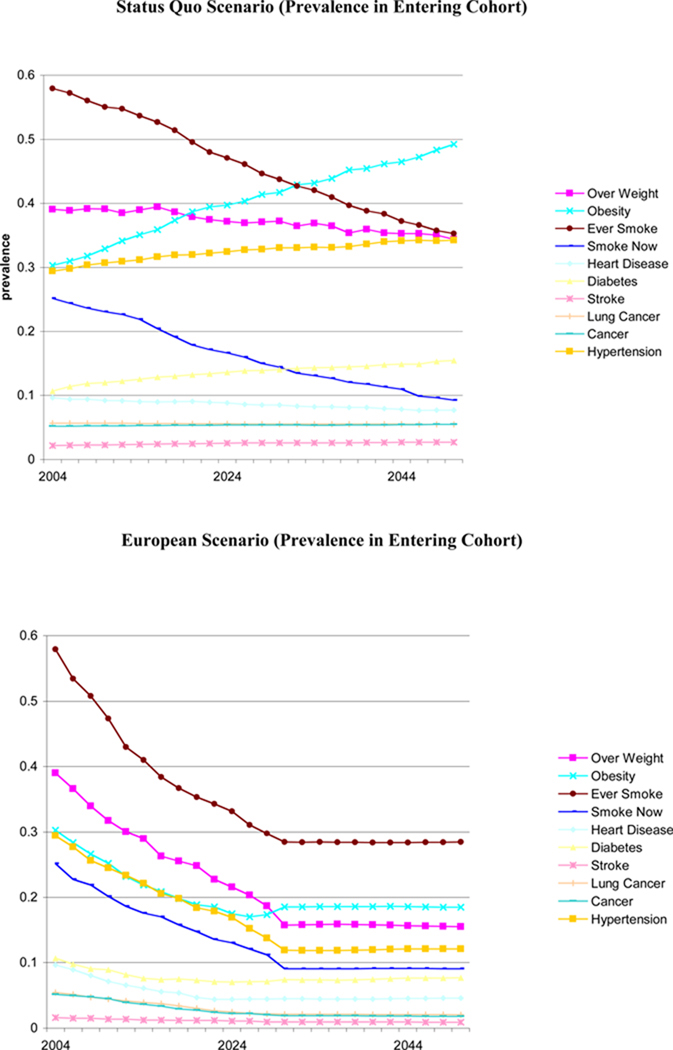
To implement a gradual scenario, we allow prevalence rates in the entering cohort to reach European levels by 2030. This is compared to a status quo scenario in which trends currently observed and projected among the new elderly persist. This scenario can be interpreted as a transition from the current steady-state where nothing is done, to a new one. Figure 4 provides the time path for various conditions among entering cohorts in the baseline and counterfactual scenarios. Each year, the population alive is representative of the age 50+ population alive in the U.S.
Figure 4.
Population Scenarios
We report the results for the status quo in Table 3. Given current trends, we project the size of the population aged 50+ will increase by nearly 75% from 80.7 million in 2004 to 145 million in 2050. As a test of validity, we found that the forecast of 81.4 million 65+ year-olds in 2050 is very close to that of the Social Security Administration, which predicts 80.8 million. Life expectancy for people at age 50 is projected to increase from 31 years in 2004, to 31.6 years in 2050, a very modest increase.
Table 3.
