Abstract
Previous studies have demonstrated the capacity of a long-acting mutant form of a naturally occurring bacterial double mutant cocaine esterase (DM CocE) to antagonize the reinforcing, discriminative, convulsant, and lethal effects of cocaine in rodents and reverse the increases in mean arterial pressure (MAP) and heart rate (HR) produced by cocaine in rhesus monkeys. This study was aimed at characterizing the immunologic responses to repeated dosing with DM CocE and determining whether the development of anti-CocE antibodies altered the capacity of DM CocE to reduce plasma cocaine levels and ameliorate the cardiovascular effects of cocaine in rhesus monkeys. Under control conditions, intravenous administration of cocaine (3 mg/kg) resulted in a rapid increase in the plasma concentration of cocaine (n = 2) and long-lasting increases in MAP and HR (n = 3). Administration of DM CocE (0.32 mg/kg i.v.) 10 min after cocaine resulted in a rapid hydrolysis of cocaine with plasma levels below detection limits within 5 to 8 min. Elevations in MAP and HR were significantly reduced within 25 and 50 min of DM CocE administration, respectively. Although slight (10-fold) increases in anti-CocE antibodies were observed after the fourth administration of DM CocE, these antibodies did not alter the capacity of DM CocE to reduce plasma cocaine levels or ameliorate cocaine's cardiovascular effects. Anti-CocE titers were transient and generally dissipated within 8 weeks. Together, these results suggest that highly efficient cocaine esterases, such as DM CocE, may provide a novel and effective therapeutic for the treatment of acute cocaine intoxication in humans.
Introduction
Cocaine abuse remains a significant public health problem with an estimated 15 million to 19 million individuals using cocaine within the past year worldwide (United Nations Office on Drugs and Crime, 2010). In the United States alone there are an estimated 1.5 million current cocaine users, with approximately 1700 people trying cocaine for the first time each day (Substance Abuse and Mental Health Services Administration, 2011). Although moderate doses of cocaine are often associated with “pleasurable” effects, large doses of cocaine can produce a variety of adverse effects including anxiety, convulsion, delirium, hypothermia, and chest pain, the latter of which results from cocaine-induced increases in mean arterial pressure (MAP) and heart rate (HR) (Olson et al., 1994; Glauser and Queen, 2007). These large-dose effects of cocaine account for the majority of all illicit drug-related emergency department (ED) visits in the United States, with recent estimates suggesting that cocaine-related ED cases are more than twice as common as those involving heroin use and four times as common as those involving other stimulants, such as methamphetamine (Substance Abuse and Mental Health Services Administration, 2011).
Despite longstanding efforts to identify small molecules capable of selectively inhibiting the reinforcing and/or toxic effects of cocaine (Dackis and O'Brien, 2003; Grabowski et al., 2004; Vocci et al., 2005; Tanda et al., 2009), there are currently no Food and Drug Administration-approved medications for the treatment of cocaine abuse or toxicity. Significant effort has been directed toward the development of cocaine-specific enzymes capable of reducing the reinforcing and/or toxic effects of cocaine by dramatically altering its pharmacokinetics. In both human and nonhuman primates, cocaine is naturally metabolized by butyrylcholinesterase (BChE) to the inactive metabolites ecgonine methyl ester and benzoic acid with an elimination half-life of ∼45 min (Mendelson et al., 1999; Mello et al., 2002). Through a series of site-directed mutagenesis studies, Zhan and colleagues identified mutant BChEs capable of hydrolyzing cocaine approximately 450 to 2000 times faster than native BChE (Pan et al., 2005; Zheng et al., 2008). In rats and mice, these mutant BChEs effectively reduced the cardiovascular, lethal, and abuse-related effects of cocaine (Brimijoin et al., 2008; Zheng et al., 2008; Carroll et al., 2011; Xue et al., 2011), suggesting that such enzymes may provide a viable strategy for treating cocaine toxicity and abuse in humans.
In a parallel series of studies, a highly efficient bacterial cocaine esterase (CocE) (kcat/Km ∼800-fold greater than BChE; Larsen et al., 2002; Turner et al., 2002) was extensively evaluated as an alternative to BChE, which is often difficult to purify or produce in the laboratory (Huang et al., 2007). Although the wild-type (wt) form of CocE dose-dependently protects mice and rats against the cardiovascular, convulsant, and lethal effects of cocaine, it is rapidly inactivated at body temperature with a half-life of ∼15 min (Cooper et al., 2006; Ko et al., 2007, 2009; Jutkiewicz et al., 2009; Wood et al., 2010). Site-directed mutations have improved thermostability and resulted in an equally efficient mutant CocE (T172R/G173Q CocE; RQ CocE; DM CocE) that retains some activity in vitro and in vivo for more than 4 h (Collins et al., 2009; Gao et al., 2009; Narasimhan et al., 2010). In addition to rapidly hydrolyzing circulating cocaine in rats and monkeys (Brim et al., 2011a, 2012), DM CocE is capable of dose-dependently inhibiting the cardiovascular, convulsant, lethal, and reinforcing effects of cocaine in rats and rhesus monkeys (Collins et al., 2009, 2011a,b).
Although these findings suggest that highly efficient cocaine-hydrolyzing enzymes, such as CocE, may provide a valuable therapeutic option for the treatment of acute cocaine toxicity, it is important to note that the repeated administration of the wt form of the bacterial CocE elicits potentially neutralizing immune responses in mice (Ko et al., 2007, 2009). Although increases in anti-CocE antibodies were observed during an initial dose-response study of DM CocE in rhesus monkeys (Collins et al., 2011a), the immunologic potential of DM CocE has yet to be systematically evaluated in any species. Thus, the current studies characterized the capacity of DM CocE to stimulate the development of anti-CocE antibodies and evaluated the potential for these anti-CocE antibodies to neutralize the effectiveness of DM CocE in reducing plasma cocaine levels and ameliorating the cardiovascular effects of cocaine during four biweekly trials in rhesus monkeys.
Materials and Methods
Subjects.
Three adult male (BE, BL, and CA), and two adult female (UR and KY) rhesus monkeys (Macaca mulatta) were used in these studies. All monkeys were singly housed in stainless-steel monkey cages in an environmentally controlled room (temperature 21 ± 3°C; relative humidity 30–70%; 10–15 air changes per hour) under a 12-h light/dark cycle with lights on at 7:00 AM. The monkeys' diet consisted of 20-50 Lab Fiber Plus Monkey Diet Chow (Lab Diet; PMI Nutrition International, LLC, Brentwood, MO), fresh fruit, and free access to water, and health checks were performed daily to ensure that all monkeys remained healthy. All experimental procedures were approved by the University of Michigan Committee on the Use and Care of Animals and performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996), as adopted and promulgated by the National Institutes of Health.
Effectiveness of DM CocE to Reduce Plasma Cocaine Concentrations after Repeated Dosing.
