Abstract
Taurine acts as a partial agonist at the glycine receptor (GlyR) in some brain regions such as the hippocampus, striatum, and nucleus accumbens. Ethanol, volatile anesthetics, and inhaled drugs of abuse are all known positive allosteric modulators of GlyRs, but their effects on taurine-activated GlyRs remain poorly understood, especially their effects on the high concentrations of taurine likely to be found after synaptic release. Two-electrode voltage-clamp electrophysiology in Xenopus laevis oocytes was used to compare the enhancing effects of ethanol, anesthetics, and inhalants on human homomeric α1-GlyR activated by saturating concentrations of glycine versus taurine. Allosteric modulators had negligible effects on glycine-activated GlyR while potentiating taurine-activated currents. In addition, inhaled anesthetics markedly enhanced desensitization rates of taurine- but not glycine-activated receptors. Our findings suggest that ethanol, volatile anesthetics, and inhalants differentially affect the time courses of synaptic events at GlyR, depending on whether the receptor is activated by a full or partial agonist.
Introduction
The glycine receptor (GlyR) is responsible for the majority of neuronal inhibition in the brainstem and spinal cord but is also found in a variety of higher brain regions, such as the basal ganglia, cerebellum, hippocampus, and the prefrontal cortex (Lynch, 2004; Baer et al., 2009; Lu and Ye, 2011). It is a member of the Cys-loop family of ligand gated ion channels composed of five subunits that coassemble around a central ion-conducting pore. Many compounds are known modulators of the GlyR, including alcohols, volatile anesthetics, zinc, and inhaled drugs of abuse (Lynch, 2004), and the GlyR has been implicated in their effects in vivo (Downie et al., 1996; Beckstead et al., 2000; Yamashita et al., 2001; Cheng and Kendig, 2002; Molander et al., 2005, 2007). Ethanol is the second most widely abused drug behind tobacco, and its use leads to depression of nervous system functioning. Volatile anesthetics are characterized by their propensities to readily vaporize at room temperature and, like ethanol, to cause central nervous system depression. In the clinical setting, they produce a myriad of effects, including analgesia, amnesia, immobility, hypnosis, and sedation. Inhalants are a heterogeneous class of industrial solvents that are often abused by adolescents because they quickly produce a rapidly reversible high (Evans and Balster, 1991).
Ethanol, anesthetics, and inhalants enhance GlyR function in a concentration-dependent manner. They act by left-shifting the glycine concentration-response curve, thus decreasing the EC50 of glycine (Mascia et al., 1996; Mihic, 1999; Beckstead et al., 2000; Welsh et al., 2010). Thus, these compounds enhance currents elicited by low concentrations of glycine but have minimal effects at saturating concentrations of glycine (Mascia et al., 1996; Beckstead et al., 2000; Welsh et al., 2010). These modulators are thought to bind in a water-filled pocket near the second transmembrane domain of each subunit of the GlyR (Mihic et al., 1997; Yamakura et al., 1999; Beckstead et al., 2001; Roberts et al., 2006).
At a saturating concentration, taurine acts as a partial agonist with ∼50% efficacy in activating the GlyR compared with glycine. This refers to the proportion of time the receptor spends in the open state (Popen) while agonist is bound. At saturating concentrations, glycine and taurine produce Popen values of 0.96 and 0.54, respectively (Lape et al., 2008). Ethanol, anesthetics, and inhalants have no effects at saturating concentrations of glycine, and one reason for this may be attributed to the GlyR already spending ∼95% of its time in the open state. Such a ceiling effect would not be expected to occur with saturating concentrations of taurine. Another possibility is that these allosteric modulators do not affect Popen, as suggested by a recent single channel study conducted by Welsh et al. (2009) and, in which case, no enhancement of GlyR function should be observed in the presence of a maximally effective concentration of taurine. In light of recently obtained evidence of a role for taurine as a GlyR activator in vivo (Dahchour et al., 1996; Mori et al., 2002; Ericson et al., 2006, 2011; Rodríguez-Navarro et al., 2009), we tested ethanol, anesthetics, and inhalants for their enhancing effects on taurine-activated GlyR. In addition, if enhancement were to be seen at saturating taurine concentrations, it would suggest differences in the mechanisms of allosteric modulation of receptors activated by full versus partial agonists.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Xenopus laevis were obtained from Nasco (Fort Atkinson, WI) and housed at 19°C on a 12-h light/dark cycle. During surgery performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care regulations, portions of ovaries were removed and placed in isolation media containing 108 mM NaCl, 1 mM EDTA, 2 mM KCl, and 10 mM HEPES. Forceps were used to manually remove the thecal and epithelial layers from stage V and VI oocytes. The oocyte follicular layer was removed using a 10-min incubation in 0.5 mg/ml type 1A collagenase (Sigma-Aldrich) in buffer containing 83 mM NaCl, 2 mM MgCl2, and 5 mM HEPES. Animal poles of oocytes were injected with 30 nl of the glycine α1-receptor subunit cDNA (1.5 ng/30 nl) in a modified pBK-cytomegalovirus vector (Mihic et al., 1997) by the “blind” method of Colman (1984), using a micropipette (10–15-μm tip size) attached to an electronically activated microdispenser. For α1β experiments, a 1:30 ratio of α- to β-cDNAs was injected to ensure incorporation of the β-subunit. Oocytes were stored in the dark at 19°C in 96-well plates containing modified Barth's saline (MBS) [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4·7H2O, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2 at pH 7.5] supplemented with 2 mM sodium pyruvate, 0.5 mM theophylline, 10 U/ml penicillin, 10 mg/l streptomycin, and 50 mg/l gentamicin and sterilized by passage through a 0.22-μm filter. Oocytes expressed the wild-type and S267F (Ye et al., 1998) GlyR within 24 h, and all electrophysiological measurements were made within 5 days of cDNA injection. Replacement of a serine residue with phenylalanine at residue 267 within the second transmembrane segment of the α1-subunit yields the S267F mutant.