Population Level Outcomes Under Status-Quo Scenario (2004–2050)
| Status Quo Estimates | |||
|---|---|---|---|
| Year | |||
| 2004 | 2030 | 2050 | |
| Population size (Million) | 80.71 | 122.13 | 145.05 |
| Population 65+ (Million) | 36.25 | 66.87 | 81.37 |
| Prevalence of selected conditions | |||
| Obesity (BMI >=30) (%) | 28.1% | 41.1% | 45.8% |
| Overweight (25<=BMI<30) (%) | 38.1% | 37.8% | 36.3% |
| Ever-smoked | 58.6% | 48.0% | 38.6% |
| Smoking now | 16.9% | 9.6% | 6.2% |
| Diabetes | 17.0% | 24.8% | 27.8% |
| Heart disease | 23.0% | 28.1% | 29.9% |
| Hypertension | 50.9% | 58.9% | 62.2% |
| Government revenues from aged 51+ (Billion $2004) | |||
| Federal personal income taxes | 216.44 | 228.62 | 249.33 |
| Social security payroll taxes | 73.82 | 86.79 | 96.63 |
| Medicare payroll taxes | 18.67 | 20.98 | 23.33 |
| Government expenditures from aged 51+ (Billion $2004) | |||
| Old Age and Survivors Insurance benefits (OASI) | 417.15 | 992.47 | 1,272.07 |
| Disability Insurance benefits (DI) | 36.99 | 36.02 | 40.77 |
| Supplementary Security Income (SSI) | 17.06 | 26.44 | 37.94 |
| Medicare costs | 290.24 | 549.44 | 735.69 |
| Medicaid costs | 118.72 | 152.66 | 228.44 |
| Total medical costs for aged 51+ (Billion $2004) | 851.05 | 1,412.58 | 1,826.03 |
Source: authors' calculation using the microsimulation model under the status quo scenario described in the text. All dollars are in 2004 values. Output reported for the years 2004, 2030 and 2050.
We compare our baseline projections to the scenario described in Figure 6—gradual movement towards European prevalence levels. Table 4 reports the results. Gradual health improvements would result in an aged 50+ population that is 4%, or 5.75 million, larger in 2050, than it would have been in the absence of those improvements. The population is also much healthier in 2030 and 2050 than under the status quo. For example, the obesity rate falls by 24 percentage points, while the prevalence of lifetime smoking and of diabetes both fall by roughly 10 percentage points each.
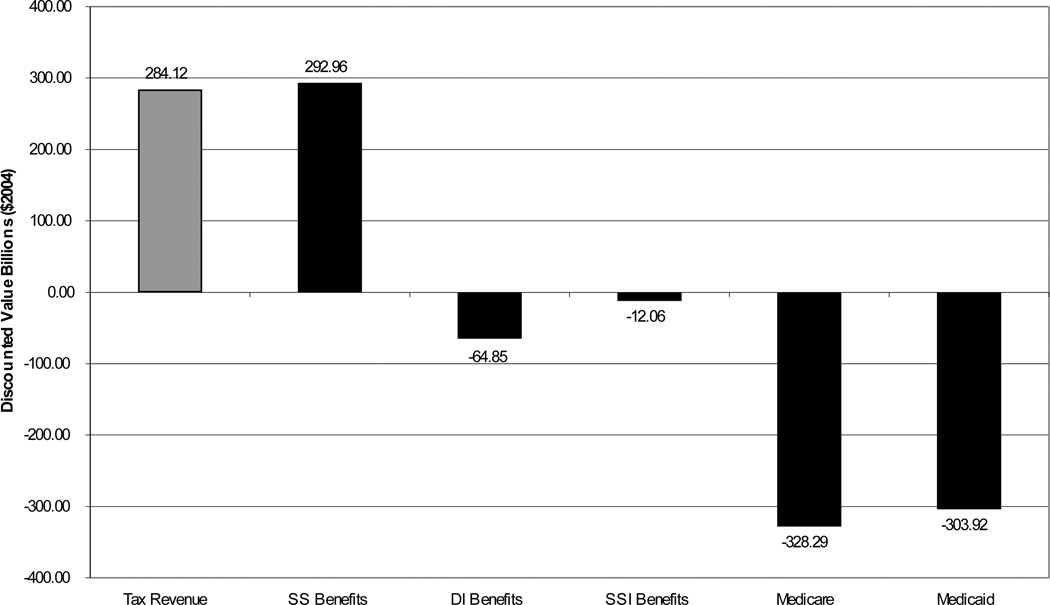
Figure 6.
Source: Authors‘ calculations using the microsimulation model. Amounts in billion $2004 and represent the difference between the European scenario and the status quo scenario as defined in Figure 4. Present discounted value calculated using a 3% real discount rate from 2004 to 2050. Tax revenue includes Federal, State and Social Security and Medicare Taxes. SS stands for Social Security Benefit Payments, DI for disability insurance payments, SSI for Supplemental Security Income payments.