One male (BE) and one female (UR) adult rhesus monkey, trained for arm-restraint chairs, were used to evaluate the time course of the plasma levels of cocaine produced by an intravenous infusion of 3 mg/kg cocaine. Each monkey was tested with this dose of cocaine five times [once with phosphate-buffered saline (PBS) and four times with DM CocE] with 14 days separating each administration. Once seated in the restraint chairs, each monkey had an acute catheter placed in the saphenous vein to allow for the administration of cocaine (3 mg/kg at t = 0 min) and 0.32 mg/kg DM-CocE or PBS, with each infusion followed by a 3-ml flush with physiologic saline to clear the catheter and ensure the entire dose had been delivered. For both monkeys PBS was delivered 10 min after cocaine; however, because of the onset of preconvulsant behaviors (e.g., tremor and drooling), BE received DM CocE at the 1-min postcocaine time point rather than the 10-min time point used for UR. These catheters also allowed for serial collection of blood samples taken 2 min before cocaine and 1, 8, 15, 30, 60, 90, and 120 min after cocaine administration. The precocaine blood sample was used to determine both anti-CocE titer levels and baseline cocaine concentrations in the plasma, whereas samples collected at later time points were used only to determine plasma concentrations of cocaine. For this reason, the precocaine sample was split so that 0.5 ml of blood was allowed to clot at room temperature before the collection of serum, with the remainder of the sample (∼2 ml) treated identically to the samples taken at later time points. Each sample was transferred into tubes containing EDTA (5 ml; BD Vacutainer K2EDTA Plus Blood Collection; BD Biosciences, San Jose, CA) and 1/10 volume of 1 M NaF to prevent clotting and eliminate further cocaine metabolism, respectively. Samples were centrifuged at 4000 rpm for 5 min at 4°C and transferred to 2-ml cryovials before being stored at −80°C. Portions of the data from these studies (PBS and DM CocE first-trial conditions) have been published previously (Brim et al., 2012).
Sample Preparation and Mass Spectral Analysis of DM CocE-Mediated Cocaine Hydrolysis.
Plasma fractions from each blood sample (50–200 μl) were added to 570 μl of acetonitrile, 20 μl of 1 M NaF, and 2 μl of internal standard solution containing 750 nM deuterium-labeled norcocaine, cocaine, benzoylecgonine, and ecgonine methyl ester (Cerilliant Corporation, Round Rock, TX). Cocaine metabolites were included as internal standards to maintain consistency between this study and other in vivo cocaine metabolite studies (Brim et al., 2011a, 2012). Samples were vortexed for 30 s and centrifuged at 25,000 relative centrifugal force at 20°C for 30 min. The supernatant was removed and added to a clean microcentrifuge tube. Samples were centrifuged a second time under the same conditions, and the supernatants were again transferred to clean tubes. Samples were evaporated to dryness in a vacuum centrifuge and stored at −80°C until analysis.
Mass spectral analysis was performed at the University of Michigan Biomedical Mass Spectrometry Facility as described previously (Brim et al., 2012). In brief, the dried samples were reconstituted with 30 μl of 10 mM ammonium formate, pH 4.6/acetonitrile (97:3; v/v) to yield a 50 nM final concentration of each internal standard. To achieve cocaine concentrations within the limits of quantification, samples were diluted further (varying along the time course) with 10 mM ammonium formate, pH 4.6/acetonitrile (97:3; v/v) and 50 nM internal standards. Samples were vortexed for 30 s, then centrifuged at 13,600 relative centrifugal force for 20 min. Aliquots of the supernatants were transferred to polypropylene autosampler vials for analysis within 12 h. Liquid chromatography/mass spectrometry was performed on a Prominence high-performance liquid chromatography system (Shimadzu, Kyoto, Japan) interfaced directly to the Turbo Ionspray source of an API 3000 triple quadrupole mass spectrometer (PerkinElmerSciex Instruments, Boston, MA). Separation was achieved with a Thermo Fisher Scientific (Waltham, MA) Hypersil Gold column (50 × 2.1 mm i.d.; 1.9-μm packing) maintained at 45°C by using a binary gradient and a flow rate of 0.45 ml/min. The injection volume was 4 μl, and the flow was split approximately 1:3.5 so that 0.13 ml/min was directed into the ionization source. Solvent A was 10 mM ammonium formate, pH 4.6, and solvent B was acetonitrile. The gradient program was as follows: 2% B at 0 min, hold 2% B for 1 min, 18% B at 2 min, 40% B at 10 min, 100% B at 11 min, 2% B at 12 min, and re-equilibrate at 2% B for 3 min. The sample tray was cooled to 10°C to prevent sample degradation, and each analysis was completed within 15 min.
Analyst software (version 1.4.2; MDS Sciex, Concord, ON, Canada) was used for instrument control, data acquisition, and quantitative analysis. Calibration curves were constructed from the standard samples. The ratio of the peak area of cocaine to the corresponding deuterium-labeled internal standards was plotted as a function of the analyte concentration normalized to the internal standard concentration. Calibration curves were generated by using a least-squares linear regression analysis with 1/× weighting. Calibration standards for cocaine (4.0–0.03 μM) were prepared in commercial plasma from untreated animals (Valley Biomedical, Winchester VA). All standards were stored at −80°C and prepared fresh for each set of experimental samples. Twenty microliters of each calibration stock was extracted with 68 μl of acetonitrile, 4 μl of 1 M NaF, and 8 μl of internal standard and prepared identically to the samples described above. Calibration standards were reconstituted to 100 μl, resulting in final internal standard concentrations of 50 nM.
Surgical Preparation.
Three of the monkeys (BL, CA, and KY) were implanted with radio-telemetric probes (D70-PCT; DSI Inc., St. Paul, MN) to allow for the collection of cardiovascular measures and an indwelling venous catheter to allow for drug delivery. Before surgery, monkeys were anesthetized with ketamine (10.0 mg/kg i.m.) and placed on a heating pad set to maintain the animal's body temperature at approximately 37°C. Monkeys were prepared by shaving the hair along the right flank, above the femoral artery on the right leg, and the left and just above the zyphoid process and to the right of the right clavicle. All areas were scrubbed with alternating betadine/alcohol swabs, and small incisions were made to allow for the implantation of the telemetric probe. A pocket was teased out to allow for placement of the probe, and the blood pressure catheter was tunneled to and implanted in the femoral artery to allow for arterial pressure measures. Electrocardiographic leads were tunneled to the incisions above the zyphoid process and clavicle and sutured to the muscle. All incisions were closed with 5-0 Ethilon suture, and monkeys were allowed 5 to 7 days to recover from surgery before implantation of an indwelling catheter in a previously unused vein (i.e., jugular or femoral vein). Monkeys were shaved between the scapula and above the vein to be catheterized, and areas were scrubbed with alternating betadine/alcohol swabs. Small incisions were made between the scapula and above the vein to be catheterized, and upon implantation the catheter was tunneled to and exited from the incision between the scapula. These monkeys were then fitted with mesh jackets and attached to a steel tether on a swivel to allow for unrestrained movement in the animal's home cage. A recovery period of at least 7 days was provided before experimentation. Catheters were flushed daily with 3 ml of saline to ensure catheter patency.
Effectiveness of DM CocE to Ameliorate Cocaine-Induced Changes in MAP, HR, Core Body Temperature, and Locomotor Activity after Repeated Dosing.
