Abstract
Mitochondrial dysfunction is a common mediator of disease and organ injury. Although recent studies show that inducing mitochondrial biogenesis (MB) stimulates cell repair and regeneration, only a limited number of chemicals are known to induce MB. To examine the impact of the β-adrenoceptor (β-AR) signaling pathway on MB, primary renal proximal tubule cells (RPTC) and adult feline cardiomyocytes were exposed for 24 h to multiple β-AR agonists: isoproterenol (nonselective β-AR agonist), (±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy] acetic acid sodium hydrate (BRL 37344) (selective β3-AR agonist), and formoterol (selective β2-AR agonist). The Seahorse Biosciences (North Billerica, MA) extracellular flux analyzer was used to quantify carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP)-uncoupled oxygen consumption rate (OCR), a marker of maximal electron transport chain activity. Isoproterenol and BRL 37244 did not alter mitochondrial respiration at any of the concentrations examined. Formoterol exposure resulted in increases in both FCCP-uncoupled OCR and mitochondrial DNA (mtDNA) copy number. The effect of formoterol on OCR in RPTC was inhibited by the β-AR antagonist propranolol and the β2-AR inverse agonist 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol hydrochloride (ICI-118,551). Mice exposed to formoterol for 24 or 72 h exhibited increases in kidney and heart mtDNA copy number, peroxisome proliferator-activated receptor γ coactivator 1α, and multiple genes involved in the mitochondrial electron transport chain (F0 subunit 6 of transmembrane F-type ATP synthase, NADH dehydrogenase subunit 1, NADH dehydrogenase subunit 6, and NADH dehydrogenase [ubiquinone] 1β subcomplex subunit 8). Cheminformatic modeling, virtual chemical library screening, and experimental validation identified nisoxetine from the Sigma Library of Pharmacologically Active Compounds and two compounds from the ChemBridge DIVERSet that increased mitochondrial respiratory capacity. These data provide compelling evidence for the use and development of β2-AR ligands for therapeutic MB.
Introduction
Mitochondrial dysfunction is associated with the etiology of multiple diseases including Alzheimer's disease, Parkinson's disease, and diabetes, as well as renal, liver, and myocardial injury (Hagen et al., 2002; Baloyannis, 2006; Civitarese and Ravussin, 2008; Seo et al., 2010; Tran et al., 2011). Mitochondrial dysfunction is also a common cause and consequence of ischemia/reperfusion, trauma, and drug/toxicant-induced organ injury. In ischemic/reperfusion and lipopolysaccharide-induced acute kidney injury there is a persistent loss of mitochondrial function (Funk et al., 2010; Tran et al., 2011). Mitochondrial damage may hinder critical energy-dependent repair mechanisms and lead to irreversible cell injury, limiting restoration of organ function. Thus, the development of therapies to promote mitochondrial biogenesis (MB) has the potential to treat multiple pathologies and restore organ function after injury.
Mitochondria are constantly being renewed through the processes of biogenesis, fusion, fission, and mitophagy (Seo et al., 2010). MB occurs under homeostatic conditions and can be induced as an adaptive response initiated by cells to meet energetic demands resulting from injury and genetic, metabolic, and dietary events, thereby affecting health and disease (Medeiros, 2008). MB is a complex process requiring the coordination of both nuclear DNA and mitochondrial DNA (mtDNA) that encode for mitochondrial proteins. A primary regulator of MB is the nuclear transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α). PGC-1α interacts with cAMP response element-binding protein and nuclear respiratory factors 1 and 2 to regulate the transcription of multiple genes (Wu et al., 2006). PGC-1α provides a direct link between external physiological stimuli and the regulation of MB and is inducible both physiologically and pharmacologically.
There are a limited number of chemicals known to induce MB. The Spiegelman group conducted a high-throughput screen that examined the effects of 3000 compounds in skeletal muscle cells, and 82 compounds (2.5%) increased PGC-1α mRNA (Arany et al., 2008). Our laboratory has reported that isoflavones, a 5-hydroxytryptamine type 2 agonist [1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride] (DOI), and a silent mating type information regulation 2 homolog 1 activator (N-[2-[3-(piperazin-1-ylmethyl)imidazo [2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide; SRT1720) induce MB in renal proximal tubular cells (RPTC) (Rasbach and Schnellmann, 2008; Funk et al., 2010; Rasbach et al., 2010). Furthermore, we determined that the stimulation of MB after the initiation of cellular injury accelerates cell repair and regeneration. For example, after t-butylhydroperoxide-induced oxidant injury, exposure of primary cultures of RPTC to 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride or SRT1720 or overexpression of PGC1-α accelerated the recovery of mitochondrial and ATP-dependent cellular functions (Rasbach and Schnellmann, 2007; Funk et al., 2010; Rasbach et al., 2010). Therefore, targeted stimulation of MB may be a valuable approach in the development of new therapies for the treatment of injury and disease characterized by mitochondrial impairment (Funk et al., 2010).
Agonists for the β2-AR have been reported to modulate oxidative metabolism, energy expenditure, lipolysis, glucose transport, and glucose oxidation (Agbenyega et al., 1995; Hagström-Toft et al., 1998; Pearen et al., 2008). Pearen et al. (2009) found that mice dosed with the β2-AR agonist formoterol showed a 5-fold mRNA induction of PGC1-α in skeletal muscle. In addition, exercise-induced increase in PGC-1α mRNA was inhibited by pretreatment with the β2-AR inverse agonist 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol hydrochloride (ICI-118,551) and the β-AR antagonist propranolol, suggesting that β-AR activation mediates exercise-induced increases in PGC-1α in skeletal muscle (Miura et al., 2007; Sutherland et al., 2009). Thus, the stimulation of the β2-AR signaling pathway may regulate mitochondrial function in multiple tissues.
β-AR receptor agonists are approved therapeutic agents for the management of asthma and other diseases. The β2-AR has been successfully targeted for drug discovery by using a ligand-based approach, resulting in the creation of multiple receptor-specific drugs (Wishart et al., 2006). Kolb et al. (2009) used the X-ray structure of the β2-AR to conduct a structure-based screen of nearly one million commercially available molecules. Using the β2-AR crystallized in complex with the inverse agonist carazozol, the researchers identified 25 novel compounds that fit the receptor, and six of the compounds were confirmed as inverse agonists. However, the therapeutic potential of β2-AR agonists as inducers of MB has not fully been explored (Ortega and Peters, 2010).
In this study, we used a unique phenotypic approach developed by Beeson et al. (2010) to examine the potential of β2-AR agonism to induce MB in both the kidney and heart. The Seahorse Biosciences (North Billerica, MA) extracellular flux analyzer was used to evaluate the effects of β2-AR agonists on maximal mitochondrial respiration in primary cultures of RPTC and cardiomyocytes, two cells highly dependent on aerobic metabolism. In vivo and ligand-based in silico studies were then used to integrate the effects of β2-AR agonists on mitochondrial function and gene expression, with detailed analysis of chemical structure. These data were used to develop a discrete pharmacophore model capable of predicting novel compounds with MB properties.
