Abstract
4-Arylpyrano-[3,2-c]-pyridones have been prepared by a one-step cyclocondensation of 4-hydroxy-1,6-dimethylpyridin-2(1H)-one with various substituted benzaldehydes and malononitrile. These heterocycles exhibit micromolar and submicromolar antiproliferative activity in HeLa and induce apoptosis in Jurkat cell lines. Structure-activity studies performed on a small library of these compounds show a pronounced cytotoxicity enhancing effect of the bromo substituent at the meta position of the C4 aromatic moiety.
Keywords: Multicomponent synthesis, Cytotoxicity, Apoptosis, Heterocycles
2-Pyridone structural unit occurs in many natural and synthetic molecules exhibiting diverse biological activities.1 A few selected examples include antitumor antibiotic diazaquinomycin A,2 a non-nucleoside HIV reverse transcriptase inhibitor L-697,6613 and a phosphodiesterase inhibitor milrinone,4 used in the clinic for the treatment of heart failure (Figure 1).
Figure 1.
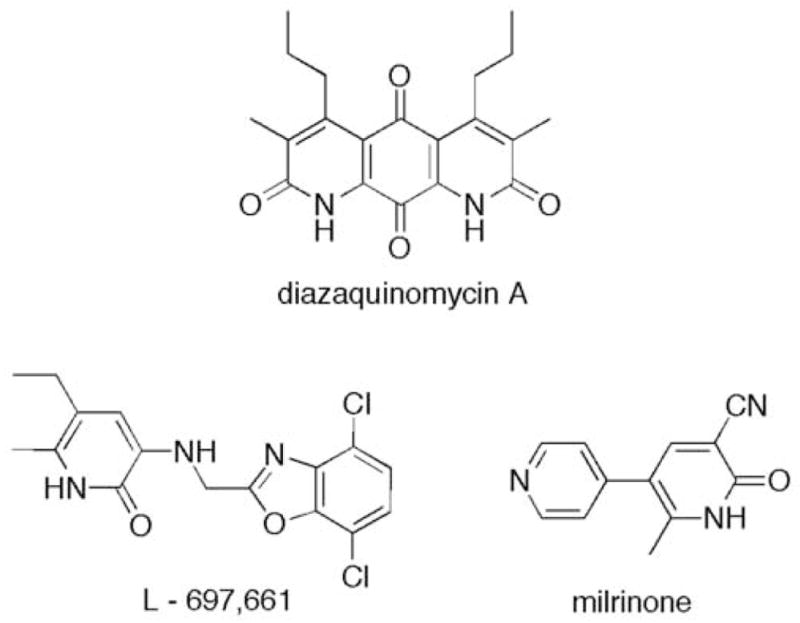
Structures of biologically active 2-pyridone-containing compounds.
Many libraries of compounds containing a 2-pyridone moiety fused with another heterocyclic ring have been prepared and tested for various biological activities. Somewhat surprisingly, the biology of 4H-pyrano-[3,2-c]-pyridin-5(6H)-ones (A, Figure 2) has not been thoroughly investigated with the exception of antibacterial properties associated with some of these compounds.5 For example, a literature search reveals that pyranopyridones B, whose preparation by way of cyclocondensation of arylidene malononitriles with 4-hydroxypyridine-2-ones has been reported on many occasions,6 have not been evaluated for biological activities. In contrast, a number of recent publications and patents have described promising anticancer activity, associated with chromenes C.7 These compounds, shown to inhibit tubulin polymerization and induce apoptosis in cancer cells, exhibit high potency against taxol- and vinblastine-resistant, P-glycoprotein overexpressing cell types.7d Furthermore, chromenes C disrupt tumor vasculature in a number of human solid tumor xenografts and they are currently in development as anticancer agents.7b.c
Figure 2.