Before electrophysiological recording oocytes were placed in a 100-μl bath with the animal poles facing upwards and impaled with two high-resistance (0.5–10 MΩ) glass electrodes filled with 3 M KCl. Cells were voltage clamped at −70 mV using an OC-725C oocyte clamp (Warner Instruments, Hamden, CT) and perfused with MBS at a rate of 2 ml/min using a Masterflex USA peristaltic pump (Cole Parmer Instrument Co., Vernon Hills, IL) through 18-gauge polyethylene tubing. All drug solutions were prepared in MBS. When saturating concentrations of agonists were applied, applications lasted for 15 s for the short-application experiments and for 13 min for the long-application experiments. Submaximally effective concentrations of agonist (glycine or taurine) were applied for 60 s. Modulators were either preapplied to oocytes for 30 s before being coapplied with agonist for an additional 15 s for short-application experiments or coapplied with agonist for 60 s for long-application experiments. All drug applications were followed by 6- to 10-min washout periods to allow for complete receptor resensitization. Loss of volatile compounds through tubing and evaporation from bath was previously measured (Mihic et al., 1994; Yamakura et al., 1999; Beckstead et al., 2000, 2002). All concentrations reported are the bath concentrations to which the oocyte was exposed. Currents were acquired using either a Powerlab 4/30 digitizer with LabChart version 7 software (ADInstruments, Bella Vista, NSW, Australia) or a strip-chart recorder (Cole Parmer Instrument Co.) with a <0.5-s full-scale pen movement response time. Peak currents were measured and used in data analysis. Currents observed in the presence of agonist plus modulators were compared with currents generated by agonist alone.
Experimental values are listed as the mean ± S.E. Significant differences between experimental conditions were determined using paired or unpaired t-tests, the Wilcoxon signed rank test or the Mann-Whitney rank sum test as indicated. The equation y = min + (max − min)/(1 + (x/EC50)−nH) was used to fit agonist and agonist + isoflurane concentration-response curves to determine the half-maximally effective concentrations (EC50). SigmaPlot version 11.0 (Systat Software, San Jose, CA) was used for statistical testing.
Results
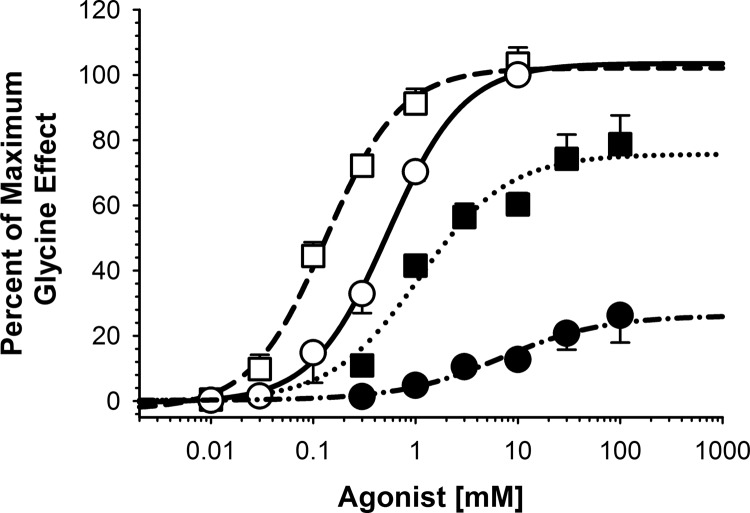
The enhancing actions of ethanol, volatile anesthetics, and inhaled drugs of abuse were compared for their effects on currents generated by glycine versus taurine on wild-type α1-homomeric GlyRs. We first tested 1.1 mM isoflurane for its abilities to enhance GlyR function in receptors activated by glycine or taurine (Fig. 1). As expected, isoflurane left-shifted the glycine concentration-response curve without having any effect at maximally effective glycine concentrations. Isoflurane also left-shifted the taurine concentration-response curve but also markedly increased the effects of maximally effective taurine concentrations.
Fig. 1.
Isoflurane decreases agonist EC50 and increases taurine-mediated peak currents. Isoflurane shifts α1-homomeric GlyR glycine and taurine concentration-response curves to the left and increases the maximal response to the partial agonist. Open symbols represent glycine-mediated responses, and filled symbols show taurine-mediated responses. Circles represent agonist applied alone, with squares showing agonist + 1.1 mM isoflurane. Each line is a logistic fit to the respective set of data. Glycine activation of the GlyR produced an EC50 of 0.54 mM with a Hill coefficient (nH) of 1.2. The addition of 1.1 mM isoflurane decreased the EC50 to 0.13 mM and an nH of 1.2. Taurine alone had an EC50 of 6.9 mM, with an nH of 0.9, decreasing to an EC50 of 1.1 mM and an nH of 1.0 in the presence of isoflurane. Data are shown as mean ± S.E.M. of four oocytes.
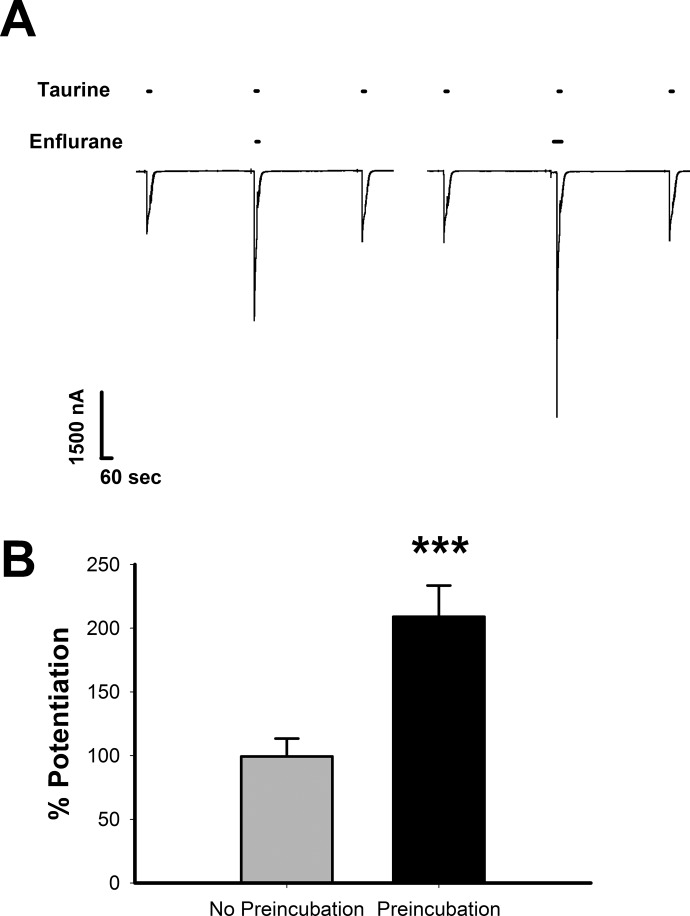
We previously examined the effects of ethanol on GlyR using short and long applications of saturating concentrations of glycine or taurine (Welsh et al., 2010) and found minor enhancing effects during long applications of the latter. In these long-application experiments, ethanol was applied for 60 s and thus had sufficient time to equilibrate within its binding pocket. In experiments involving brief applications of saturating concentrations of agonists, the peak current is reached in less than 100 ms in our hands, concurrent with rapid desensitization of the GlyR. If ethanol is coapplied with agonist, the ethanol may not have sufficient time to reach equilibrium within the binding pocket. Therefore, we first tested whether preincubation of modulator was necessary for this equilibrium to occur before desensitization predominated, allowing us to see the full effects of the modulator on the activated GlyR. Because isoflurane was previously shown to have enhancing effects on saturating concentrations of taurine (Downie et al., 1996), we chose to use the structurally similar anesthetic enflurane for these experiments. Figure 2A, left panel, shows sample tracings of the effects of 100 mM taurine coapplied with 1.2 mM enflurane for 15 s, with 15-s applications of 100 mM taurine alone (before and after application). The tracing in the right panel shows the effects of a 30-s preincubation of 1.2 mM enflurane immediately before coapplication with taurine. Coapplication of 100 mM taurine plus 1.2 mM enflurane resulted in a 99.4 ± 14% increase in current above that produced by 100 mM taurine alone (Fig. 2B). However, preincubation of 1.2 mM enflurane before coapplication with 100 mM taurine resulted in a current 209 ± 24.4% above that produced by taurine alone, suggesting that preincubation with anesthetics is required for enflurane to come to equilibrium with receptors before agonist is coapplied. There was a statistically significant effect of preincubation [t(4) = 9.3, P < 0.001].