Materials and Methods
All experiments were performed in accordance with national and institutional guidelines for animal welfare, adhering to protocols approved by the institutional subcommittee on research animal care.
Chemistry
All solvents and reagents, unless otherwise stated, were supplied by Sigma-Aldrich (St. Louis, MO) and used as supplied.
(±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy] acetic acid sodium hydrate (BRL 37344), (R*,R*)-N-[2-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide fumarate (formoterol furmarate dihydrate), and (R)-3,4-dihydroxy-α-(isopropylaminomethyl)benzyl alcohol hydrochloride (isoproterenol hydrochloride) were purchased from Sigma-Aldrich and confirmed to be 98% pure by HPLC. The Library of Pharmacologically Active Compounds (LOPAC) was purchased from Sigma-Aldrich, and the compounds were confirmed to be 95% pure by HPLC. The ChemBridge DIVERSet 50,000-compound library was purchased from the ChemBridge Corporation (San Diego, CA), and the compounds were confirmed to be 95% pure by HPLC.
Biological Evaluation of Compounds
Isolation of Proximal Tubules.
Female New Zealand white rabbits (1.5–2.0 kg) were purchased from Myrtle's Rabbitry (Thompson Station, TN). Rabbit renal tubules were isolated by using the iron oxide perfusion method as described previously (Nowak et al., 1995). The resulting proximal tubules were plated on 100-mm tissue culture-grade plastic Petri dishes constantly swirled on an orbital shaker at 80 rpm. The culture medium was a 50:50 mixture of Dulbecco's modified Eagle's essential medium and Ham's F12 (lacking glucose, phenol red, and sodium pyruvate; Invitrogen, Carlsbad, CA) supplemented with 5 μg/ml human transferrin, 5 ng/ml selenium, 50 nM hydrocortisone, and 10 nM bovine insulin. After 3 days the de-differentiated cells were trypsinized and replated onto XF-96 polystyrene cell culture microplates (Seahorse Bioscience) at a concentration of 18,000 cells/well and maintained in a 37°C incubator for 2 days before experimentation (Beeson et al., 2010).
Isolation of Primary Adult Feline Cardiomyocytes.
Adult feline cardiomyocytes (AFC) were isolated to 95% purity as described previously (Mann et al., 1989). In the present study, we used glass-bottom dishes (MatTek, Ashland, MA) coated with laminin for culturing cardiomyocytes in Piper's medium, which was prepared in M199 cell culture medium (Invitrogen) containing the following additional ingredients: 2% bovine serum albumin, 5 mM creatine, 2 mM l-carnitine, 5 mM taurine, 0.25 mM l-ascorbate, 10 μM cytosine arabinoside, 200 units/ml penicillin, and 200 mg/ml streptomycin (Invitrogen). The freshly isolated adult cardiomyocytes were plated into XF-96 polystyrene cell culture microplates and maintained in a 37°C incubator for 2 days before experimentation.
Respirometry Assay.
The oxygen consumption rate (OCR) measurements were performed by using a Seahorse Bioscience XF-96 instrument according to the protocol outlined in Beeson et al. (2010). Each experimental plate was treated with vehicle controls (DMSO <0.5%), a positive control (SRT1720, 10 μM), blank controls, and the appropriate concentration of the compound of interest. The XF-96 protocol consists of five measurements of basal OCR (1 measurement/1.5 min), injection of p-trifluoromethoxyphenylhydrazone (FCCP) (0.5 μM), and three measurements of uncoupled OCR (1 measurement/1.5 min). The consumption rates were calculated from the continuous average slope of the O2 partitioning among plastic, atmosphere, and cellular uptake (Gerencser et al., 2009). Quality-control evaluations considered the basal and uncoupled rates of the vehicle control, positive control, and variances between duplicate treatment wells. Based on preliminary studies the positive threshold value was >1.15 for the mean ratio of chemical treatment FCCP-OCR/vehicle control FCCP-OCR. This threshold is ≥1 S.D. above the historic mean for the vehicle control.
Dosing of C57BL/6 Mice.
Male C57BL/6 mice (National Cancer Institute, Bethesda, MD) were between 6 and 8 weeks old. The mice were housed in groups of three in a temperature-controlled room under a 12-h light/dark cycle. Mice were randomly assigned to either saline control or formoterol-treated groups (n = 3–6 mice/group). Treated mice received daily intraperitoneal injections of formoterol for up to 72 h (100 μg/kg/day), and control mice received an equivalent volume of sterile saline.
Real-Time Reverse Transcription-PCR.
Total RNA was isolated from renal cortex and heart by using TRIzol reagent (Invitrogen). cDNA was synthesized from 2 μg of RNA template by using a SuperScript II Reverse Transcriptase kit (Invitrogen). PCR products were amplified from 5 μl of cDNA template in a 25-μl reaction containing 12.5 μl of 2× SYBR Premix (Agilent Technologies, Santa Clara, CA) and 400 nM of each primer (Integrated DNA Technologies, Inc., Coralville, IA) (Supplemental Table 1). The average fold induction was calculated by comparing the CT (threshold cycle) of the target gene to that of tubulin (the reference gene). The gene expression of the reference gene remained consistent throughout each treatment. The CT of each of the technical replicates was averaged, and that average was used in the following formulas:
Mitochondrial DNA Content.
Real-time PCR was used to determine relative quantities of mtDNA content in both RPTC and mouse kidney and heart tissues. Genomic DNA was extracted by using the DNeasy Blood and Tissue kit (QIAGEN, Valencia, CA). PCR products were amplified from 25 ng of cellular DNA in a 25-μl reaction containing 12.5 μl of 2× SYBR Premix and 400 nM of each primer. For estimation of mtDNA in RPTC the NADH dehydrogenase subunit 6 (ND6) gene was amplified. The nuclear-encoded tubulin gene was used for normalization (Supplemental Table 1). For estimation of mtDNA in mice the control region (D loop) of mouse mtDNA was amplified. The nuclear-encoded apolipoprotein B gene was used for normalization (Supplemental Table 1) (Fuke et al., 2011).
Computational Procedures
Molecular Modeling of Potential Mitochondrial Biogenic Compounds.
Modeling, simulations, and visualizations were performed by using MOE version 2010.10 (Chemical Computing Group Inc., Montreal, Canada). The Sigma LOPAC and the ChemBridge DiverSet were searched by using the Tanimoto score with MACCS structural keys (166 keys) fingerprinting (Maccs II; Molecular Design Ltd., Memphis, TN). Using tables of the compounds represented as Simplified Molecular Input Line Entry System strings were imported into MOE as an mdb database. Molecules were rigidly aligned manually, and then subjected to MOE flexible body refinement (configuration limit 100, α1, gradient test 0.01, RMSD tolerance 0.5, maximum steps 500). The ChemBridge DIVERSet conformers were a stochastic force field-based library generated by using MOE conformation import with no import filters. The total number of possible conformations per molecule was 50 and used default settings including a strain limit of 4 kcal/mol, RMSD test for structural diversity of 0.15, and an energy minimization gradient test of 0.01 kcal/mol. Transconformations were enforced. Consensus pharmacophores were calculated by using a distance parameter (tolerance) maintained at the default value of 1.2 Å and a threshold of 100%. Pharmacophore feature projections including aromatic ring projections (PiN), H-bond acceptor projections (Don2), and H-bond acceptor projections (Acc2) were not included in the analysis.