Three adult rhesus monkeys, two males (BL and CA) and one female (KY), were used to evaluate the cardiovascular effects of intravenous cocaine (3 mg/kg). Each monkey was tested with this dose of cocaine six times, with each test separated by 14 days to reduce the possibility of the development of tolerance to the cardiovascular effects of cocaine and to increase the possibility of observing the development of anti-CocE antibodies. Each dose of cocaine was followed immediately by a 5-ml saline flush to ensure the entire dose was delivered. Experimental treatments (PBS or 0.32 mg/kg DM CocE) were administered intravenously, 10 min after cocaine, and were similarly followed by a 5-ml saline flush. PBS served as the vehicle control and was always evaluated during the first and last test sessions (sessions 1 and 6) to allow for the development of tolerance or sensitization to cocaine's effects to be observed. The effects of 0.32 mg/kg i.v. DM CocE were always evaluated during the intervening four test sessions (sessions 2- 5). Test sessions were performed between 1:00 PM and 5:00 PM, with real-time measures of MAP, HR, core body temperature, and locomotor activity collected at 1-s intervals for at least 45 min before and 120 min after cocaine administration. To determine whether monkeys were developing anti-DM CocE antibodies, serum samples were collected from each monkey at 2-week intervals throughout the course of the study, beginning with the initial cocaine versus PBS condition. During weeks in which monkeys were tested 2 ml of blood was collected via the saphenous vein 24 h before the test session. Blood samples were collected without preservatives and stored at room temperature for 60 min before centrifugation at 4000 rpm for 5 min at 4°C. Serum was then collected and pipetted into 2-ml cryovials and stored at −80°C until being assayed for anti-CocE antibody titer determinations.
Immunologic Determinations.
To determine whether monkeys were developing anti-DM CocE antibodies, a direct enzyme-linked immunosorbent assay specific for anti-CocE antibodies was set up by using a standard protocol. CocE was used (1 g/ml) to coat a 96-well microtiter plate by using borate-buffered saline (1.5 M NaCl, 0.5 M H3BO3, and 1.0 M NaOH) to resuspend CocE (50 μl/well). The coating plates were left overnight at 4°C. The coating buffer was removed the next morning, and the plates were blocked with 2% normal goat serum in phosphate-buffered saline for 1 h at 37°C and washed three times. Serum from the various monkeys was serially diluted in 50 μl of phosphate-buffered saline in the wells in a range of 102 to 107 and run in duplicate. The plates were covered and incubated for 1 h at 37°C. Subsequently, the plates were washed three times, and 50 μl/well of goat anti-mouse IgG peroxidase-labeled antibody was diluted 1:400. The plates were then washed three times, and 100 μl of peroxidase substrate solution (O-phenylenediamine-dissolved citrate/phosphate buffer) was added to each well. After a 5- to 10-min incubation (based on color development in the positive controls), the reaction was stopped by using 3 M H2SO4 (50 μl/well). The plates were read at 490 nm, and titer was determined by the highest dilution that showed increases over background absorbance.
Drugs.
Cocaine HCl was obtained from Mallinckrodt (Hazelwood, MO) and dissolved in 0.9% sterile saline to a concentration of 10.0 mg/ml and administered on a milligram/kilogram basis over 30 s. DM CocE (T172R/G173Q CocE) was prepared as described previously (Brim et al., 2012) and stored at −80°C until needed. Endotoxin levels for these preparations were assessed by using an endpoint Limulus Amebocyte Lysate assay (Charles River, Margate, Kent, UK) according to the manufacturer's specifications and were less than 30 EU/ml (∼0.2 ng/ml or less than 2 EU/kg at the 0.32 mg/kg dose of DM CocE). Before administration, DM CocE (5.0 mg/ml) was thawed on ice and administered on a milligram/kilogram basis over 10 s.
Data Analysis.
Real-time measures of MAP, HR, body temperature (°C), and locomotor activity were collected at 1-s intervals, beginning at least 45 min before cocaine administration. Baseline measures for each of the parameters represent the mean ± S.E.M. of the 15 min before each infusion for each measure with the exception of locomotor activity, which represents the total locomotor activity observed during the 10-min period immediately before the infusion of cocaine. Cardiovascular and physiologic parameters were collected for at least 120 min after cocaine infusion, and the effects of cocaine or CocE on each of the parameters are reported as the change from baseline for each 5-min block of time (mean of 5-min block − mean of 15 min before infusion). Locomotor activity was summed over 10-min blocks and reported as the total locomotor counts/10 min. Two-way ANOVA with repeated measures and post hoc Bonferroni tests were used to determine whether DM CocE administration produced significant alterations in the cardiovascular or physiologic effects of 3 mg/kg i.v. cocaine for each 5-min bin over the 120-min period after cocaine infusion and to determine whether the effectiveness of DM CocE was altered with repeated administration. Plasma cocaine levels are reported as the mean ± S.E.M. concentration of cocaine (nanograms/milliliters) for each time point. Two-way ANOVA with repeated measures and post hoc Bonferroni tests were used to determine whether DM CocE administration significantly plasma cocaine concentrations associated with 3 mg/kg i.v. cocaine at each time point and whether the effectiveness of DM CocE was altered with repeated administration. Physiologic and locomotor data are also presented as the mean ± S.E.M. (change in MAP, HR, and temperature) or total (total locomotor activity) for the 110-min period after DM CocE (or PBS) administration. Significant differences among conditions were determined by one-way ANOVA with repeated measures and post hoc Bonferroni tests. Immunologic data are expressed as the mean ± S.E.M. change from baseline (pre-CocE) in the anti-CocE antibody titer (using a log 10 dilution). One-way ANOVA with repeated measures and post hoc Dunnett's tests were used to determine whether there was a significant effect of DM CocE administration on anti-CocE titer levels over time.
Results
Effectiveness of DM CocE to Reduce Plasma Cocaine Concentrations after Repeated Dosing.
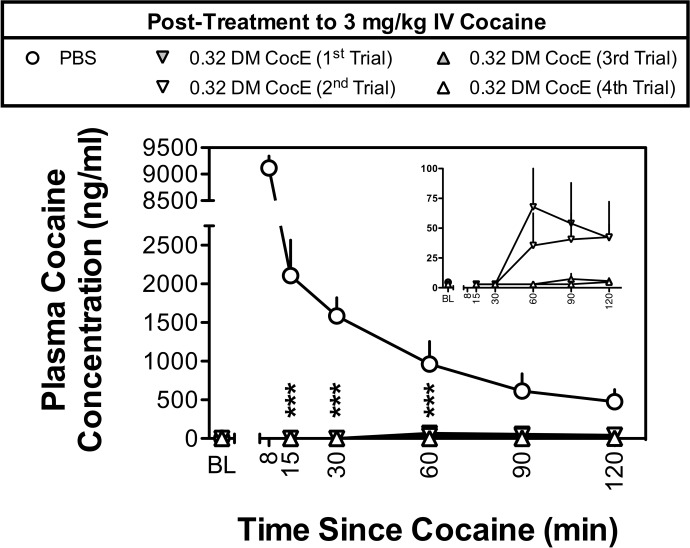
As shown in Fig. 1, intravenous administration of 3 mg/kg cocaine, followed 10 min later by PBS, resulted in large increases in plasma cocaine concentrations, with cocaine concentrations of 9115.8 ± 227.5, and 2106.8 ± 459.6 ng/ml plasma at the 8- and 15-min time points, respectively. Assuming the high concentrations of cocaine obtained at the 8-min time point were caused by the bolus of cocaine not being fully distributed or contamination of the sample with residual cocaine in the sampling catheter, cocaine exhibited normal first-order kinetics with an elimination half-life of t1/2 = 50.7 min.