Fig. 2.
Preincubation enhances the potentiating effects of enflurane on taurine-activated GlyRs. A, sample tracings showing enflurane potentiation of taurine responses with and without preincubation. The left tracing shows coapplication of 100 mM taurine and 1.2 mM enflurane, with applications of 100 mM taurine alone (before and after). The right tracing shows the effect of preincubation with 1.2 mM enflurane, immediately followed by coapplication of 100 mM taurine and 1.2 mM enflurane. Applications of 100 mM taurine alone preceded and followed the enflurane response. Horizontal bars over tracings indicate the time of exposure to taurine or enflurane. B, summary of the effects of 100 mM taurine and 1.2 mM enflurane with and without preincubation. The y-axis represents the percentage increase in current observed in the presence of enflurane compared with that produced by taurine alone. Data are shown as mean ± S.E.M. of five oocytes. ***, P < 0.001.
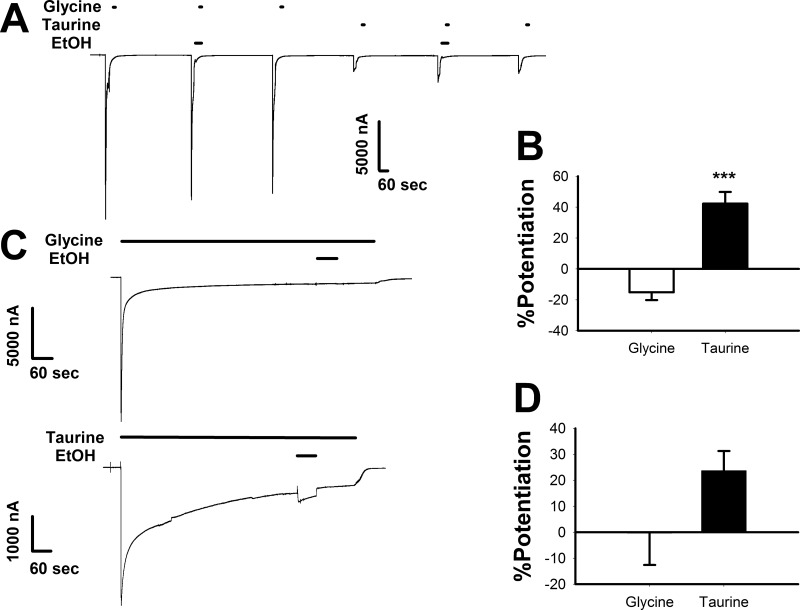
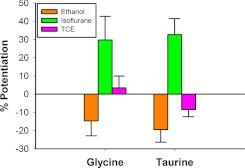
We have previously demonstrated that ethanol acts as an allosteric modulator by left-shifting glycine and taurine concentration-response curves without an effect when maximally effective concentrations of either glycine or taurine were tested (Welsh et al., 2010). Here, we further investigated the effects of ethanol on maximally effective concentrations of glycine and taurine using two different experimental designs. Since we showed that preincubation was necessary to reveal the full enhancing effect of enflurane (Fig. 2), we also tested ethanol using the same preincubation experimental design (Fig. 3A). Preincubation of 200 mM ethanol for 30 s was immediately followed by coapplication with a maximally effective concentration of agonist (10 mM glycine or 100 mM taurine) for 15 s. Each coapplication was preceded and followed by 15-s applications of glycine or taurine as appropriate (Fig. 3A). Ethanol (200 mM) slightly inhibited the glycine response, decreasing the peak current by 15.2 ± 5% (Fig. 3B). In contrast, ethanol moderately increased the peak taurine current by 42.2 ± 7.6%. The taurine + ethanol response was significantly different from the glycine + ethanol response [t(5) = 8.81, P < 0.001].
Fig. 3.
Ethanol affects currents elicited by maximally effective concentrations of taurine but not glycine. A, sample tracing showing the effects of maximally effective concentrations of glycine and taurine applied with or without 200 mM ethanol. The tracing shows 30-s preincubations with 200 mM EtOH followed immediately by 15-s applications of either 10 mM glycine or 100 mM taurine plus EtOH. Each of these applications was preceded and followed by applications of 10 mM glycine or 100 mM taurine, respectively. Horizontal bars over tracings indicate time of exposure to glycine, taurine, or ethanol. B, summary of the effects of 200 mM ethanol on 15-s applications of maximally effective concentrations of glycine or taurine. The y-axis represents the percentage change in current observed in the presence of ethanol compared with that produced by glycine or taurine applied alone. Data are shown as mean ± S.E.M. of six oocytes. ***, P < 0.001. C, sample tracings showing the effects of 200 mM ethanol coapplied with saturating concentrations of glycine or taurine, after the neurotransmitters had already been applied for 10 min. In the top tracing 10 mM glycine was applied for 10 min followed immediately by coapplication of 10 mM glycine plus 200 mM ethanol for 1 min. The bottom tracing shows a similar experiment using 100 mM taurine instead of glycine. D, summary graph of the effects of 200 mM ethanol during long applications of saturating concentrations of glycine and taurine. The y-axis represents the percentage change in current observed in the presence of ethanol compared with the glycine or taurine current observed immediately preceding ethanol coapplication. Data are shown as mean ± S.E.M. of four to five oocytes.