Statistical Analysis.
Data are presented as means ± S.E.M. and were tested for normality. Data that were confirmed to have a normal distribution were subjected to one-way analysis of variance. The respiration data failed a normality test; therefore, a Kruskal-Wallis one-way analysis of variance on ranks was conducted. Multiple means were compared by using Dunn's post hoc test and considered statistically different at P < 0.05. RPTC and AFC isolated from a single animal represented an individual experiment (n = 1) and were repeated until n ≥ 4 was obtained. Rodent studies were repeated until n ≥ 3 was obtained.
Results
β2-Adrenergic Agonist Induces MB In Vitro.
Kidney proximal tubules require aerobic metabolism to maintain high levels of ATP for transport processes. The primary cultures of RPTC used in this study were grown under improved culture conditions with optimized glucose-free media supplemented with 6 mM sodium lactate and increased oxygen supply (Nowak and Schnellmann, 1995, 1996). RPTC grown under these conditions remain polarized, maintain their differentiated functions, and exhibit respiration and gluconeogenesis rates comparable with in vivo renal proximal tubule cells. The primary culture of AFC used in the study also maintained differentiated function and exhibited mitochondrial respiration similar to that observed in vivo (Mitcheson et al., 1998).
Primary cultures of RPTC and AFC have been optimized for use with the Seahorse Biosciences 96-well extracellular flux analyzer (XF-96), a multiwell plate-based assay platform that addresses the need for higher-throughput cellular respirometric measurements (Ferrick et al., 2008; Beeson et al., 2010). The XF-96 instrument uses fluorescent detectors to measure OCRs and can be used to identify compounds that alter mitochondrial respiration. Injection of the proton ionophore carbonylcyanide FCCP uncouples the mitochondrial membrane potential from the production of ATP, increasing the OCR. We determined that this maximum respiratory capacity could be used as a measure of MB (Beeson et al., 2010).
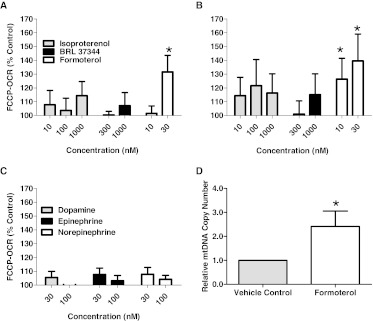
The FCCP-OCR increased in RPTC after a 24-h exposure to formoterol (30 nM) relative to the vehicle control (Fig. 1A). The FCCP-uncoupled OCR was also increased in AFC after a 24-h exposure to formoterol (10 and 30 nM) (Fig. 1B). No significant changes in FCCP-OCR were observed after exposure to isoproterenol (nonselective β-AR agonist; 10, 100, and 1000 nM) or BRL 37344 (selective β3-AR agonist; 100, 300, and 1000 nM). The catecholamines dopamine, epinephrine, and norepinephrine are nonselective agonists for all three β-adrenoceptors (Hall et al., 1990). No significant changes in FCCP-OCR were observed in the RPTC after exposure to dopamine, epinephrine, or norepinephrine (30 and 100 nM) (Fig. 1C).
Fig. 1.
Formoterol exposure increases uncoupled OCR and mtDNA copy number in RPTC and AFC. A, RPTC were exposed to isoproterenol (nonspecific β-AR agonist), BRL 37344 (β3-AR agonist), and formoterol (β2-AR agonist) for 24 h and evaluated for changes in FCCP-OCR. B, AFC were exposed to isoproterenol (nonspecific β-AR agonist), BRL 37344 (β3-AR agonist), and formoterol (β2-AR agonist) for 24 h and evaluated for changes in FCCP-OCR. C, RPTC were exposed to the nonspecific β-AR agonists dopamine, epinephrine, and norepinephrine for 24 h and evaluated for changes in FCCP-OCR. D, RPTC were exposed to 30 nM formoterol for 24 h and evaluated for changes in mtDNA copy number relative to DMSO controls. Data are represented as mean ± S.E.M. of four biological replicates. *, P < 0.05.
To further document that the increased OCR values were the result of MB, mtDNA copy number was assessed. Relative mtDNA copy number was determined by using quantitative real-time PCR and the ratio of a mitochondrial-encoded gene (ND6) to a nuclear-encoded gene (tubulin). Formoterol (30 nM)-treated cells exhibited a significant 2.5-fold increase in mitochondrial copy number (Fig. 1D). These results provide strong evidence that β2-adrenergic receptor activation induces MB in RPTC and AFC and formoterol is a potent agonist.
β2-Adrenergic Agonist Induces MB In Vivo.
To determine whether β2-AR agonism in vivo produces MB, male C57BL/6 mice were dosed intraperitoneally with 100 μg/kg formoterol every day for 1 or 3 days. Kidney and heart tissues were collected from the animals, and MB was determined by assessing mtDNA copy number and the mRNA levels of multiple mitochondrial proteins.
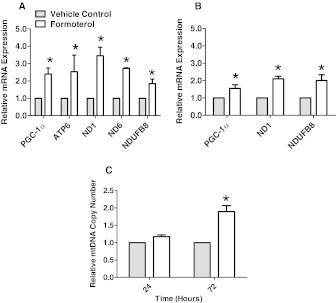
Twenty four hours after formoterol exposure, mRNA levels of multiple genes involved in mitochondrial regulation and function increased in kidneys. PGC-1α was induced 2.5-fold and mitochondrially encoded ATP synthase F0 subunit 6 of transmembrane F-type ATP synthase (ATP6), ND1, and ND6 were induced 2.5-, 4-, and 2.5-fold, respectively (Fig. 2A). The kidneys of formoterol-exposed animals also showed 2-fold induction of the nuclear-encoded mitochondrial protein NADH dehydrogenase [ubiquinone] 1β subcomplex subunit 8 (NDUFB8). After repeated daily exposure to 100 μg/kg formoterol for 72 h, the kidneys exhibited 1.5-fold induction of PGC-1α, as well as a 2-fold induction of NDUFB8 and ND1 (Fig. 2B). The mtDNA copy number was increased in the kidneys of mice 72 h after repeated daily formoterol exposure (Fig. 2C).
Fig. 2.
Formoterol exposure induced the expression of mitochondrial genes and mtDNA copy number in the kidney cortex of CB57BL/6 mice. A, mRNA expression was evaluated in the kidney cortex of CB57BL/6 mice 24 h after a single intraperitoneal injection with 100 μg/kg formoterol. B, mRNA expression was evaluated in the kidney cortex of CB57BL/6 mice 72 h after daily repeated intraperitoneal injections with 100 μg/kg formoterol. C, mtDNA copy number was evaluated in the kidney cortex 24 and 72 h after daily repeated intraperitoneal injection with 100 μg/kg formoterol. Values indicate fold change relative to DMSO controls. Data are represented as mean ± S.E.M. of three to six biological replicates. *, P < 0.05.