Table 4.
Population Level Outcomes under European Scenario (2004–2050)
| European Scenario | Absolute Change |
||||
|---|---|---|---|---|---|
| 2030 | 2050 | 2030 | 2050 | ||
| Population size (Million) | 123.74 | 150.81 | 1.605 | 5.752 | |
| Population 65+ (Million) | 67.95 | 86.52 | 1.072 | 5.147 | |
| Prevalence of selected conditions | |||||
| obesity (BMI >=30) (%) | 27.5% | 21.6% | −0.136 | −0.242 | |
| over weight (25<=BMI<30) (%) | 36.4% | 35.6% | −0.013 | −0.007 | |
| Ever-smoked | 38.2% | 28.0% | −0.098 | −0.107 | |
| Smoking now | 7.9% | 5.3% | −0.016 | −0.01 | |
| Diabetes | 19.1% | 17.0% | −0.057 | −0.108 | |
| Heart disease | 25.1% | 25.7% | −0.03 | −0.042 | |
| Hypertension | 50.3% | 48.3% | −0.086 | −0.139 | |
| Government revenues from aged 51+ (Billion $2004) | |||||
| Federal personal income taxes | 244.59 | 275.97 | 15.970 | 26.644 | |
| Social security payroll taxes | 88.75 | 99.11 | 1.957 | 2.481 | |
| Medicare payroll taxes | 21.46 | 23.95 | 0.480 | 0.620 | |
| Government expenditures from aged 51+ (Billion $2004) | |||||
| Old Age and Survivors Insurance benefits (OASI) | 1 004.97 | 1 342.42 | 12.499 | 70.358 | |
| Disability Insurance benefits (DI) | 32.29 | 35.94 | −3.732 | −4.837 | |
| Supplementary Security Income (SSI) | 25.55 | 38.83 | −0.890 | 0.890 | |
| Medicare costs | 527.96 | 699.21 | −21.481 | −36.488 | |
| Medicaid costs | 133.50 | 201.49 | −19.158 | −26.951 | |
| Net Fiscal Effect | 51.169 | 26.773 | |||
| Total medical costs for aged 51+ (Billion $2004) | 1 327.72 | 1 701.61 | −84.854 | −124.422 | |
Source: authors' calculations using the microsimulation model under the European scenario described in the text. All dollars in 2004 values. Output reported for the years 2004, 2030 and 2050.
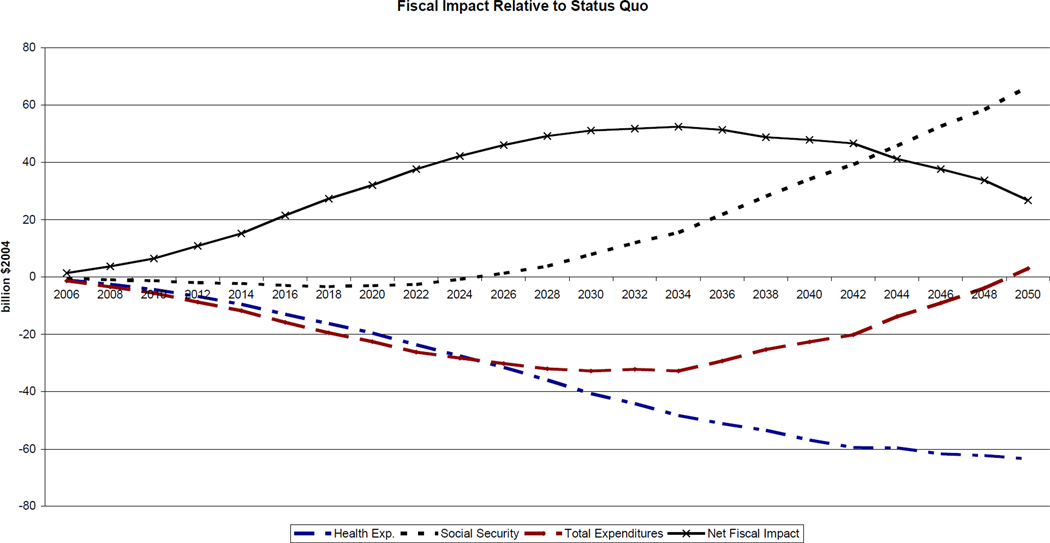
Government revenue rises by 10%, or $30 billion, by 2050, as a result of the longevity gains. As before, there are two offsetting expenditure effects. On one hand, longer lives imply larger annuity burdens: OASI benefits go up by $70.4 billion. On the other hand, medical costs decrease by $124 billion, or 6.7%, in 2050. Medicare saves $36.4 billion, despite the increase in longevity. The overall effect on expenditures is initially negative, but turns positive by 2050. As found in Michaud et al. (2009), a transition to better health first decreases expenditures, but gains in longevity eventually exert upward pressure on spending.