We next examined the effects of ethanol using a saturating concentration of agonist applied for 10 min to allow for a pseudo-equilibrium between the open and desensitized states of channels to become established before the coapplication of ethanol (Fig. 3C). After a 10-min application of either 10 mM glycine (Fig. 3C, top) or 100 mM taurine (Fig. 3C, bottom), 200 mM ethanol was coapplied for 1 min followed by agonist alone for 2 min. The effects of ethanol were similar to those seen in the preincubation experiment but to a lesser degree. Ethanol had a negligible effect on the saturating 10 mM glycine response (0.3 ± 12.3%) while potentiating the saturating 100 mM taurine response by 23.6 ± 7.6% (Fig. 3D), although these responses were not significantly different [Mann-Whitney U-Test U = 4, n1 = 4, n2 = 5, P > 0.05].
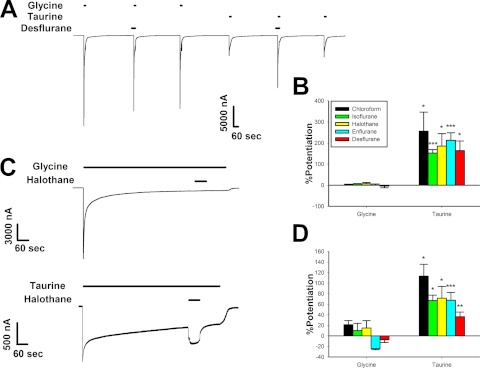
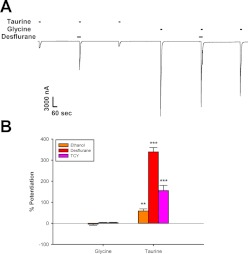
We next examined the effects of volatile anesthetics using the same experimental protocols used for ethanol. The concentrations of volatile anesthetics tested were 1.5 mM chloroform, 0.55 mM isoflurane, 0.5 mM halothane, 1.2 mM enflurane, and 1.76 mM desflurane. In Fig. 4A, desflurane was preapplied for 30 s before coapplication with a saturating concentration of either glycine or taurine. None of the anesthetics tested in this manner showed any effect on 10 mM glycine responses, but all markedly enhanced currents elicited by 100 mM taurine (Fig. 4B). Each anesthetic showed significantly greater potentiation of maximal taurine responses compared with maximal glycine responses. Chloroform [Wilcoxon Signed-Rank Test W = 28, Z = 2.4, P < 0.05], isoflurane [t(6) = 8.32, P < 0.001], halothane [t(6) = 3.11, P < 0.05], enflurane [t(6) = 6.31, P < 0.001], and desflurane [t(5) = 3.61, P < 0.05] all produced significantly greater enhancement of maximal taurine responses.
Fig. 4.
Anesthetics affect currents elicited by maximally effective concentrations of taurine but not glycine. A, sample tracing of maximally effective concentrations of glycine and taurine applied with and without 1.76 mM desflurane. The tracing shows 30-s preincubations with 1.76 mM desflurane followed immediately by 15-s applications of either 10 mM glycine or 100 mM taurine plus desflurane. Each of these applications was preceded and followed by applications of 10 mM glycine or 100 mM taurine, respectively. Horizontal bars over tracings indicate time of exposure to glycine, taurine, or desflurane. B, summary of the effects of various anesthetics on 15-s applications of maximally effective concentrations of glycine or taurine. The y-axis represents the percentage change in current observed in the presence of anesthetic compared with that produced by glycine or taurine applied alone. Data are shown as mean ± S.E.M. of six to seven oocytes for each anesthetic. C, sample tracings showing the effects of 0.5 mM halothane coapplied with saturating concentrations of glycine or taurine after the neurotransmitters had already been applied for 10 min. In the top tracing, 0.5 mM halothane was coapplied with 10 mM glycine after 10-min exposure to a saturating glycine concentration. The bottom tracing shows the same experiment, with 100 mM taurine instead of glycine. D, summary graph of the effects of various anesthetics during long applications with saturating concentrations of glycine or taurine. The y-axis represents the percentage change in current observed in the presence of anesthetic compared with the glycine or taurine current measured immediately before anesthetic coapplication. Data are shown as mean ± S.E.M. of four to eight oocytes.*, P < 0.05, **, P < 0.01, ***, P < 0.001 comparing glycine to taurine.
The results for experiments involving long exposures to saturating concentrations of agonists were similar to the preincubation experiments, with marked potentiation of 100 mM taurine responses and minimal effects on 10 mM glycine responses (Fig. 4, C and D). Again, for each anesthetic, potentiation of maximal taurine responses was significantly increased compared with their effects on maximal glycine responses. Chloroform [Mann-Whitney U-Test U = 0, n1 = 4, n2 = 4, P < 0.05], isoflurane [t(6) = 3.38, P < 0.05], halothane [Mann-Whitney U-Test U = 6, n1 = 6, n2 = 8, P < 0.05], enflurane [t(7) = 5.51, P < 0.001], and desflurane [t(8) = 3.7, P < 0.01] displayed significantly greater enhancement of maximal taurine responses.
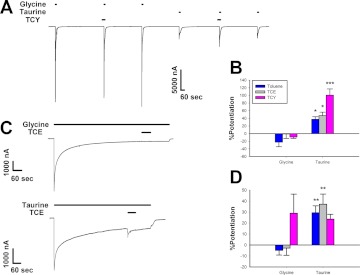
The inhaled drugs of abuse, toluene, 1,1,1-trichloroethane (TCE), and trichloroethylene (TCY) were also assayed using the same experimental protocols as before. The bath concentrations of these inhalants were 0.42 mM toluene, 0.56 mM TCE, and 0.39 mM TCY. Figure 5, A and C, shows tracings of these experiments by use of a sample inhalant in each case. Each of the inhalants slightly inhibited 10 mM glycine responses while potentiating 100 mM taurine responses in preincubation experiments (Fig. 5B). All three inhalants displayed significantly greater enhancement of maximal taurine responses: toluene [t(4) = 4.31, P < 0.05], TCE [t(5) = 3.58, P < 0.05], and TCY [t(6) = 6.65, P < 0.001].
Fig. 5.