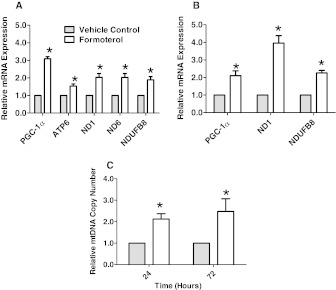
The hearts of mice exposed to 100 μg/kg formoterol for 24 h exhibited a marked induction of PGC-1α, ATP6, ND1, ND6, and NDUFB8 (3-, 1.5-, 2-, 2-, and 2-fold, respectively) (Fig. 3A). After a repeated daily exposure to 100 μg/kg formoterol for 72 h, the hearts of formoterol-exposed animals exhibited a 2-fold induction of PGC-1α, a 4-fold induction of mitochondrially encoded ND1, and a 2-fold induction of nuclear-encoded NDUFB8 (Fig. 3B). The mtDNA copy number was increased in the hearts of mice 24 and 72 h after daily formoterol exposure (Fig. 3C). These results provide strong evidence that β2-adrenergic receptor activation induces MB in the kidney and heart.
Fig. 3.
Formoterol exposure induced the expression of mitochondrial genes and mtDNA copy number in the hearts of CB57BL/6 mice. A, mRNA expression was evaluated in the hearts of CB57BL/6 mice 24 h after a single intraperitoneal injection with 100 μg/kg formoterol. B, mRNA expression was evaluated in the hearts of CB57BL/6 mice 72 h after daily repeated intraperitoneal injections with 100 μg/kg formoterol. C, mtDNA copy number was evaluated in the heart 24 and 72 h after daily repeated intraperitoneal injection with 100 μg/kg formoterol. Values indicate fold change relative to DMSO controls. Data are represented as mean ± S.E.M. of three to six biological replicates. *, P < 0.05.
Formoterol-Based Pharmacophore Identifies Novel Mitochondrial Biogenics.
Using formoterol as a basis for chemical similarity and pharmacophore analysis, we explored chemical space that would otherwise be impractical to explore given the current limitations in biological assay techniques. Pharmacophores are defined as a collection of steric and electronic features required for molecular interactions with a specific biological target structure, resulting in the activation or deactivation of its biological response (Horvath, 2011). Pharmacophore models have been developed to identify biologically active chemicals responsible for therapeutic activity (Ebalunode et al., 2011). The resulting structures can be used to predict potential pharmacology based on the assumption that compounds containing the same pharmacophore are likely to cause similar effects by targeting the same active site (Horvath, 2011). Currently, there are no known pharmacophores associated with MB. We used formoterol in a ligand-based approach to identify novel compounds with a similar chemical structure and determine whether they induced MB.
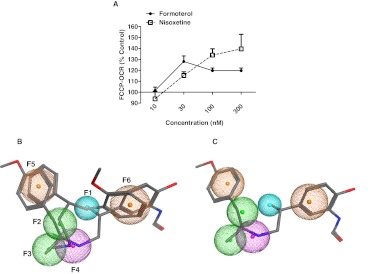
We used formoterol to conduct a similarity search of the 1280-compound Sigma LOPAC. Similarity was measured by using the Tanimoto Coefficient (TC). TC is a similarity metric of one-dimensional chemical descriptors that identifies the presence of molecular elements and facilitates rapid initial comparisons (Willett, 2006). A TC similarity search of LOPAC found 23 compounds 60% similar to formoterol. RPTC were treated with the 23 identified LOPAC compounds (5 μM) for 24 h and examined for changes in FCCP-uncoupled OCR. This assay revealed that nisoxetine increases FCCP-uncoupled OCR above vehicle control (Table 1). A subsequent concentration response evaluation determined that nisoxetine increased FCCP-uncoupled OCR in a concentration-dependent manner with a minimum effective dose of 30 nM (Fig. 4A).
TABLE 1.
Formoterol pharmacophore extraction of the 1280-compound Sigma LOPAC
Chemical fingerprints defined within the MOE software package were used to cluster compounds based on molecular similarity as measured from the TC. Analysis of the data using a TC of 60% identified 23 compounds of 1280 (or 1.8%) compounds from LOPAC that matched formoterol based on chemical similarity. RPTC were treated with the 23 identified LOPAC compounds (5 μM) for 24 h and examined for changes in FCCP-OCR. This assay identified nisoxetine, which increases FCCP-OCR 15% above vehicle control.
| Structure | Name | Average Uncoupled OCR Ratio | CAS Number |
|---|---|---|---|
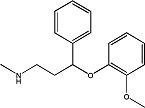 |
Nisoxetine | 1.2 ± 0.08 | 57754-86-6 |
 |
Olvanil | 1.12 ± 0.08 | 58493-49-5 |
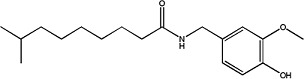 |
Dihydrocapsaicin | 1.12 ± 0.1 | 19408-84-5 |
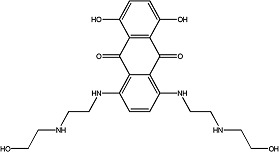 |
Mitoxantrone | 1.1 ± 0.06 | 65271-80-9 |
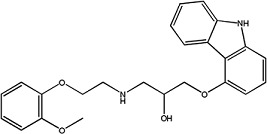 |
Carvedilol | 1.09 ± 0.03 | 72956-09-3 |
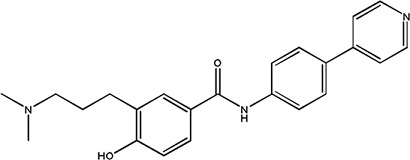 |
GR 55562 | 1.09 ± 0.09 | 159533-26-3 |
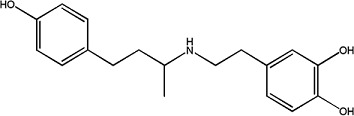 |
Dobutamine | 1.09 ± 0.14 | 49745-95-1 |
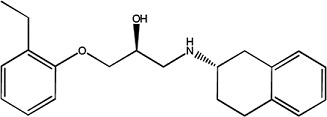 |
SR 59230A | 1.09 ± 0.07 | 174689-39-5 |
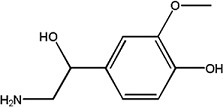 |
(±)-Normetanephrine | 1.06 ± 0.03 | 1011-74-1 |
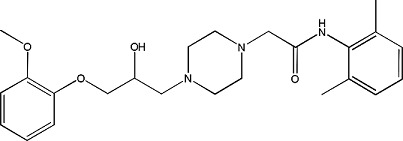 |
Ranolazine | 1.03 ± 0.03 | 95635-56-6 |
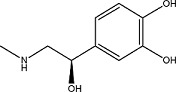 |
(−)-Epinephrine | 1.03 ± 0.1 | 51-42-3 |
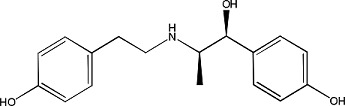 |
Ritodrine | 1.02 ± 0.09 | 23239-51-2 |
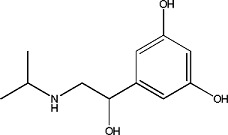 |
Metaproterenol | 1.02 ± 0.06 | 586-06-1 |