Fig. 1.
Plasma concentrations of cocaine produced by intravenous doses of 3 mg/kg cocaine during five biweekly trials in two rhesus monkeys. Plasma cocaine concentrations were assessed by mass spectrometry ∼2 min before cocaine (baseline; BL) and at 8, 15, 30, 60, 90, and 120 min after the administration of cocaine. ○ represent the mean ± S.E.M. plasma concentrations of cocaine when PBS was administered 10 min after 3 mg/kg cocaine. Plasma concentrations of cocaine during the first (▾), second (▿), third (▴), and fourth (▵) trials in which 0.32 mg/kg DM CocE was administered as a post-treatment to 3 mg/kg cocaine are shown. Data from the 8-min time point were excluded because of differences in the post-treatment times (UR, 10 min post-treatment; BE, 1 min post-treatment). Inset shows plasma cocaine concentrations during the four DM CocE conditions plotted on a y axis with a smaller range of concentrations. ***, p < 0.001. Significant differences in plasma cocaine concentrations were determined by two-way ANOVA with repeated measures and post hoc Bonferroni tests.
Although the experimental design called for DM CocE to be administered 10 min after cocaine, the timing of DM CocE administrations had to be adjusted because of differences in the sensitivities of the monkeys to the large dose effects of cocaine (BE exhibited tremor, sialorrhea, and decreased respiration shortly after the bolus administration of 3 mg/kg i.v. cocaine). For this reason, BE received DM CocE 1 min after cocaine, whereas UR received DM CocE 10 min after cocaine. Despite this difference, the administration of DM CocE (0.32 mg/kg i.v.) significantly reduced (treatment: F4,20 = 71.4, p < 0.001; time: F4,20 = 5.8, p < 0.05) the plasma cocaine concentrations to below the limits of quantification within the first 5 to 7 min after administration. Although cocaine levels remained below quantification limits at the 30-min time point, a slight rise in plasma cocaine concentrations was observed over the second hour of sampling during the first two tests with DM CocE (Fig. 1 Inset). There were no significant differences observed when the plasma levels of cocaine were compared across the four DM CocE test sessions. Data from the PBS and DM CocE first-trial conditions have been published previously (Brim et al., 2012).
Effectiveness of DM CocE to Ameliorate Cocaine-Induced Changes in MAP, HR, Core Body Temperature, and Locomotor Activity after Repeated Dosing.
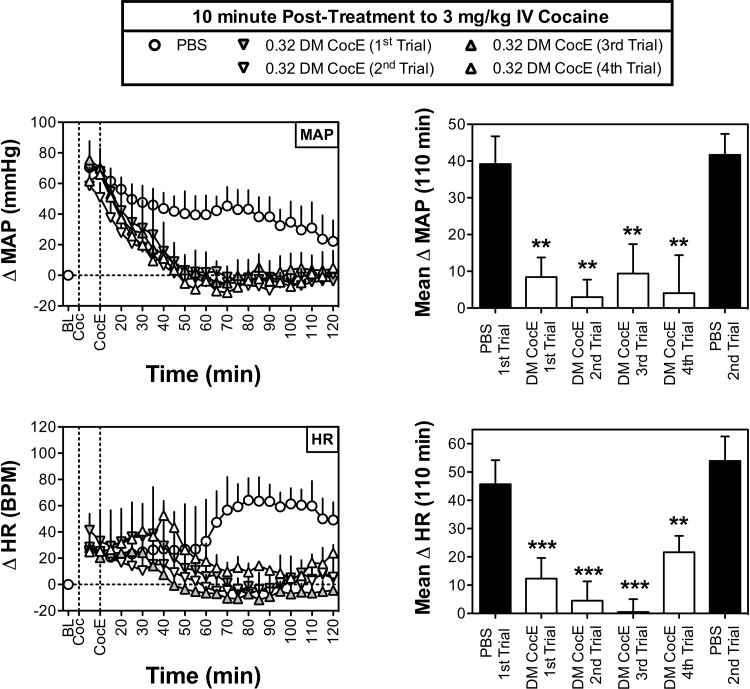
Figure 2 depicts the cardiovascular effects of 3 mg/kg i.v. cocaine during six biweekly sessions in which PBS or 0.32 mg/kg i.v. DM CocE was administered 10 min after cocaine. Although cocaine produced rapid increases in MAP that peaked (69.2 ± 2.7 mm Hg above baseline) within the first 5 to 10 min of cocaine administration, the cocaine-induced increases in HR were slower to develop with peak increases (73.7 ± 9.1 bpm) in HR typically observed within 80 min of cocaine administration.
Fig. 2.
Effects of repeated doses of DM CocE (0.32 mg/kg i.v.) or PBS on the cocaine-induced changes in MAP (top) and HR (bottom) when administered 10 min after an intravenous dose of 3 mg/kg cocaine (n = 3) during six biweekly trials. Left, data points represent the mean ± S.E.M. change from baseline for MAP or HR over successive 5-min blocks of time during sessions in which 3 mg/kg cocaine was followed by PBS (○) or 0.32 mg/kg DM CocE (triangles). Right, data represent the mean ± S.E.M. change from baseline for MAP or HR over the entire 110 min after the administration of PBS (filled bars) or 0.32 mg/kg DM CocE (open bars). **, p < 0.001; ***, p < 0.001. Significant differences in mean change in MAP or HR among conditions were determined by one-way ANOVA with repeated measures with post hoc Bonferroni tests.
As shown in Fig. 2, top left, administration of 0.32 mg/kg i.v. DM CocE 10 min after cocaine resulted in a significant (treatment: F4,176 = 135.0, p < 0.001; time: F4,176 = 4.6, p < 0.001) and rapid amelioration of cocaine's hypertensive effects with significant reductions in MAP observed within 25 to 35 min of DM CocE administration. Cocaine-induced increases in MAP returned to baseline within 30 min of DM CocE administration where they remained for the remainder of the 120-min session. As shown in Fig. 2, top right, DM CocE also significantly reduced (F5,10 = 14.1; p < 0.001) the mean change in MAP over the final 110 min of each of the four repeated trials with 0.32 mg/kg i.v. DM CocE. It is noteworthy that the effectiveness of DM CocE to reduce the hypertensive effects of cocaine did not change across the four trails.