Inhalants affect currents elicited by maximally effective concentrations of taurine but not glycine. A, sample tracing demonstrating the effects of maximally effective concentrations of glycine and taurine applied with or without 0.39 mM TCY. The tracing shows 30-s preincubations with TCY followed immediately by 15-s applications of either 10 mM glycine or 100 mM taurine plus TCY. Each of these applications was preceded and followed by applications of 10 mM glycine or 100 mM taurine, respectively. Horizontal bars over tracings indicate time of exposure to glycine, taurine, or TCY. B, summary of the effects of various inhalants on 15-s applications of maximally effective concentrations of glycine or taurine. The y-axis represents the percentage change in current observed in the presence of inhalant compared with that produced by glycine or taurine applied alone. Data are shown as mean ± S.E.M. of five to seven oocytes for each inhalant. C, sample tracings showing the effects of 0.56 mM TCE coapplied with saturating concentrations of glycine or taurine, after the neurotransmitters had already been applied for 10 min. In the top tracing, 0.56 mM TCE was coapplied with 10 mM glycine after 10-min exposure to a saturating glycine concentration. The bottom tracing shows the same for a sustained application of 100 mM taurine instead of glycine. D, summary graph of the effects of various inhalants during long applications of saturating concentrations of glycine and taurine. The y-axis represents the percentage change in current observed in the presence of inhalant compared with the glycine or taurine current seen immediately before inhalant coapplication. Data are shown as mean ± S.E.M. of four to eight oocytes.*, P < 0.05, **, P < 0.01, ***, P < 0.001 comparing glycine with taurine.
The results for the long exposures to saturating concentrations of agonists were similar to the preincubation experiments, with marked potentiation of 100 mM taurine responses observed but small effects on 10 mM glycine responses, with the exception of TCY (Fig. 5D). TCY produced a potentiation of maximal glycine and maximal taurine responses that were not significantly different [t(8) = 0.36, P > 0.7], although the TCY + glycine responses were highly variable (Fig. 5D). For toluene [t(10) = 3.59, P < 0.01] and TCE [t(9) = 3.38, P < 0.01], potentiation of maximal taurine responses was significantly greater than inhalant effects on maximal glycine responses.
We performed the same experimental protocol of modulator preincubation followed by short application of agonist on α1β-heteromeric GlyR to confirm that the modulator effects seen on the homomeric α1-GlyR were also seen in the presence of the β-subunit. For these experiments, we chose a representative compound from each of the categories of modulators tested previously. Ethanol, desflurane, and TCY were used at the same bath concentrations as in the previous experiments. Figure 6A shows tracings of the effects of 1.76 mM desflurane on maximally effective concentrations of both glycine- and taurine-mediated currents. Figure 6B shows a summary of the effects of each compound tested, with all three compounds showing negligible effects on maximal glycine responses and large potentiating effects on maximal taurine responses. Potentiation of maximal taurine responses was significantly greater than for glycine for all three modulators: ethanol [Wilcoxon Signed-Rank Test W = 45, Z = 2.7, P < 0.01]; desflurane [t(8) = 17.15, P < 0.001]; and TCY [t(7) = 6.31, P < 0.001)].
Fig. 6.
Positive allosteric modulators affect α1β-heteromeric GlyR in a similar manner to homomeric α1-GlyR. A, sample tracing demonstrating the effects of maximally effective concentrations of glycine and taurine applied with and without 1.76 mM desflurane. The tracing shows 30-s preincubations with desflurane followed immediately by 15-s applications of either 10 mM glycine or 100 mM taurine plus desflurane. Each of these applications was preceded and followed by applications of 10 mM glycine or 100 mM taurine, respectively. Horizontal bars over tracings indicate time of exposure to glycine, taurine, or desflurane. B, summary of the effects of 200 mM ethanol, 1.76 mM desflurane, or 0.39 mM TCY on 15-s applications of maximally effective concentrations of glycine or taurine. The y-axis represents the percentage change in current observed in the presence of modulator compared with that produced by glycine or taurine applied alone. Data are shown as mean ± S.E.M. of eight to nine oocytes for each modulator tested. **, P < 0.01, ***, P < 0.001 comparing glycine with taurine.
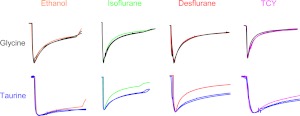
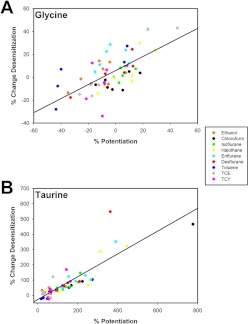
We compared tracings from the brief application experiments to contrast the effects of modulators on desensitization rates after activation by glycine or taurine. Figure 7 shows sample tracings for four of the modulators tested: ethanol, isoflurane, desflurane, and TCY. None of the modulators affected desensitization rates after receptor activation by glycine, which was expected because none of the modulators had large effects on glycine peak currents in these brief application experiments. However, both isoflurane and desflurane markedly increased desensitization rates when GlyR was activated by taurine. A summary of the effects of modulators on glycine- and taurine-mediated desensitization is provided in Table 1. We hypothesized that there might be a direct correlation between the degree of potentiation of peak responses produced by allosteric modulators and their effects on desensitization. Figure 8 shows that, as the modulator-induced percentage enhancement of peak current responses increases, so does the rate of desensitization produced by the modulator.
Fig. 7.
Anesthetics increase desensitization rates of taurine- but not glycine-activated GlyR. Each set of the sample tracings shown depicts 15-s applications of agonist + modulator as well as the agonist-alone tracings that preceded and followed the agonist + modulator application. Oocytes were preincubated with modulators for 30 s before the incubations with agonists. For each set of tracings all responses were normalized to the peak response produced by the first application of glycine or taurine. Each column contains one of four different allosteric modulators: ethanol, isoflurane, desflurane, or TCY. Each row shows one of the agonists tested, glycine (black) or taurine (blue). Tracings are color coded by compound, and each column shows tracings from a single oocyte.
TABLE 1.