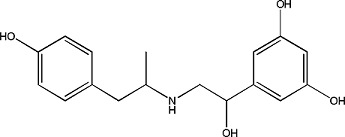 |
Fenoterol | 0.98 ± 0.04 | 12/3/1944 |
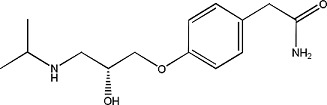 |
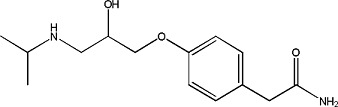
S(−)-Atenolol | 0.98 ± 0.12 | 93379-54-5 |
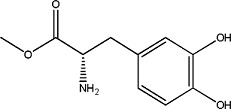 |
l-3,4-Dihydroxyphenylalanine methyl ester | 0.95 ± 0.07 | 7101-51-1 |
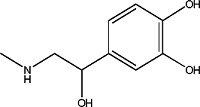 |
(±)-Epinephrine | 0.92 ± 0.03 | 329-63-5 |
 |
(±)-Atenolol | 0.92 ± 0.15 | 29122-68-7 |
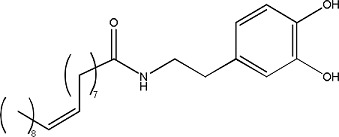 |
N-oleoyldopamine | 0.9 ± 0.09 | 105955-11-1 |
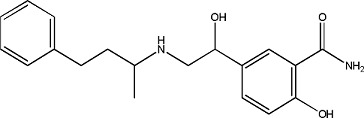 |
Labetalol | 0.86 ± 0.11 | 32780-64-6 |
 |
N-methyldopamine | 0.82 ± 0.13 | 62-32-8 |
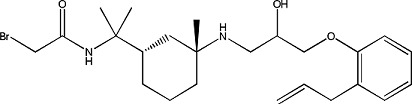 |
Bromoacetyl alprenolol menthane | 0.68 ± 0.2 | 958697-99-9 |
Fig. 4.
Cheminformatic analysis of formoterol identified nisoxetine, which induces MB in RPTC. A, RPTC were exposed to formoterol and nisoxetine (10–300 nM) for 24 h and examined for changes in FCCP-OCR. Values indicate a percentage of fold change relative to DMSO controls. Data are represented as mean ± S.E.M. of four biological replicates. B, pharmacophore based on the alignment of formoterol and nisoxetine. Formoterol and nisoxetine aligned with superimposed chemical features. F1 is a proton acceptor, F2 and F3 are hydrophobic, F4 is a mixed feature with a cationic and proton donor, and F5 and F6 are mixed features with aromatic or hydrophobic characteristics. F1 and F4 were marked as essential while requiring that at least five features matched the model. C, formoterol aligned with superimposed pharmacophore.
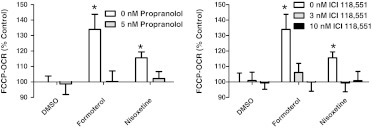
To confirm the specificity of formoterol on β2-AR, we pretreated cells for 1 h with the β-AR antagonist propranolol (5 nM) or the β2-AR inverse agonist ICI 115,881 (3 and 10 nM) before a 24-h treatment with formoterol (30 nM). As seen in Fig. 5, both propranolol and ICI 115,881 attenuated the formoterol-induced increase in FCCP-OCR in RPTC. Nisoxetine is a norepinephrine reuptake inhibitor, and there are no direct published data suggesting nisoxetine activates β2-AR. Nevertheless, because there is a report that some effects of nisoxetine are blocked by propranolol (Springer et al., 1994), we determined whether propranolol and ICI 115,881 blocked the MB effects of nisoxetine. Propranolol (5 nM) and ICI 115,881 (3 and 10 nM) blocked the increase in FCCP-OCR produced by nisoxteine (Fig. 5).
Fig. 5.
The effects of formoterol and nisoxetine are inhibited by β2 antagonism. A, RPTC were pre-exposed to the β-AR antagonist propranolol (5 nM) 1 h before exposure to 30 nM formoterol or nisoxetine and evaluated for changes in FCCP-OCR. B, RPTC were pre-exposed to the β2-AR inverse agonist ICI-118,551 (3 and 10 nM) 1 h before exposure to 30 nM formoterol or nisoxetine and evaluated for changes in FCCP-OCR. Values indicate a percentage of fold change relative to DMSO controls. Data are represented as mean ± S.E.M. of four biological replicates. *, P < 0.05.
Formoterol and nisoxetine were aligned in first two and then three dimensions based on the presence of consensus chemical features within 100% of the compounds. This alignment resulted in a pharmacophore containing six features of conserved chemical similarity (Fig. 4, B and C). F1 is a proton acceptor, F2 and F3 are hydrophobic, F4 is a mixed feature with a cationic and proton donor, and F5 and F6 are mixed features with aromatic and hydrophobic characteristics. Distances of the model are: F1–F2, 2.53 Å; F2–F4, 2.02 Å; F4–F3, 1.77 Å; F3–F5, 4.55 Å; F5–F6, 6.56 Å, and F6–F4, 4.76 Å.
The six-point pharmacophore model was used to search the ChemBridge DIVERSet for similar compounds. This library contains 50,080 unique drug-like compounds that represent a wide range of chemical diversity. These compounds were used to create a 1,420,467-entry conformer library. An in silico search identified 16 compounds containing all six chemical features with a RMSD <1 Å. No compounds with an RMSD >1 Å were identified, but this was expected because each feature was only ∼1 Å in diameter and six features were absolutely required, leading to a minimum possible RMSD. Note that the TC search and pharmacophore search of ChemBridge had no overlapping compounds.
RPTC were exposed to the 16 identified ChemBridge compounds (5 μM) for 24 h and examined for changes in FCCP-OCR. This assay identified three novel compounds (CB1, CB2, and CB3) that increase FCCP-OCR above the vehicle control (Table 2). In a previous study, our laboratory tested 480 random compounds from the ChemBridge DIVERSet on the XF-96. From these 480 random compounds, 13 were determined to be biogenic (unpublished data). We extrapolate that the spontaneous hit rate of the entire data set would be approximately 2.7%. Our pharmacophore performed with a hit rate of 18.8% of the compounds it chose. Using the enrichment factor equation from Pearlman and Charifson (2001), our pharmacophore selected a subset of the database 6.9 times richer in biogenic compounds than the original database:
TABLE 2.