Figure 5 shows that the gains in health expenditure materialize quickly, while the annuity burden takes longer to emerge. This is essentially a timing issue: cost savings due to lower disability appear before cost increases due to extensions in life expectancy. The total effect on expenditures is largest around 2030 and goes to zero by 2050. Since revenue rises as well, the net fiscal effect is positive in 2050 but slowly converges to zero. Hence, the transition to better health involves important fiscal effects, but these largely vanish once the new equilibrium is reached.
Figure 5.
Source: Authors‘ own calculations using the microsimulation model. Health expenditures include Medicare and Medicaid. Social Security includes SSI, DI and OASI expenditures. Net Fiscal Impact is the revenue change minus the total expenditure change. All amounts in billions $2004 and refer to the difference between the European scenario and the status quo defined in Figure 4.
Figure 6 shows that, in present value terms from the 2004 perspective, the increase in tax revenue almost entirely offsets the annuity burden. The effect on health expenditures remains. The present discounted value of Medicare and Medicaid savings combined is $632 billion, or 1.6 years of combined 2004 spending on the two programs. In terms of total medical spending, the present value of those savings is $1.1 trillion dollars. The fact that these are such large amounts illustrates the potential for savings by improving population health.
Robustness to Cross-Country Differences in Diagnosis
An alternative interpretation of cross-country differences in health focuses on differences in rates of diagnosis, rather than real differences in health status. The literature documents under-diagnosis of diseases like diabetes and hypertension in the US (Smith, 2007b), but it is difficult to find comparable European studies. However, one direct analysis of this question suggests that differences in diagnosis are relatively modest. Banks et al. (2006) compare objective clinical diagnosis among men, using commonly used thresholds on biomarkers, to self-reported measures of whether respondents have previously been diagnosed. For diabetes among those aged 40–70, they find a clinical prevalence of 4.8% in the UK and 8.9% in the US, but self-reported prevalence of 4.4% and 8.6%, respectively. The cross-country difference is similar using self-reports or clinical measurements (4.2% versus 4.1%). They find a similar result for hypertension. These discrepancies are not large relative to the differences we observe in the data.
Of course, the Banks et al. evidence is somewhat narrow in its focus on diabetes and hypertension, and on the US-UK difference specifically. Differences in screening and diagnosis might be more important for other diseases like cancer, as documented in Howard et al. (2009). To test the sensitivity of our results to diagnostic differences, we ran the cohort analysis under various assumptions about differential diagnosis. We allowed the “diagnosis effect” to account for between 0% and 100% of the total difference in measured health across the US and Europe, in 6 alternative sensitivity analyses. In these sensitivity analyses, we kept differences in obesity and smoking constant, as differential reporting across countries seems less clearly linked to systematic institutional factors. We calculate that if all of the difference we observed was due to under-reporting of disease in Europe, US life expectancy would increase by 0.25 years, as a result of differences in baseline obesity and smoking. On the other hand, if there are no differences in the rates at which diseases are diagnosed across countries, the effect is 1.2 years. If the Banks et al. result holds more generally, and there is at most a 5% difference in diagnosis, the effect drops to about 1.1 year. This suggests that the differences in health are likely to remain meaningful under reasonable assumptions about differential diagnosis.