The effects of positive allosteric modulators on glycine- and taurine-activated GlyR desensitization rates
Currents were measured at the peak responses and 5 s later. The percentage drop in current observed within 5 s in the presence of the modulator was divided by the percentage drop seen in the absence of the modulator, multiplied by 100, and then had 100 subtracted to indicate the percentage change in desensitization produced by the modulator. Data are shown as the mean ± S.E.M. of five to seven oocytes per modulator per agonist.
| Glycine | Taurine | |
|---|---|---|
| % | ||
| Ethanol | 2.3 ± 4.5 | 16.3 ± 10.5 |
| Chloroform* | −4.5 ± 2.5 | 120.0 ± 58.4 |
| Isoflurane* | 3.6 ± 1.9 | 68.6 ± 14.0 |
| Halothane | 3.9 ± 6.2 | 104.5 ± 52.5 |
| Enflurane* | 20.5 ± 4.7 | 128.3 ± 39.1 |
| Desflurane* | 2.9 ± 5.8 | 136.2 ± 83.2 |
| Toluene | 2.3 ± 9.4 | −4.9 ± 10.1 |
| TCE | 6.7 ± 11.4 | 27.6 ± 19.4 |
| TCY* | −4.9 ± 6.4 | 56.4 ± 23.1 |
P < 0.05 comparing glycine and taurine columns.
Fig. 8.
Correlation between the degree of positive allosteric modulator enhancement of peak current levels and desensitization rates. A, correlation between positive allosteric modulator effects on peak current percentage potentiation and change in desensitization rate for glycine-activated GlyR. Each data point is from a single oocyte and a single modulator according to the color key shown. The x-axis represents the percentage change in peak current produced by modulator, and the y-axis represents the percentage change in desensitization rate seen with each modulator. The solid line is a linear regression through the data set with r = 0.67. Each modulator was tested on five to seven oocytes. B, correlation between positive allosteric modulator effects on percentage potentiation and change in desensitization for taurine-activated GlyR. The axes and color coding are the same as in A. The solid line is a linear regression through the data set with r = 0.87. Each modulator was tested on five to seven oocytes.
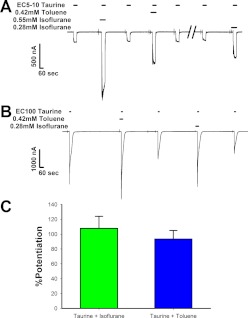
We next investigated the differences in the magnitudes of effects of the volatile anesthetics and the inhaled drugs of abuse. Although all drugs in both categories showed enhancing effects when applied with maximal concentrations of taurine, the degree of potentiation was much larger for the volatile anesthetics than that for the inhaled drugs of abuse. We further examined this difference by testing whether the degree of efficacy inherent in each compound was simply concentration dependent by comparing sample modulators from each group. From the anesthetics, we chose isoflurane because of its high potentiation of maximal taurine responses coupled with a small error in the preincubation experiments, and from the inhalant class, we chose toluene because of its relatively low enhancement of maximal taurine effects in the preincubation experiments. For this experiment, the concentration of taurine producing a 5 to 10% effect (EC5–10) of a maximally effective taurine response was first determined in each oocyte. The concentrations of isoflurane and toluene producing ∼200% potentiation of the EC5–10 response were determined next (Fig. 9A). These concentrations of isoflurane and toluene were then tested using the maximally effective concentration of taurine (EC100), and the effects of isoflurane and toluene were compared (Fig. 9B). Concentrations of 0.42 mM toluene and 0.28 mM isoflurane produced ∼200% potentiation of the EC5–10 taurine response. When tested at EC100 taurine concentrations, isoflurane potentiated the taurine response by 107.7 ± 16.4%, and toluene potentiated the taurine response by 93.4 ± 11.5%; these responses were not significantly different from one another [t(3) = 0.57, P > 0.6].
Fig. 9.
Equi-effective concentrations of modulators at a low taurine concentration also have equal efficacy at a saturating taurine concentration. A, sample tracing showing how the concentrations of toluene and isoflurane that yield an ∼200% potentiating effect of EC5–10 taurine were determined. The tracing shows the effect of EC5–10 taurine with and without several concentrations of isoflurane or toluene. Toluene (0.42 mM) potentiated EC5–10 taurine ∼200%, whereas 0.55 mM isoflurane potentiated to a higher degree, and 0.28 mM isoflurane produced ∼200% potentiation of EC5–10 taurine. EC5–10 taurine applications preceded and followed each coapplication with a modulator. Horizontal bars above tracings indicate the times of exposure to taurine, toluene, or isoflurane at the concentrations indicated. B, sample tracing demonstrating the enhancing effects of the toluene and isoflurane concentrations found in A to produce ∼200% potentiation of taurine-mediated currents, but this time applied with a saturating concentration (EC100) of taurine. Coapplications of taurine and modulator were preceded and followed by applications of EC100 taurine alone. C, summary of the effects of EC100 taurine plus 0.42 mM toluene or 0.28 mM isoflurane. The y-axis represents the percentage increase in current observed in the presence of modulator compared with that produced by taurine alone. Data are shown as mean ± S.E.M. of four oocytes.
Lastly, we investigated whether mutation of a residue in the second transmembrane segment of the α1-subunit previously shown to alter alcohol modulation of glycine-activated GlyR would similarly affect receptors activated by taurine. Figure 10 shows that ethanol, isoflurane, and TCE had minimal effects on S267F homomeric α1-GlyR activated by subsaturating concentrations (EC5–10) of glycine or taurine.
Fig. 10.
The S267F mutation in the second transmembrane segment of the α1-subunit similarly affects modulator enhancement of glycine- and taurine-mediated GlyR function. The effects of 200 mM ethanol, 0.55 mM isoflurane, or 0.56 mM TCE were assayed with EC5–10 concentrations of glycine or taurine. The y-axis represents the percentage change in current observed in the presence of modulator compared with that produced by glycine or taurine applied alone. Data are shown as mean ± S.E.M. of four to five oocytes for each modulator.
Discussion
Glycine receptors, traditionally thought to be responsible for inhibitory neurotransmission only in the brainstem and spinal cord, are also found in higher brain regions (Lynch, 2004). As a result, they have come to be considered possible sites of action for ethanol, volatile anesthetics, and inhaled drugs of abuse (Mihic, 1999; Bowen et al., 2006; Franks, 2008; Lubman et al., 2008; Chau, 2010; Yevenes and Zeilhofer, 2011). After release, the postsynaptic GlyR is initially exposed to millimolar concentrations of glycine, which then rapidly decrease. Enhancement of GlyR function by allosteric modulators is not seen at millimolar glycine concentrations because these compounds left-shift glycine concentration-response curves and exert their greatest effects at low glycine concentrations (Downie et al., 1996; Mascia et al., 1996; Beckstead et al., 2000). Thus, allosteric modulators function to extend the durations of synaptic effects at the GlyR. The partial agonist taurine may be an endogenous ligand at GlyR expressed in the hippocampus (Shibanoki et al., 1993; Mori et al., 2002), nucleus accumbens (Dahchour et al., 1996; Ericson et al., 2006, 2011), and the ventral tegmental area (Wang et al., 2005) where glycine has also been shown to work. This suggests the possibility that ethanol, volatile anesthetics, and inhaled drugs of abuse exert some of their effects through the taurine-activated GlyR and that these responses may differ from those of receptors activated by glycine.