The six-point formoterol pharmacophore model was used to search the ChemBridge DIVERSet for similar compounds
The ChemBridge DIVERSet contains 50,080 unique drug-like compounds that cover pharmacophore diversity. These compounds were used to create a 1,420,467-entry conformer library. An in silico search identified 16 compounds (or 0.03%) containing all six chemical features with a RMSD < 1 Å to the pharmacophore model for all features. RPTC were exposed to the 16 identified ChemBridge compounds (5 μM) for 24 h and examined for changes in FCCP-OCR. This assay identified three novel compounds (CB1, CB2, and CB3) that increase FCCP-OCR 15% above the vehicle control.
| Structure | ID | Average Drug FCCP OCR/Control FCCP OCR | CAS Number |
|---|---|---|---|
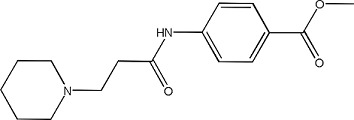 |
CB3 - 6431725 | 1.22 ± 0.04 | 398470-83-2 |
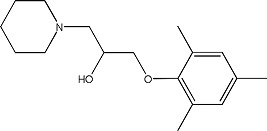 |
CB2 - 5144525 | 1.22 ± 0.08 | 300852-45-3 |
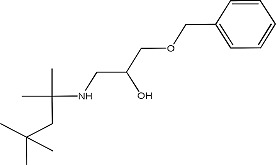 |
CB1 - 5144510 | 1.19 ± 0.08 | 765894-01-7 |
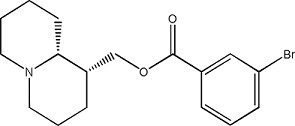 |
5402373 | 1.14 ± 0.05 | 329720-38-9 |
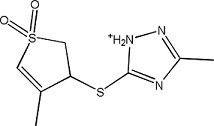 |
6384391 | 1.14 ± 0.08 | 443652-15-1 |
 |
6434119 | 1.09 ± 0.03 | 339228-37-4 |
 |
6433097 | 1.09 ± 0.06 | 42528-76-7 |
 |
5144511 | 1.08 ± 0.06 | 314027-94-6 |
 |
6435349 | 1.01 ± 0.05 | 313962-89-9 |
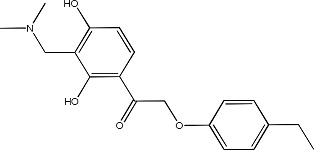 |
6632718 | 1 ± 0.05 | 377074-32-3 |
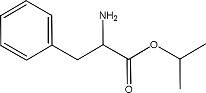 |
5108436 | 1 ± 0.11 | 81084-82-4 |
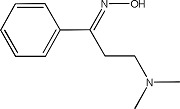 |
5533967 | 0.99 ± 0.07 | 309251-09-0 |
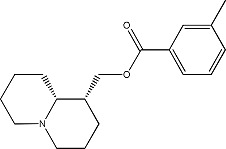 |
5402374 | 0.94 ± 0.04 | 1209049-21-7 |
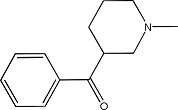 |
5244600 | 0.92 ± 0.1 | 70029-33-3 |
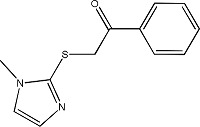 |
6111462 | 0.89 ± 0.04 | 263869-50-7 |
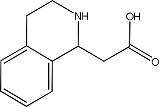 |
7562564 | 0.85 ± 0.04 | 105400-81-5 |
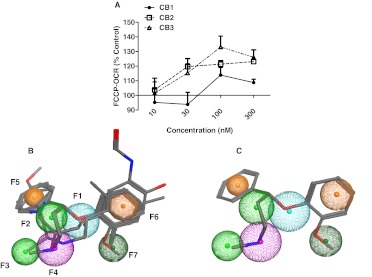
RPTC were exposed for 24 h to 10 to 3000 nM of CB1, CB2, and CB3 to determine potency in increasing FCCP-OCR. CB2 and CB3 increased FCCP-OCR in RPTC with maximal effects at 30 and 100 nM, respectively, whereas CB1 was not effective at this concentration range (Fig. 6A). These data were incorporated into the original model and used to further refine the pharmacophore. Formoterol, nisoxetine, CB2, and CB3 were aligned in both two and three dimensions and matched all six of the original features, suggesting the model accurately described all of the essential features (Fig. 6B). Analysis of the final alignment revealed a unique hydrophobic feature (F7) found in nisoxetine and CB2 (Fig. 6C; Table 3).
Fig. 6.
Formoterol pharmacophore identified two ChemBridge compounds that induce MB in RPTC. A, RPTC were exposed to ChemBridge compounds 1, 2, and 3 (10–300 nM) for 24 h and evaluated for changes in FCCP-OCR. Values indicate a percentage of fold change relative to DMSO controls. Data are represented as mean ± S.E.M. of four biological replicates. B, refined pharmacophore based on the alignment of formoterol, nisoxetine, CB2, and CB3. In this alignment, F1 is a proton acceptor, F2 and F3 are hydrophobic, F4 is a mixed feature with a proton donor and a cationic or proton acceptor, and F5 and F6 are mixed features with aromatic or hydrophobic characteristics. F7 is a unique hydrophobic feature found in nisoxetine and CB2. F1, F2, F4, F5, and F6 are essential features. C, nisoxetine aligned with superimposed pharmacophore.
TABLE 3.
Formoterol, nisoxetine, and (CB2) ChemBridge 5144525
The original pharmacophore was built by using formoterol and nisoxetine. CB1, CB2, and CB3 were found by using the original pharmacophore from a search of the ChemBridge DIVERSet 50,000 small-molecule conformational library. CB2 and CB3 were confirmed to be biogenic; however, CB2 was the only compound to add features to the pharmacophore model.
| Structure | Molecule Name | CAS Number |
|---|---|---|
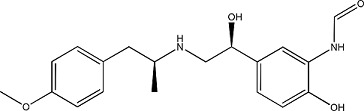 |
Formoterol hemifumarate | 183814–30-4 |
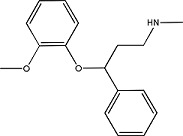 |
Nisoxetine | 57754–86-6 |
 |
CB2 | 463929–24-0 |
Discussion
The mitochondrion is an intricate organelle, with components derived from both the nuclear and mitochondrial genomes, whose activity must be carefully coupled to cellular metabolism and signaling (Wagner et al., 2008). Mitochondrial health is essential for cell and organ function. Multiple diseases are characterized by mitochondrial impairment including diabetes, Alzheimer's disease, Parkinson's disease, and diet-induced obesity. Ischemia/reperfusion injury of the kidney, heart, and liver all show deleterious consequences on mitochondrial function. Therefore, the development of novel pharmaceuticals that induce MB may have broad usage in diverse diseases.
Despite the potential for treating disorders characterized by mitochondrial impairment, very few therapies target the mitochondria to promote its function (Funk et al., 2010). Likewise, there are no current databases matching mitochondrial biogenic activity to chemical pharmacophores or specific chemotypes. Those studies that have focused on MB as a therapeutic target have used skeletal muscle cells and assay systems using surrogate markers of MB (e.g., PGC-1α mRNA). Surrogate markers may suffer from false positives and negatives, and the use of cell lines that are highly glycolytic with limited aerobic capacity may not be sensitive to MB. The use of primary cultures of primary RPTC grown under improved conditions resolves the cell line issue, and the use of a phenotypic respirometric assay minimizes the limitations of surrogate markers.