Administration of 0.32 mg/kg DM CocE produced a similar and significant reduction (treatment: F4,176 = 153.9, p < 0.001; time: n.s.) in the tachycardic effects of cocaine when evaluated across 5-min blocks of time. Although significant reductions in cocaine's HR-stimulating effects were not observed until 45 to 50 min after DM CocE administration, it is important to note that these effects correspond to the portion of the sessions when the effects of cocaine's tachycardic effects were largest. As was observed with MAP, DM CocE significantly reduced the mean change in HR observed over the final 110 min of the session (F5,10 = 41.7; p < 0.001). Although there were no significant differences in the effectiveness of DM CocE to ameliorate the tachycardic effects of cocaine across the four biweekly tests, cocaine did seem to produce a slightly larger mean HR response during the fourth trial. Although it is possible that this may represent a loss of activity, it is important to note that HR responses seemed to spike at the 40-min time point before decreasing to near baseline-like levels. There were no significant differences between the MAP- or HR-stimulating effects of cocaine during the PBS test sessions that were conducted before and after DM CocE tests.
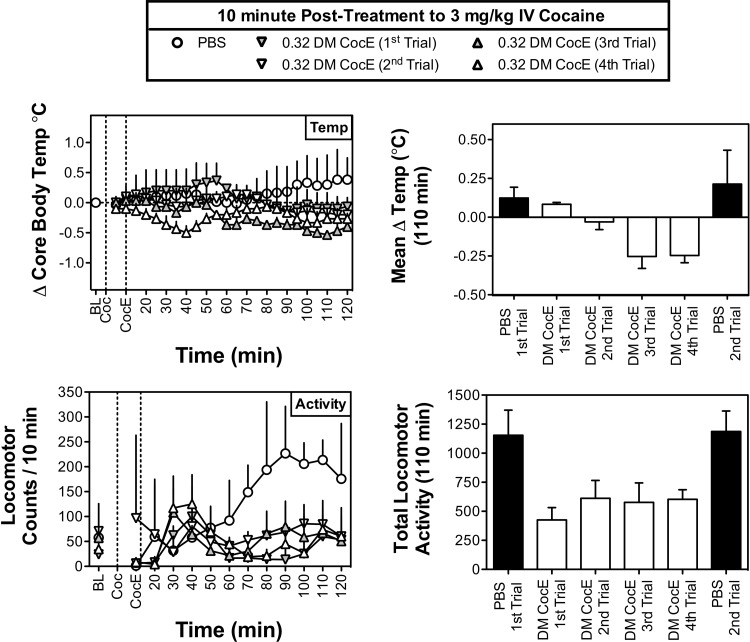
In addition to the cardiovascular effects described above, the intravenous administration of 3 mg/kg cocaine stimulated locomotor activity and produced a modest increase in core body temperature (Fig. 3). Not only was the administration of DM CocE (0.32 mg/kg i.v.) 10 min after cocaine effective at reducing cocaine's hyperthermic effects when evaluated over individual 5-min bins (treatment: F4,176 = 44.0, p < 0.001; time: n.s.), but a similar reduction in the mean change in body temperature was also observed over the final 110 min of the session (F5,10 = 5.6; p < 0.05). Although there were no significant differences in the body temperature responses observed across the four DM CocE trials, the mean temperatures obtained during the third and fourth trials with DM CocE were significantly lower than those obtained in the PBS condition (p < 0.01 for both), suggesting that the administration of DM CocE may have produced a mild hypothermia. Finally, the administration of 0.32 mg/kg i.v. DM CocE was similarly effective at reducing the locomotor stimulatory effects of 3 mg/kg cocaine. Not only was DM CocE effective at reducing cocaine-stimulated activity when evaluated across 10-min bins (treatment: F4,88 = 15.4, p < 0.001; time: n.s.), but DM CocE also reduced the locomotor effects of cocaine when they were summed across the final 110 min of the session (F5,10 = 3.7; p < 0.05). Although DM CocE seemed to produce a consistent inhibition of cocaine's locomotor stimulatory effects, post hoc tests failed to reveal any significant differences among the treatments.
Fig. 3.
Effects of repeated doses of DM CocE (0.32 mg/kg i.v.) or PBS on the cocaine-induced changes in core body temperature (°C; top) and locomotor activity (bottom) when administered 10 min after an intravenous dose of 3 mg/kg cocaine (n = 3) during six biweekly trials. Left, data points represent the mean ± S.E.M. change from baseline for temperature or sum of locomotor activity over successive 5-min blocks of time during sessions in which 3 mg/kg cocaine was followed by PBS (○) or 0.32 mg/kg DM CocE (triangles). Right, data represent the mean ± S.E.M. change from baseline for temperature or sum of locomotor activity over the entire 110 min after the administration of PBS (filled bars) or 0.32 mg/kg DM CocE (open bars).
Immunologic Effects of Repeated Dosing with DM CocE.
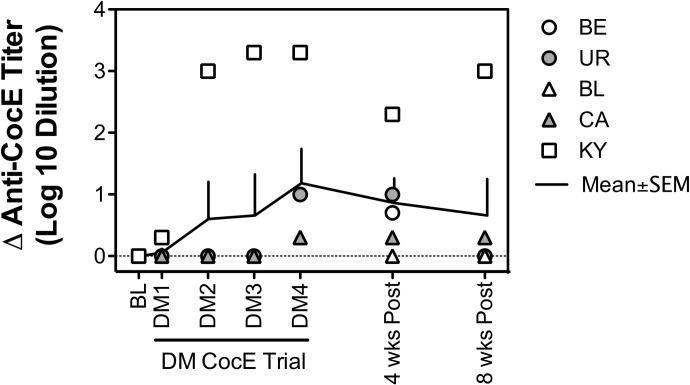
Figure 4 shows the effects of repeated administration of 0.32 mg/kg i.v. DM CocE on the development, and subsequent disappearance, of anti-CocE antibodies for each of the five monkeys. Although the first three administrations of DM CocE failed to increase anti-CocE titers in four of the five monkeys, each of the five monkeys displayed some level of immunologic response after the fourth trial, with a mean increase in anti-CocE titer of ∼10-fold. The strongest response (∼1000-fold increase in anti-CocE titer) was observed in KY, the female monkey that participated in the cardiovascular portion of the study, whereas the weakest responses (∼3-fold increase in anti-CocE titer) was observed in monkeys BL and CA, the two male monkeys that participated in the cardiovascular portion of the study. Although the administration of DM CocE tended to result in a gradual increase in anti-CocE titer over the course of the four trials, these effects failed to reach significance when the data were grouped. Just as anti-CocE titers gradually increased with the repeated administration of DM CocE, discontinuation of treatment led to a gradual decline in anti-CocE titer levels, with four of the five monkeys showing little or no immunologic response by 8 weeks after treatment.
Fig. 4.
Time course for the development of anti-CocE antibody titers after the repeated administration of 0.32 mg/kg DM CocE. Serum samples were collected 24 h before each of six biweekly trials (PBS and DM CocE) and 4 and 8 weeks after the final DM CocE administration. Symbols represent anti-CocE antibody titers for each of the five monkeys. Solid line represents the mean ± S.E.M. of the anti-CocE antibody titers (n = 5).