On the basis of the importance of modulator preincubation as shown in Fig. 2, we replicated the saturating agonist and ethanol experiments that we had performed previously (Welsh et al., 2010) but with modifications to the experimental design. We previously tested whether ethanol had any effects on applications of saturating agonist concentrations on the GlyR, and as expected, 200 mM ethanol had no effects on saturating glycine responses or on responses elicited by brief applications of a maximally effective concentration of taurine (Figs. 2 and 3A in Welsh et al., 2010). However, during a long application of a saturating concentration of taurine, 100 mM ethanol seemed to have a small enhancing effect (Fig. 3B in Welsh et al., 2010). Therefore, we tested the effect of preincubation of 200 mM ethanol in a short application protocol and compared its effects with those in the long application experiments. We used this concentration of ethanol to allow us to compare results with the approximately equivalent anesthetizing concentrations used for the anesthetics that we also tested. With preincubation of ethanol, followed by a brief coapplication with agonist, we found no effects on saturating glycine responses and modest (∼40%) potentiating effects in the presence of a saturating concentration of taurine. Ethanol had no effect when combined with glycine, consistent with previous findings that ethanol does not affect the Popen for individual glycine-activated GlyRs (Welsh et al., 2009). However, the modest potentiation of taurine-activated currents suggests that ethanol does increase Popen when receptors are activated by this partial agonist. An increase in single channel conductance could conceivably also explain the potentiation seen here, but it is unlikely given that partial and full agonists elicit the same conductance (Gardner et al., 1984; Lewis et al., 2003). In addition, ethanol does not affect unitary conductance of glycine-activated GlyRs (Welsh et al., 2009).
We sought to further examine the effects of ethanol by coapplying it with maximally effective concentrations of glycine or taurine for 10 min. During constant exposure to a saturating agonist concentration, essentially all receptors enter a fully liganded state, thus minimizing any possible modulator effects on agonist binding or unbinding. When fully liganded, the GlyR exists in one of the three general states: closed, open, or desensitized. Channel activity is observed as a cluster of transitions between closed and open states terminated by receptor desensitization (Beato et al., 2004). Upon resensitization in the presence of a saturating concentration of agonist, the receptor will almost immediately initiate a new cluster of opening/closing events, meaning that for all practical purposes, all transitions leaving the desensitized state almost instantly lead to a fully liganded open state. Thus, long applications of saturating concentrations of agonist result in a pseudo-equilibrium of clusters of channel-opening events separated by sojourns into desensitized states, with no receptors found in the closed unliganded state. Any increases in current produced by allosteric modulators under these conditions could not be attributed to changes in agonist association or dissociation rates. Positive allosteric modulators must instead be acting by increasing the rates of entering (or decreasing the rates of leaving) open states within clusters to increase Popen or by increasing the rates of leaving (or decreasing the rates of entering) desensitized states. Ethanol did not affect currents elicited by long applications of glycine, consistent with our previous data showing that ethanol only affects glycine unbinding rates (Welsh et al., 2009). However, ethanol did seem to slightly potentiate taurine-mediated currents during long applications (Fig. 3, C and D). This is not attributed to ethanol decreasing the rates of desensitization (Fig. 7; Table 1), so the most parsimonious explanation is that ethanol modestly affects taurine-activated intracluster Popen.
We next performed these same experiments using a series of inhaled anesthetics. For these experiments, we tested the alkyl halides chloroform and halothane and the halogenated ethers isoflurane, enflurane, and desflurane. None of the anesthetics tested had any effects on glycine-mediated currents in the brief application experiments, but all produced striking (>150% potentiation) effects on taurine-mediated currents (Fig. 4). Similar to Downie et al. (1996), we found that isoflurane has no effects on maximally effective glycine concentrations but enhances maximal taurine currents. Because the anesthetics enhanced the rates of taurine- but not glycine-mediated desensitization (Fig. 7), this suggests that they increase Popen. If anything, one would expect a slowing of desensitization to increase peak heights. These structurally dissimilar anesthetics had very similar effects, suggesting a common mechanism of action. Lastly, we tested the effects of three inhaled drugs of abuse using the same experimental design, finding that the inhalants produced enhancement of taurine-mediated currents and desensitization rates similar to those seen with ethanol.
The different classes of modulators we tested produced variable enhancement of taurine-activated GlyR currents. We hypothesized that this may be simply attributed to differences in the concentrations used. To test this, we compared the weakly enhancing inhaled drug of abuse toluene with the strongly enhancing anesthetic isoflurane. We first identified the concentrations of each that produced ∼200% potentiation of the effects of low (EC5–10) concentrations of taurine. We next tested those modulator concentrations using a maximally effective taurine concentration, observing almost identical effects of the two. Thus, these classes of modulators may all act via a similar mechanism to enhance taurine currents. The greater percentage enhancement seen at low taurine concentrations probably reflects the dual effects of allosteric modulators on taurine unbinding as well as increasing Popen, whereas only the latter effect was observed at a maximally effective taurine concentration.
In summary, all modulators tested produced enhancement of peak current responses elicited by a saturating concentration of taurine but not glycine. In addition, enhancement of desensitization rates was seen when modulators were applied with taurine but not glycine. Allosteric modulators potentiate both taurine- and glycine-elicited currents when either agonist is present at low concentrations. However, the concentration of either agonist found at the GlyR in vivo is probably high either through the synaptic release of glycine or the osmotic release of taurine. The effects of these modulators on saturating concentrations of taurine are especially interesting given that taurine is the second most abundant amino acid in the brain (Albrecht and Schousboe, 2005) and has also been shown to be released through an osmoresistant calcium-mediated mechanism in the hippocampus (Rodríguez-Navarro et al., 2009). Our findings suggest that allosteric modulators differentially affect the time courses of synaptic events at GlyR, depending on whether the receptor is activated by a full or partial agonist.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant 1R03AA018197]; and the Waggoner Center for Alcohol and Addiction Research.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- GlyR
- glycine receptor
- EC#
- effective concentration giving number percentage of the response of a maximally effective concentration
- Popen
- probability of channel being open
- MBS
- modified Barth's saline
- TCE
- 1,1,1-trichloroethane
- TCY
- trichloroethylene.