We demonstrate that formoterol, a potent and selective agonist for the β2-AR, stimulates MB in both RPTC and AFC within 24 h of a 30-nM exposure (Fig. 1). Neither a selective β3-AR agonist (BRL 37344) nor nonselective β-AR agonists (dopamine, epinephrine, norepinephrine, or isoproterenol) stimulate MB in RPTC. In addition, the ability of formoterol to increase FCCP-OCR was inhibited by the β-AR antagonist propranolol and the β2-AR inverse agonist ICI 115,881. These data provide further evidence that specific stimulation of the β2-AR induces MB in vitro.
Researchers have shown that exercise-induced increases in PGC-1α mRNA in skeletal muscle is blocked by the β2-AR inhibitor ICI-118,551 and the β-AR inhibitor propranolol (Miura et al., 2007; Sutherland et al., 2009). In addition, PGC-1α in skeletal muscle 24 h increased after injection of formoterol (100 μg/kg) (Pearen et al., 2009). In our studies, the same dose of formoterol induced MB in the kidney and heart of male mice as indicated by increased mRNA expression of multiple mitochondrial proteins that are both nuclear and mitochondrially encoded (NDUFB8, AT6, ND1, and ND6) (Figs. 2 and 3). Furthermore, PGC1-α, the master regulator of MB, was induced both 24 and 72 h after formoterol exposure. Using the aforementioned markers of MB, these data suggest that formoterol does induce MB in vivo and in both the kidney and heart.
We used the chemical structure of formoterol to search the Sigma LOPAC of 1280 compounds to find current pharmacological compounds that were similar in structure to formoterol and therefore may induce MB. We identified 29 compounds that were tested for their ability to increase the FCCP-OCR in RPTC after a 24-h 5-μM exposure. We identified nisoxetine as a potent inducer (30–300 nM) of MB. Nisoxetine is reported to be a potent and selective inhibitor of noradrenaline uptake with little or no affinity for a range of other similar neurotransmitter receptors (Wong and Bymaster, 1976; Cheetham et al., 1996; Mochizucki, 2004). Currently there are no data suggesting a role for nisoxetine in inducing MB. It is noteworthy that nisoxetine was aligned with formoterol, and a preliminary pharmacophore was developed with six features in common (Fig. 4B). In addition, propranolol and ICI-118,551 blocked the MB effects of both compounds (Fig. 5). These data provide strong evidence that nisoxteine is an agonist at the β2-AR receptor.
In addition to finding pharmacologically active compounds with MB activity, we used the formoterol-based pharmacophore to probe diverse chemical space. The pharmacophore designed from formoterol and nisoxetine was used to screen the 50,000 small-molecule ChemBridge DIVERSet. The in silico screen identified only 16 compounds that matched all six chemical features that defined the pharmacophore. When tested using RPTC, three of the compounds (19% accuracy in prediction, enrichment factor of 6.9) increased FCCP-OCR 24 h after a 5-μM exposure. A concentration response curve for the three compounds revealed CB2 and CB3 increased MB at 30 nM, whereas CB1 had no effect at concentrations below 5 μM. The positive compounds were aligned, resulting in the development of a refined pharmacophore with six features present within 100% of the compounds (Fig. 6B). Negative data are not included, because no clear consensus to exclusion space was reached. This improved pharmacophore can be used to search libraries such as the World Drug Index to find other compounds that match the pharmacophore and induce MB. In addition, future studies will attempt to identify a broader range of novel agonists for β2-AR-mediated MB by using a structure-based approach of the β2-AR as outlined by Kolb et al. (2009).
There is an abundance of evidence showing that exercise induces MB in skeletal muscle, and data suggest that β-AR agonism is required for exercise-mediated alterations in mitochondrial function (Miura et al., 2007; Higashida et al., 2008; Sutherland et al., 2009; Little et al., 2011). However, the potential use for β2-AR agonists for the treatment of mitochondrial dysfunction and injury has not yet been explored. Although evidence suggests that chronic exposure to formoterol decreases oxidative capacity in the heart, acute exposures to β2-AR agonists have the potential to activate MB after insult with limited detrimental effects (Ortega and Peters, 2010; Léger et al., 2011). Agonists for the β2-AR are promising therapeutics to treat mitochondrially related organ dysfunction found in diseases, including diabetes, and to promote recovery after injury, including acute kidney injury. We have identified a role of formoterol in the induction of MB in both the kidney and heart. In addition, we have used the chemical structure of formoterol to identify four new mitochondrial biogenic compounds. Future studies will focus on the potential of formoterol exposure to promote recovery of kidney and heart mitochondrial function in both disease and injury models.
Supplementary Material
Acknowledgments
We thank the staff at the Medical University of South Carolina Drug Discovery Core for access to the ChemBridge DIVERSet and the Medical University of South Carolina/Seahorse Biosciences Academic Core Facility.
L.P.W. is funded by the National Institutes of Health National Cancer Institute [Grant T32 CA119945-04] and the National Institutes of Health National Institute of Environmental Health Sciences [Grant F32 ES020103-01]. R.G.S. is funded by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK062028, DK071997], the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES012878], the National Institutes of Health National Institute of General Medical Sciences [Grant GM084147], and the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs. Animal facilities were funded by the National Institutes of Health National Center for Research Resources [Grant C06 RR-015455].
Portions of this work have been presented previously: Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, and Schnellmann RG (2011) Characterization of a β2 adrenoceptor pharmacophore that predicts mitochondrial biogenesis, at the American Society of Nephrology Annual Meeting; 2011 Nov 8–13; Philadelphia, PA. American Society of Nephrology, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- MB
- mitochondrial biogenesis
- AFC
- adult feline cardiomyocytes
- ATP6
- F0 subunit 6 of transmembrane F-type ATP synthase
- β-AR
- β-adrenoceptor
- CB
- ChemBridge
- Ct
- threshold cycle
- DMSO
- dimethyl sulfoxide
- FCCP
- carbonylcyanide p-trifluoromethoxyphenylhydrazone
- HPLC
- high-performance liquid chromatography
- LOPAC
- Library of Pharmacologically Active Compounds
- mtDNA
- mitochondrial DNA
- ND1
- NADH dehydrogenase subunit 1
- ND6
- NADH dehydrogenase subunit 6
- NDUFB8
- NADH dehydrogenase [ubiquinone] 1β subcomplex subunit 8
- OCR
- oxygen consumption rate
- PCR
- polymerase chain reaction
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator 1-α
- RMSD
- root mean square deviation
- RPTC
- renal proximal tubule cells
- SRT1720
- N-[2-[3-(piperazin-1-ylmethyl)imidazo[2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide
- TC
- Tanimoto Coefficient
- BRL 37344
- (±)-(R*,R*)-[4-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy] acetic acid sodium hydrate
- ICI-118,551
- 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol hydrochloride.
Authorship Contributions
Participated in research design: Wills, Peterson, C. C. Beeson, and Schnellmann.
Conducted experiments: Wills, Trager, and G. C. Beeson.
Contributed new reagents or analytic tools: Peterson.
Performed data analysis: Wills, Trager, Lindsey, Peterson, C. C. Beeson, and Schnellmann.