Discussion
We have described previously the capacity of DM CocE to rapidly hydrolyze cocaine, both in vitro and in vivo, as well as the effectiveness of DM CocE to ameliorate the reinforcing, cardiovascular, convulsant, and lethal effects of cocaine in a variety of species including mice, rats, and rhesus monkeys (Collins et al., 2009, 2011a,b; Gao et al., 2009; Brim et al., 2010, 2011a, 2012; Narasimhan et al., 2010). The current studies systematically evaluated 1) the immunologic responses to repeated doses of DM CocE, 2) the effectiveness of repeated doses of DM CocE to reduce circulating concentrations of cocaine, and 3) the effectiveness of repeated doses of DM CocE to ameliorate the cardiovascular and psychomotor stimulatory effects of cocaine in rhesus monkeys during four biweekly trials in which 0.32 mg/kg DM CocE was administered as a 10-min post-treatment to an intravenous bolus of 3 mg/kg cocaine. There were five main findings. First, DM CocE greatly accelerated the elimination of cocaine from the circulation, with plasma concentrations of cocaine reduced below detectable limits within 5 to 7 min. Second, DM CocE significantly reduced cocaine-induced increases in MAP and HR, with baseline-like cardiovascular responses generally recovered within 25 to 30 min. Third, DM CocE significantly reduced the locomotor stimulatory effects of the 3 mg/kg dose of cocaine. Fourth, repeated dosing with DM CocE stimulated the development of anti-CocE antibodies; however, these effects were relatively mild with increases in titer levels of 10-fold or less observed in four of five monkeys. Fifth, there was no evidence to suggest that anti-CocE antibodies were capable of neutralizing the effectiveness of DM CocE to hydrolyze cocaine or ameliorate the cardiovascular and locomotor stimulatory effects of cocaine in any of the rhesus monkeys. Finally, when taken together with the results of previous studies, these findings strongly suggest that highly efficient bacterial cocaine esterases, such as DM CocE, should provide a safe and effective method to rapidly eliminate the symptoms of acute cocaine intoxication in humans, even if an individual requires multiple treatments over a relatively short period of time.
Although it has been difficult to establish a clear dose-response function for the toxic effects of cocaine in humans, blood levels obtained from patients in the ED and cocaine overdose fatalities (Finkle and McCloskey, 1978; Karch et al., 1998; Blaho et al., 2000; Koehler et al., 2005) suggest that cocaine concentrations at the time of ED admission may well exceed 1000 ng/ml. Similarly high concentrations of cocaine were observed in the current studies, with a single intravenous dose of 3 mg/kg cocaine, during the PBS condition, resulting in plasma cocaine levels in excess of 2000 ng/ml during the first 15 min and in excess of 1000 ng/ml for the entirety of the first 60 min after cocaine administration. In addition to confirming previous estimates of cocaine's elimination half-life (∼51 min in the current studies) in rhesus monkeys (Mello et al., 2002), these data provide further validation for using the 3 mg/kg i.v. dose of cocaine as a rhesus monkey model of acute cocaine intoxication. Although concerns for the well being of one of the monkeys (BE) precluded our ability to administer DM CocE at the same post-treatment time in both monkeys, these studies clearly demonstrate the effectiveness of 0.32 mg/kg DM CocE to rapidly eliminate high concentrations of cocaine from the circulation. Moreover, these findings extend previous reports (Brim et al., 2011a, 2012) by demonstrating that the hydrolytic effects of DM CocE are not affected by repeated administrations with DM CocE.
In addition to the increased blood levels of cocaine, intravenous administration of 3 mg/kg cocaine produced a variety of cardiovascular and psychomotor stimulatory effects, including rapid increases in MAP that gradually decreased over the 2-h period and a more gradual increases in HR and locomotor activity that tended to peak during the second 60 min of observation. A close correspondence was observed between the elevations in MAP and the plasma concentrations of cocaine produced by 3 mg/kg i.v. cocaine. Given that blood levels of cocaine are also closely linked to the cardiovascular stimulation and cocaine-associated chest pain that is commonly reported with cocaine-related ED cases (Javaid et al., 1978; Brody et al., 1990; Foltin and Fischman, 1991; Mittleman et al., 1999), these findings provide further validation of the current model of acute cocaine intoxication in rhesus monkeys. Treatment with 0.32 mg/kg DM CocE 10 min after cocaine resulted in a rapid amelioration of the hypertensive effects of cocaine and virtually eliminated the increases in HR and locomotor activity that were observed during the second hour of the PBS conditions. In addition to confirming the results of our initial dose-response studies with DM CocE in rhesus monkeys (Collins et al., 2011a), these studies provide the first demonstration that the effectiveness of DM CocE in ameliorating the cardiovascular and psychomotor stimulatory effects of cocaine does not change with repeated administrations.
That DM CocE retained its effectiveness across multiple administrations is important for several reasons. First, in mice the repeated administration of wt CocE has been shown to stimulate the production of anti-CocE antibodies and reduce the effectiveness of wt CocE to protect against cocaine-induced lethality (Ko et al., 2007, 2009). Unlike the relatively large increases observed in these early studies (≥1000-fold), DM CocE failed to stimulate large increases in anti-CocE titers, with four of the five monkeys displaying increases of 10-fold or less. The current studies are also in agreement with previous findings in which minimal increases in titers were observed in rhesus monkeys treated with three doses of DM CocE (0.32, 1.0, and 3.2 mg/kg) at 1-week intervals (Collins et al., 2011a). It is noteworthy that in both studies the increased titer levels were transient, with baseline anti-CocE titer levels observed within 6 to 10 weeks of the final treatment.
Although it is possible that species differences contributed to the milder immune response observed in the current and past studies (Collins et al., 2011a), that DM CocE failed to elicit strong immune responses also suggests that the immunogenic potential of CocE is not necessarily caused by its bacterial origins (Bresler et al., 2000). Rather, it seems likely that the comparatively strong immune potential of wt CocE resulted from a contamination of earlier formulations with higher levels of endotoxin, a consequence of using a Gram-negative, Escherichia coli expression system. Even though endotoxin levels were not reported in the previous studies with wt CocE, that the current formulation of DM CocE (present study; Collins et al., 2011a) required extensive purification to reduce endotoxin to levels below the 5 EU/kg limit (current formulation was 2 EU/kg) imposed by the United States Pharmacopoeia and the Food and Drug Administration (Malyala and Singh, 2008) suggests that endotoxin probably enhanced the immune responses observed with wt CocE. Nevertheless, studies in mice suggest that any potentially neutralizing effect of anti-CocE antibodies could be easily surmounted by larger doses of CocE (Ko et al., 2009).
When taken together with previous reports (Collins et al., 2009, 2011a,b; Brim et al., 2011a,b, 2012), the results of the current study suggest that DM CocE would provide distinct advantages over benzodiazepines, the current standard of care for acute cocaine intoxication (McCord et al., 2008). Unlike the symptomatic relief provided by the anxiolytic and/or analgesic properties of benzodiazepines, highly efficient cocaine hydrolases aim to treat cocaine-associated chest pain and/or psychiatric symptoms by rapidly eliminating the underlying cause of these symptoms, that being cocaine. In addition to ameliorating the cardiovascular and psychostimulant effects of cocaine, therapeutics such as DM CocE would limit the direct, cardiotoxic effects of cocaine, such as myocardial ischemia, left ventricular hypertrophy, and dilated cardiomyopathy (Hollander, 1995; Hollander et al., 1995; Hollander and Henry, 2006), thereby reducing the patient's risk of future heart failure. Moreover, rapidly eliminating cocaine would have the added benefit of reducing, or even eliminating, the need for lengthy observational periods to allow patients to naturally clear cocaine from their system, thus reducing the cost of treatment and freeing up ED resources for other cases.