Authorship Contributions
Participated in research design: Kirson, Todorovic, and Mihic.
Conducted experiments: Kirson and Todorovic.
Performed data analysis: Kirson and Mihic.
Wrote or contributed to the writing of the manuscript: Kirson and Mihic.
References
- Albrecht J, Schousboe A. (2005) Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30:1615–1621 [DOI] [PubMed] [Google Scholar]
- Baer K, Waldvogel HJ, Faull RL, Rees MI. (2009) Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. (2004) The activation mechanism of alpha1 homomeric glycine receptors. J Neurosci 24:895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. (2000) Glycine and gamma-aminobutyric acidA receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol 57:1199–1205 [PubMed] [Google Scholar]
- Beckstead MJ, Phelan R, Mihic SJ. (2001) Antagonism of inhalant and volatile anesthetic enhancement of glycine receptor function. J Biol Chem 276:24959–24964 [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Phelan R, Trudell JR, Bianchini MJ, Mihic SJ. (2002) Anesthetic and ethanol effects on spontaneously opening glycine receptor channels. J Neurochem 82:1343–1351 [DOI] [PubMed] [Google Scholar]
- Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. (2006) The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol 28:636–647 [DOI] [PubMed] [Google Scholar]
- Chau PL. (2010) New insights into the molecular mechanisms of general anaesthetics. Br J Pharmacol 161:288–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kendig JJ. (2002) Pre- and postsynaptic volatile anaesthetic actions on glycinergic transmission to spinal cord motor neurons. Br J Pharmacol 136:673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A. (1984) Expression of exogenous DNA in Xenopus oocytes, in Transcription and Translation: A Practical Approach (Hames BD, Higgins SJ. eds) pp 49–69, Oxford Press, Washington, DC [Google Scholar]
- Dahchour A, Quertemont E, De Witte P. (1996) Taurine increases in the nucleus accumbens microdialysate after acute ethanol administration to naïve and chronically alcoholised rats. Brain Res 735:9–19 [DOI] [PubMed] [Google Scholar]
- Downie DL, Hall AC, Lieb WR, Franks NP. (1996) Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurons and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol 118:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Molander A, Stomberg R, Söderpalm B. (2006) Taurine elevates dopamine levels in the rat nucleus accumbens: antagonism by strychnine. Eur J Neurosci 23:3225–3229 [DOI] [PubMed] [Google Scholar]
- Ericson M, Chau P, Clarke RB, Adermark L, Söderpalm B. (2011) Rising taurine and ethanol concentrations in nucleus accumbens interact to produce dopamine release after ethanol administration. Addict Biol 16:377–385 [DOI] [PubMed] [Google Scholar]
- Evans EB, Balster RL. (1991) CNS depressant effects of volatile organic solvents. Neurosci Biobehav Rev 15:233–241 [DOI] [PubMed] [Google Scholar]
- Franks NP. (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386 [DOI] [PubMed] [Google Scholar]
- Gardner P, Ogden DC, Colquhoun D. (1984) Conductances of single ion channels opened by nicotinic agonists are indistinguishable. Nature 309:160–162 [DOI] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. (2008) On the nature of partial agonism in the nicotinic receptor superfamily. Nature 454:722–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TM, Schofield PR, McClellan AM. (2003) Kinetic determinants of agonist action at the recombinant human glycine receptor. J Physiol 549:361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye JH. (2011) Glycine-activated chloride currents of neurons freshly isolated from the prefrontal cortex of young rats. Brain Res 1393:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Lawrence AJ. (2008) Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol 154:316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84:1051–1095 [DOI] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. (1996) A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol 50:402–406 [PubMed] [Google Scholar]
- Mihic SJ, McQuilkin SJ, Eger EI, 2nd, Ionescu P, Harris RA. (1994) Potentiation of gamma-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol 46:851–857 [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389 [DOI] [PubMed] [Google Scholar]
- Mihic SJ. (1999) Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int 35:115–123 [DOI] [PubMed] [Google Scholar]
- Molander A, Löf E, Stomberg R, Ericson M, Söderpalm B. (2005) Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res 29:38–45 [DOI] [PubMed] [Google Scholar]
- Molander A, Lidö HH, Löf E, Ericson M, Söderpalm B. (2007) The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol 42:11–18 [DOI] [PubMed] [Google Scholar]
- Mori M, Gähwiler BH, Gerber U. (2002) Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol 539:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Phelan R, Erlichman BS, Pillai RN, Ma L, Lopreato GF, Mihic SJ. (2006) Occupancy of a single anesthetic binding pocket is sufficient to enhance glycine receptor function. J Biol Chem 281:3305–3311 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro JA, Gonzalo-Gobernado R, Herranz AS, Gonźlez-Vigueras JM, Solís JM. (2009) High potassium induces taurine release by osmosensitive and osmoresistant mechanisms in the rat hippocampus in vivo. J Neurosci Res 87:208–217 [DOI] [PubMed] [Google Scholar]
- Shibanoki S, Kogure M, Sugahara M, Ishikawa K. (1993) Effect of systemic administration of N-methyl-d-aspartic acid on extracellular taurine level measured by microdialysis in the hippocampal CA1 field and striatum of rats. J Neurochem 61:1698–1704 [DOI] [PubMed] [Google Scholar]
- Wang F, Xiao C, Ye JH. (2005) Taurine activates excitatory non-synaptic glycine receptors on dopamine neurones in ventral tegmental area of young rats. J Physiol 565:503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh BT, Goldstein BE, Mihic SJ. (2009) Single-channel analysis of ethanol enhancement of glycine receptor function. J Pharmacol Exp Ther 330:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh BT, Kirson D, Allen HM, Mihic SJ. (2010) Ethanol enhances taurine-activated glycine receptor function. Alcohol Clin Exp Res 34:1634–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Mihic SJ, Harris RA. (1999) Amino acid volume and hydropathy of a transmembrane site determine glycine and anesthetic sensitivity of glycine receptors. J Biol Chem 274:23006–23012 [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ueno T, Akaike N, Ikemoto Y. (2001) Modulation of miniature inhibitory postsynaptic currents by isoflurane in rat dissociated neurons with glycinergic synaptic boutons. Eur J Pharmacol 431:269–276 [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. (1998) Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position alpha267. J Biol Chem 273:3314–3319 [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Zeilhofer HU. (2011) Allosteric modulation of glycine receptors. Br J Pharmacol 164:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]