Discussion
There is a growing longevity gap between the US and Europe with no settled interpretation. We have demonstrated that differences in observed disease prevalence can almost entirely account for this difference. In this sense, the international longevity gap appears much easier to explain than the racial or socioeconomic longevity gaps, which are not well explained by health differences. Internationally, there is no “American-specific” effect on longevity, above and beyond differences in disease at age 50. This suggests further that addressing the health gap across the Atlantic will likely erase the longevity gap, although the same cannot necessarily be said for analogous disparities within countries. The expansion of the gap in longevity and health coincided with relative increases in obesity among the US population. Policies that target obesity and better management of chronic health conditions such as diabetes and hypertension could be effective in improving health. Moreover, near-elderly cohorts of Americans took up smoking at much higher rates than their European counterparts and while the prevalence of smoking is likely to continue decreasing, more could be done.
As we demonstrate, the gap in health and longevity has obvious private costs to the citizens suffering from disease. There are also significant public finance consequences, on the order of $17,800 in per capita medical costs, and net public finance costs of roughly five thousand dollars per capita. Gradual transitions of US cohorts towards European levels could generate large fiscal benefits. In the long-run, medical expenditures may fall by $1.1 trillion on a present value basis. Our results suggest that prevention, in the form of lowering health risks prior to age 50 may yield important benefits. This is in line with results we published on the private value of prevention for major risk factors (Goldman et al., 2009). In that research, we found that treating 50 year-old obese individuals could be worth approximately $50,000 while preventing diabetes would be worth close to $200,000. It is not clear which policies could help reach this goal. But the historical success of anti-smoking campaigns suggests that behavior change is possible. The costs of such policies will need to be weighed against the welfare and economic consequences we have analyzed in this paper.
Supplementary Material
Acknowledgments
We are grateful to the U.S. Department of Labor, the National Institute on Aging (RC4G039036 and 7P30AG024968) and the MacArthur Research Network on an Aging Society for financial support. We also thank Michael Hurd, James Smith, Maggie Weden, Samuel Preston, James Banks and Arthur van Soest as well as seminar participants at Tilburg University and the Michigan RRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pierre-Carl Michaud, Email: michaud.pierre_carl@uqam.ca, Université du Québec à Montréal (UQAM) and RAND Corporation.
Dana Goldman, University of Southern California and RAND Corporation.
Darius Lakdawalla, University of Southern California and RAND Corporation.
Adam Gailey, RAND Corporation.
Yuhui Zheng, Harvard School of Public Health.
References
- Adams P, Hurd MD, et al. Healthy, wealthy, and wise? Tests for direct causal paths between health and socioeconomic status. Journal of Econometrics. 2003;112(1):3–56. [Google Scholar]
- Andreyeva T, Michaud P-C, van Soest A. Obesity and Health in Europeans aged 50 years and older. Public Health. 2007;121:497–509. doi: 10.1016/j.puhe.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Banks J, Marmot M, et al. Disease and Disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Shang B, et al. Technological advances in cancer and future spending by the elderly. Health Affairs. 2005;24(Suppl 2):W5R53–W5R66. doi: 10.1377/hlthaff.w5.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz G. Weight Gain as a Risk Factor for Clinical Diabetes Mellitus in Women. Annals of International Medicine. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- Cutler DM, Glaeser EL, Shapiro JM. Why Have Americans Become More Obese? Journal of Economic Perspectives. 2003;17(3):93–118. [Google Scholar]
- Cutler DM, Glaeser EL. Why Do Europeans Smoke More than Americans? 2006 NBER working paper 12124. [Google Scholar]
- Goldman D, Hurd M, et al. Santa Monica, CA: RAND Corporation; 2004. Health status and medical treatment of the future elderly: Final Report. [Google Scholar]
- Goldman DP, Shang B, et al. Consequences of health trends and medical innovation for the future elderly. Health Affairs. 2005;24(Suppl 2):W5R5–W5R17. doi: 10.1377/hlthaff.w5.r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Michaud P-C, Lakdawalla D, Zheng Y, Gailey A. The Fiscal Consequences of Trends in Population Health. National Tax Journal. 2010;63(2):307–330. [Google Scholar]
- Goldman D, Zheng Y, Girosi F, Michaud P-C, Olshansky SJ, Cutler D, Rowe J. The Benefits of Risk Factor Prevention in Americans Aged 51 and Older. American Journal of Public Health. 2009;vol. 99(11):2096–2101. doi: 10.2105/AJPH.2009.172627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Warner DF. Demography of Population Health. In: Poston DL Jr, Micklin M, editors. The Handbook of Demography. New York: Springer; 2005. pp. 809–825. [Google Scholar]
- Howard D, Richardson L, Thorpe K. Cancer Screening and Age in the United States and Europe. Health Affairs. 2009;28(6):1838–1847. doi: 10.1377/hlthaff.28.6.1838. [DOI] [PubMed] [Google Scholar]
- Lakdawalla D, Philipson T. The Growth of Obesity and Technological Change. Economics and Human Biology. doi: 10.1016/j.ehb.2009.08.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. Mortality Forecasts and Linear Life Expectancy Trends. [March 25, 2003];Center for the Economics and Demography of Aging. 2003 CEDA Papers: Paper 2003-0003CL http://repositories.cdlib.org/iber/ceda/papers/2003-0003CL.