In conclusion, the results of the current study clearly demonstrate that even though DM CocE is mildly antigenic in rhesus monkeys it retained its effectiveness to hydrolyze cocaine and ameliorate the cardiovascular and psychostimulant effects of cocaine across each of four biweekly tests. Although these findings suggest that immunologic responses to repeated administrations of DM CocE are not likely to affect its therapeutic effectiveness, recent attempts to improve the duration and further reduce the immunogenicity of CocE have resulted in a polyethylene glycol-modified mutant form of CocE (PEG-CCRQ CocE) capable of reducing the reinforcing, cardiovascular, convulsant, and lethal effects of cocaine for up to 48 h without eliciting increases in anti-CocE antibodies (Narasimhan et al., 2011; Collins et al., 2012). It is noteworthy that even though self-administration studies in rats (Collins et al., 2009, 2012) suggest that human cocaine abusers would be able to surmount the protective effects of CocE (by ingesting approximately 10 times more cocaine), the capacity of CocE to rapidly metabolize large doses of cocaine (>100 mg/kg i.v. self-administered over 90 min) would probably protect these individuals from the adverse effects that would otherwise be associated with such large doses of cocaine. When taken together with the results of previous studies demonstrating the effectiveness of DM CocE to protect against and/or reverse acute cocaine toxicity in mice, rats, and rhesus monkeys the current findings strongly suggest that highly efficient cocaine hydrolases, such as DM CocE, could provide a safe and effective option for the treatment of acute cocaine intoxication in humans.
Acknowledgments
We thank Joseph Nichols, Kathy Carey, Eric Hu, Colette Cremeans, and Aaron Berlin for assistance in completing these studies.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA023213].
Portions of this work have been presented previously: Collins GT, Brim RL, Noon KR, Narasimhan D, Lukacs NW, Sunahara RK, Woods JH, and Ko MC (2011) Repeated administration of a long-acting mutant cocaine esterase: effects on cardiovascular activity, plasma cocaine levels, and immune responses in rhesus monkeys, at the American Society of Pharmacology and Experimental Therapeutics Annual Meeting; 2011 April 9–13; Washington, DC; American Society of Pharmacology and Experimental Therapeutics, Bethesda, MD. Brim RL, Noon KR, Stein A, Nance MR, Nichols J, Narasimhan D, Sunahara RK, and Woods JH (2011) Quantification of rapid in vivo cocaine hydrolysis by bacterial cocaine esterase, at the American Society of Pharmacology and Experimental Therapeutics Annual Meeting; 2011 April 9–13; Washington, DC; American Society of Pharmacology and Experimental Therapeutics, Bethesda, MD. Brim RL (2011) Investigations into the Therapeutic Potential of a Bacterial Cocaine Esterase for the Treatment of Cocaine Toxicity and Cocaine Abuse, Ph.D. Thesis, University of Michigan, Ann Arbor, MI. Collins GT, Brim RL, Narasimhan D, Noon KR, Lukacs NW, Sunahara RK, Woods JH, and Ko MC (2011) Repeated administration of a mutant cocaine esterase: effects on plasma cocaine levels, cocaine-induced cardiovascular activity, and immune responses in rhesus monkeys, at the College on Problems of Drug Dependence Annual Meeting; 2011 June 18–23; Hollywood FL; College on Problems of Drug Dependence, Philadelphia, PA. Brim RL, Noon KR, Collins GT, Stein A, Nichols J, Narasimhan D, Ko MC, Woods JH, and Sunahara RK (2012) The fate of bacterial cocaine esterase (CocE): an in vivo study of CocE-mediated cocaine hydrolysis, CocE pharmacokinetics, and CocE elimination. J Pharmacol Exp Ther 340:83–95.
R.K.S. and J.H.W. have served as consultants to Reckitt Benckiser Pharmaceuticals (Richmond, VA). R.L.B., D.N., R.K.S., J.H.W., and M.-C.K. are listed as inventors on patent number PCT/US2008/069659 for DM CocE.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- MAP
- mean arterial pressure
- ANOVA
- analysis of variance
- BChE
- butyrylcholinesterase
- CocE
- cocaine esterase
- DM CocE
- double mutant CocE
- ED
- emergency department
- HR
- heart rate
- PBS
- phosphate-buffered saline
- n.s.
- not significant
- wt
- wild type
- EU
- endotoxin units.
Authorship Contributions
Participated in research design: Collins, Brim, Noon, Narasimhan, Lukacs, Sunahara, Woods, and Ko.
Conducted experiments: Collins, Brim, and Noon.
Contributed new reagents or analytic tools: Noon and Narasimhan.
Performed data analysis: Collins, Brim, and Noon.
Wrote or contributed to the writing of the manuscript: Collins, Brim, Woods, and Ko.