Wrote or contributed to the writing of the manuscript: Wills, Trager, Peterson, and Schnellmann.
References
- Agbenyega ET, Morton RH, Hatton PA, Wareham AC. (1995) Effect of the β2-adrenergic agonist clenbuterol on the growth of fast- and slow-twitch skeletal muscle of the dystrophic (C57BL6J dy2J/dy2J) mouse. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 111:397–403 [DOI] [PubMed] [Google Scholar]
- Arany Z, Wagner BK, Ma Y, Chinsomboon J, Laznik D, Spiegelman BM. (2008) Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1α and oxidative phosphorylation. Proc Natl Acad Sci U S A 105:4721–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloyannis SJ. (2006) Mitochondrial alterations in Alzheimer's disease. J Alzheimer's Dis 9:119–126 [DOI] [PubMed] [Google Scholar]
- Beeson CC, Beeson GC, Schnellmann RG. (2010) A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem 404:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham SC, Viggers JA, Butler SA, Prow MR, Heal DJ. (1996) [3H]nisoxetine–a radioligand for noradrenaline reuptake sites: correlation with inhibition of [3H]noradrenaline uptake and effect of DSP-4 lesioning and antidepressant treatments. Neuropharmacology 35:63–70 [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Ravussin E. (2008) Mitochondrial energetics and insulin resistance. Endocrinology 149:950–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebalunode JO, Zheng W, Tropsha A. (2011) Application of QSAR and shape pharmacophore modeling approaches for targeted chemical library design. Methods Mol Biol 685:111–133 [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, Beeson C. (2008) Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13:268–274 [DOI] [PubMed] [Google Scholar]
- Fuke S, Kubota-Sakashita M, Kasahara T, Shigeyoshi Y, Kato T. (2011) Regional variation in mitochondrial DNA copy number in mouse brain. Biochim Biophys Acta 1807:270–274 [DOI] [PubMed] [Google Scholar]
- Funk JA, Odejinmi S, Schnellmann RG. (2010) SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther 333:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. (2009) Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem 81:6868–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Moreau R, Suh JH, Visioli F. (2002) Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-l-carnitine and/or lipoic acid. Ann NY Acad Sci 959:491–507 [DOI] [PubMed] [Google Scholar]
- Hagström-Toft E, Enoksson S, Moberg E, Bolinder J, Arner P. (1998) β-Adrenergic regulation of lipolysis and blood flow in human skeletal muscle in vivo. Am J Physiol Endocrinol Metab 275:E909–E916 [DOI] [PubMed] [Google Scholar]
- Hall JA, Kaumann AJ, Brown MJ. (1990) Selective β1-adrenoceptor blockade enhances positive inotropic responses to endogenous catecholamines mediated through β2-adrenoceptors in human atrial myocardium. Circ Res 66:1610–1623 [DOI] [PubMed] [Google Scholar]
- Higashida K, Higuchi M, Terada S. (2008) Potential role of lipin-1 in exercise-induced mitochondrial biogenesis. Biochem Biophys Res Commun 374:587–591 [DOI] [PubMed] [Google Scholar]
- Horvath D. (2011) Pharmacophore-based virtual screening, in Chemoinformatics and Computational Chemical Biology (Bajorath J. ed) pp 261–298, Humana Press, New York: [DOI] [PubMed] [Google Scholar]
- Kolb P, Rosenbaum DM, Irwin JJ, Fung JJ, Kobilka BK, Shoichet BK. (2009) Structure-based discovery of β2-adrenergic receptor ligands. Proc Natl Acad Sci U S A 106:6843–6848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger B, Koopman R, Walrand S, Gehrig SM, Murphy KT, Lynch GS. (2011) Chronic formoterol administration reduces cardiac mitochondrial protein synthesis and oxidative capacity in mice. Int J Cardiol 146:270–272 [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. (2011) An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300:R1303–R1310 [DOI] [PubMed] [Google Scholar]
- Mann DL, Kent RL, Cooper G., 4th (1989) Load regulation of the properties of adult feline cardiocytes: growth induction by cellular deformation. Circ Res 64:1079–1090 [DOI] [PubMed] [Google Scholar]
- Medeiros DM. (2008) Assessing mitochondria biogenesis. Methods 46:288–294 [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Hancox JC, Levi AJ. (1998) Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties.. Cardiovasc Res 39:280–300 [DOI] [PubMed] [Google Scholar]
- Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. (2007) An increase in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in response to exercise is mediated by β-adrenergic receptor activation. Endocrinology 148:3441–3448 [DOI] [PubMed] [Google Scholar]
- Mochizucki D. (2004) Serotonin and noradrenaline reuptake inhibitors in animal models of pain. Hum Psychopharmacol 19 (Suppl 1):S15–S19 [DOI] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. (1995) Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol Cell Physiol 268:C1053–C1061 [DOI] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. (1996) L-ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol Cell Physiol 271:C2072–C2080 [DOI] [PubMed] [Google Scholar]
- Ortega VE, Peters SP. (2010) β2 Adrenergic agonists: focus on safety and benefits versus risks. Curr Opin Pharmacol 10:246–253 [DOI] [PubMed] [Google Scholar]
- Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. (2008) The orphan nuclear receptor, NOR-1, a target of β-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology 149:2853–2865 [DOI] [PubMed] [Google Scholar]
- Pearen MA, Ryall JG, Lynch GS, Muscat GE. (2009) Expression profiling of skeletal muscle following acute and chronic β2-adrenergic stimulation: implications for hypertrophy, metabolism and circadian rhythm. BMC Genomics 10:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman DA, Charifson PS. (2001) Improved scoring of ligand-protein interactions using OWFEG free energy grids. J Med Chem 44:502–511 [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG. (2010) 5-Hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J Pharmacol Exp Ther 332:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. (2007) PGC-1α over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355:734–739 [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. (2008) Isoflavones promote mitochondrial biogenesis. J Pharmacol Exp Ther 325:536–543 [DOI] [PubMed] [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. (2010) New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123:2533–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JP, Kropp BP, Thor KB. (1994) Facilitatory and inhibitory effects of selective norepinephrine reuptake inhibitors on hypogastric nerve-evoked urethral contractions in the cat: a prominent role of urethral β-adrenergic receptors. J Urol 152:515–519 [DOI] [PubMed] [Google Scholar]
- Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. (2009) Exercise and adrenaline increase PGC-1α mRNA expression in rat adipose tissue. J Physiol 587:1607–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, et al. (2011) PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121:4003–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BK, Kitami T, Gilbert TJ, Peck D, Ramanathan A, Schreiber SL, Golub TR, Mootha VK. (2008) Large-scale chemical dissection of mitochondrial function. Nat Biotechnol 26:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett P. (2006) Similarity-based virtual screening using 2D fingerprints. Drug Discov Today 11:1046–1053 [DOI] [PubMed] [Google Scholar]
- Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. (2006) DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34:D668–D672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP. (1976) Effect of nisoxetine on uptake of catecholamines in synaptosomes isolated from discrete regions of rat brain. Biochem Pharmacol 25:1979–1983 [DOI] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. (2006) Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A 103:14379–14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.