- Lee RD. The Lee-Carter Method for Forecasting Mortality, With Various Extensions and Applications. North American Actuarial Journal. 2000;4:80–91. [Google Scholar]
- Levy D. University of Baltimore; 2006. Trends in Smoking Rates Under Different Tobacco Control Policies:Results from the SimSmoke Tobacco Policy Simulation Model. working paper. [Google Scholar]
- Manton K, Singer B, et al. Forecasting the Health of Elderly Populations. New York: Springer-Verlag; 1993. [Google Scholar]
- Manton KG, Gu XL, Lowrimore G. Cohort Changes in Active Life Expectancy in the U.S. Elderly Population: Experience From the 1982–2004 National Long-Term Care Survey. The Journals of Gerontology: Series B. 2008;63(5):S269–S281. doi: 10.1093/geronb/63.5.s269. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Oshlansky SJ, Carnes B. The Quest for Immortality: Science at the Frontiers of Aging. New York: W.W. Norton; 2001. [Google Scholar]
- Preson SH, Ho J. Low Life Expectancy in the United States: Is the Health Care System at Fault? 2009 NBER Working Paper 15213. [Google Scholar]
- Rogers RG, Powell-Griner E. Life Expectancies of Cigarette Smokers and Nonsmokers in the United States. Social Science and Medicine. 1991;32(10):1151–1159. doi: 10.1016/0277-9536(91)90092-q. [DOI] [PubMed] [Google Scholar]
- Ruhm C. Current and Future Prevalence of Obesity and Severe Obesity in the United States. Forum for Health Economics & Policy. 2007;Vol. 10(Iss. 2) (Obesity), Article 6. [Google Scholar]
- Smith J. The Impact of Socioeconomic Status on Health over the Life-Course. Journal of Human Resources. 2007a;42(4):739–764. [Google Scholar]
- Smith J. Nature and Causes of Trends in Male Diabetes Prevalence, Undiagnosed Diabetes, and the Socioeconomic Status Health Gradient. Proceedings of the National Academy of Sciences. 2007b;104(33) doi: 10.1073/pnas.0611234104. 13225-1323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe KE, Howard DH, Galactionova K. Differences in Disease Prevalence as a Source of the U.S.-European Health Care Spending Gap. Health Affairs. 2007;26(6):678–686. doi: 10.1377/hlthaff.26.6.w678. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar S, Li N, Boe C. A Universal Pattern of Mortality Decline in the G7 Countries. Nature. 2000;405:789–792. doi: 10.1038/35015561. [DOI] [PubMed] [Google Scholar]
- Viscusi K, Aldy JE. The Value of a Statistical Life: A Critical Review of Market Estimates throughout the World. Journal of Risk and Uncertainty. 2003;Vol. 27(No. 1):5–76. [Google Scholar]
- White K. Longevity Advances in High-Income Countries, 1955–1996. Population and Development Review. 2002;28(1):59–76. [Google Scholar]
- Willett W. Weight, Weight Change and Coronary Hearth Disease in Women. Jama. 1995;273(6):461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.