References
- Blaho K, Logan B, Winbery S, Park L, Schwilke E. (2000) Blood cocaine and metabolite concentrations, clinical findings, and outcome of patients presenting to an ED. Am J Emerg Med 18:593–598 [DOI] [PubMed] [Google Scholar]
- Bresler MM, Rosser SJ, Basran A, Bruce NC. (2000) Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl Environ Microbiol 66:904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim RL, Nance MR, Youngstrom DW, Narasimhan D, Zhan CG, Tesmer JJ, Sunahara RK, Woods JH. (2010) A thermally stable form of bacterial cocaine esterase: a potential therapeutic agent for treatment of cocaine abuse. Mol Pharmacol 77:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim RL, Noon KR, Collins GT, Nichols J, Narasimhan D, Sunahara RK, Woods JH. (2011a) The ability of bacterial cocaine esterase to hydrolyze cocaine metabolites and their simultaneous quantification using high-performance liquid chromatography-tandem mass spectrometry. Mol Pharmacol 80:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim RL, Noon KR, Collins GT, Stein A, Nichols J, Narasimhan D, Ko MC, Woods JH, Sunahara RK. (2012) The fate of bacterial cocaine esterase (CocE): an in vivo study of CocE-mediated cocaine hydrolysis, CocE pharmacokinetics, and CocE elimination. J Pharmacol Exp Ther 340:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim RL, Noon KR, Nichols J, Narasimhan D, Woods JH, Sunahara RK. (2011b) Evaluation of the hydrolytic activity of a long-acting mutant bacterial cocaine in the presence of commonly co-administered drugs. Drug Alcohol Depend 119:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. (2008) A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SL, Slovis CM, Wrenn KD. (1990) Cocaine-related medical problems: consecutive series of 233 patients. Am J Med 88:325–331 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Gao Y, Brimijoin S, Anker JJ. (2011) Effects of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacology (Berl) 213:817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Brim RL, Narasimhan D, Ko MC, Sunahara RK, Zhan CG, Woods JH. (2009) Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J Pharmacol Exp Ther 331:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Carey KA, Narasimhan D, Nichols J, Berlin AA, Lukacs NW, Sunahara RK, Woods JH, Ko MC. (2011a) Amelioration of the cardiovascular effects of cocaine in rhesus monkeys by a long-acting mutant form of cocaine esterase. Neuropsychopharmacology 36:1047–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Narasimhan D, Cunningham AR, Zaks ME, Nichols J, Ko MC, Sunahara RK, Woods JH. (2012) Long-lasting effects of a PEGylated mutant cocaine esterase (CocE) on the reinforcing and discriminative stimulus effects of cocaine in rats. Neuropsychopharmacology 37:1092–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Zaks ME, Cunningham AR, St Clair C, Nichols J, Narasimhan D, Ko MC, Sunahara RK, Woods JH. (2011b) Effects of a long-acting mutant bacterial cocaine esterase on acute cocaine toxicity in rats. Drug Alcohol Depend 118:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Narasimhan D, Sunahara RK, Mierzejewski P, Jutkiewicz EM, Larsen NA, Wilson IA, Landry DW, Woods JH. (2006) Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol Pharmacol 70:1885–1891 [DOI] [PubMed] [Google Scholar]
- Dackis C, O'Brien C. (2003) Glutamatergic agents for cocaine dependence. Ann NY Acad Sci 1003:328–345 [DOI] [PubMed] [Google Scholar]
- Finkle BS, McCloskey KL. (1978) The forensic toxicology of cocaine (1971–1976). J Forensic Sci 23:173–189 [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. (1991) Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther 257:247–261 [PubMed] [Google Scholar]
- Gao D, Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara RK, Zhan CG. (2009) Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol 75:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser J, Queen JR. (2007) An overview of non-cardiac cocaine toxicity. J Emerg Med 32:181–186 [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464 [DOI] [PubMed] [Google Scholar]
- Hollander JE. (1995) The management of cocaine-associated myocardial ischemia. N Engl J Med 333:1267–1272 [DOI] [PubMed] [Google Scholar]
- Hollander JE, Henry TD. (2006) Evaluation and management of the patient who has cocaine-associated chest pain. Cardiol Clin 24:103–114 [DOI] [PubMed] [Google Scholar]
- Hollander JE, Hoffman RS, Gennis P, Fairweather P, Feldman JA, Fish SS, DiSano MJ, Schumb DA, Dyer S. (1995) Cocaine-associated chest pain: one-year follow-up. Acad Emerg Med 2:179–184 [DOI] [PubMed] [Google Scholar]
- Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Côté M, Herskovits P, et al. (2007) Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci U S A 104:13603–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Javaid JI, Fischman MW, Schuster CR, Dekirmenjian H, Davis JM. (1978) Cocaine plasma concentration: relation to physiological and subjective effects in humans. Science 202:227–228 [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Cooper ZD, Narasimhan D, Sunahara RK, Woods JH. (2009) A bacterial cocaine esterase protects against cocaine-induced epileptogenic activity and lethality. Ann Emerg Med 54:409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch SB, Stephens B, Ho CH. (1998) Relating cocaine blood concentrations to toxicity–an autopsy study of 99 cases. J Forensic Sci 43:41–45 [PubMed] [Google Scholar]
- Ko MC, Bowen LD, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Cooper ZD, Woods JH. (2007) Cocaine esterase: interactions with cocaine and immune responses in mice. J Pharmacol Exp Ther 320:926–933 [DOI] [PubMed] [Google Scholar]
- Ko MC, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Woods JH. (2009) Effects of cocaine esterase following its repeated administration with cocaine in mice. Drug Alcohol Depend 101:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SA, Ladham S, Rozin L, Shakir A, Omalu B, Dominick J, Wecht CH. (2005) The risk of body packing: a case of a fatal cocaine overdose. Forensic Sci Int 151:81–84 [DOI] [PubMed] [Google Scholar]
- Larsen NA, Turner JM, Stevens J, Rosser SJ, Basran A, Lerner RA, Bruce NC, Wilson IA. (2002) Crystal structure of a bacterial cocaine esterase. Nat Struct Biol 9:17–21 [DOI] [PubMed] [Google Scholar]
- Malyala P, Singh M. (2008) Endotoxin limits in formulations for preclinical research. J Pharm Sci 97:2041–2044 [DOI] [PubMed] [Google Scholar]
- McCord J, Jneid H, Hollander JE, de Lemos JA, Cercek B, Hsue P, Gibler WB, Ohman EM, Drew B, Philippides G, et al. (2008) Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 117:1897–1907 [DOI] [PubMed] [Google Scholar]
- Mello NK, Bowen CA, Mendelson JH. (2002) Comparison of plasma cocaine levels during a “binge” pattern of cocaine administration in male and female rhesus monkeys. Psychopharmacology (Berl) 164:19–26 [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. (1999) Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology 21:294–303 [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Mintzer D, Maclure M, Tofler GH, Sherwood JB, Muller JE. (1999) Triggering of myocardial infarction by cocaine. Circulation 99:2737–2741 [DOI] [PubMed] [Google Scholar]
- Narasimhan D, Collins GT, Nance MR, Nichols J, Edwald E, Chan J, Ko MC, Woods JH, Tesmer JJ, Sunahara RK. (2011) Subunit stabilization and polyethylene glycolation of cocaine esterase improves in vivo residence time. Mol Pharmacol 80:1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan D, Nance MR, Gao D, Ko MC, Macdonald J, Tamburi P, Yoon D, Landry DM, Woods JH, Zhan CG, et al. (2010) Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng Des Sel 23:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR, Kearney TE, Dyer JE, Benowitz NL, Blanc PD. (1994) Seizures associated with poisoning and drug overdose. Am J Emerg Med 12:392–395 [DOI] [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. (2005) Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci U S A 102:16656–16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2011) Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41, HHS Publication SMA-11-4658 Substance Abuse and Mental Health Services Administration, Rockville, MD [Google Scholar]
- Tanda G, Newman AH, Katz JL. (2009) Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57:253–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, Larsen NA, Basran A, Barbas CF, 3rd, Bruce NC, Wilson IA, Lerner RA. (2002) Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry 41:12297–12307 [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2010) World Drug Report 2010. United Nations Publication E.10.XI.13, United Nations, New York [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. (2005) Medication development for addictive disorders: the state of the science. Am J Psychiatry 162:1432–1440 [DOI] [PubMed] [Google Scholar]
- Wood SK, Narasimhan D, Cooper Z, Sunahara RK, Woods JH. (2010) Prevention and reversal by cocaine esterase of cocaine-induced cardiovascular effects in rats. Drug Alcohol Depend 106:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Ko MC, Tong M, Yang W, Hou S, Fang L, Liu J, Zheng F, Woods JH, Tai HH, et al. (2011) Design, preparation, and characterization of high-activity mutants of human butyrylcholinesterase specific for detoxification of cocaine. Mol Pharmacol 79:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. (2008) Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc 130:12148–12155 [DOI] [PMC free article] [PubMed] [Google Scholar]