Structures of the scaffold A, pyranopyridone library B and chromene library C.
Extensive SAR studies performed with chromenes C show that substituents on the benzene ring at positions 7 and 8 (R7 and R8) are well tolerated, while the introduction of subsituents at positions 5 and 6 (R5 and R6) results in inactive compounds. Furthermore, replacement of the benzene ring of the chromene scaffold with a heterocyclic moiety has not been reported to the best of our knowledge. We reasoned that pyridones B would represent an interesting test because of the partial aromatic character of this ring brought about by the amide resonance conjugation. In addition, the amide group would be placed into the part of the structure that is most sensitive to alterations. Finally, amide resonance conjugation would make the compounds more polar addressing water solubility issues of the parent chromenes C.8
The synthesis of the pyridones B library is shown in Figure 3. Pyridone 1 was prepared by treating the corresponding commercially available pyrone with aqueous MeNH2 following a literature procedure.9 A three-component reaction of pyridone 1 with malononitrile and various aromatic aldehydes in a 1:1:1 ratio proceeds smoothly in refluxing ethanol containing a small quantity of Et3N.10 Pyranopyridones 2–12 precipitate directly from the refluxing reaction mixtures and require no further purification. The product yields are given in Table 1.11,12
Figure 3.
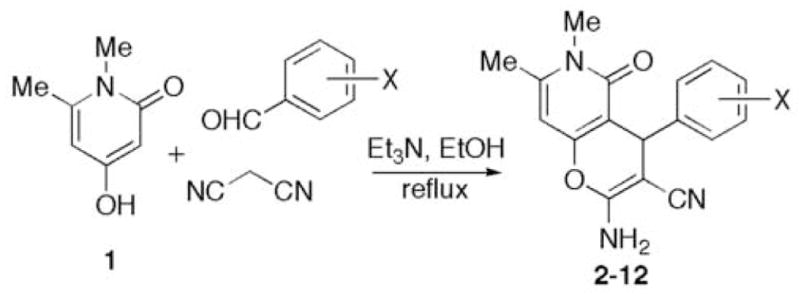
Three-component synthesis of pyrano-[3,2-c]-pyridones.
Table 1.
Synthetic yields and antiproliferative activity of pyrano-[3,2-c]-pyridones.
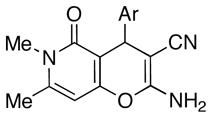
|
Synthesis | Compound concentration required to reduce HeLa cell viability by 50 % after 48 h treatment relative to 100 % DMSO control as assessed with MTT assay in three independent experiments | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
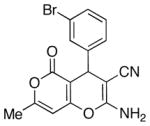
| Analogue | Ar | % Yield | IC50 (1), μM | IC50 (2), μM | IC50 (3), μM | IC50, μM | SD, μM |
| 2 |

|
83 | 0.3 | 0.3 | 0.4 | 0.33 | 0.06 |
| 3 |

|
87 | 0.75 | 0.5 | 0.5 | 0.58 | 0.14 |
| 4 |

|
88 | 2 | 0.75 | 0.5 | 1.08 | 0.8 |
| 5 |

|
75 | 2 | 2 | 4 | 2.67 | 1.1 |
| 6 |
|
83 | 4 | 2 | 4.5 | 3.5 | 1.3 |
| 7 |

|
84 | 7 | 7 | 5 | 6.33 | 1.1 |
| 8 |

|
97 | 7.5 | 7 | 5 | 6.5 | 1.3 |
| 9 |

|
98 | 20 | 15 | 20 | 18.3 | 2.9 |
| 10 |

|
97 | 40 | 50 | 40 | 43.3 | 5.1 |
| 11 |

|
81 | > 100 | > 100 | > 100 | > 100 | n/a |
| 12 |

|
97 | 20 | 25 | 60 | 35.0 | 21.8 |
 13 |
87 | > 100 | > 100 | > 100 | > 100 | n/a | |
 14 |
80 | 30 | 18 | 20 | 22.7 | 6.4 | |
The analogue library was tested for antiproliferative activity using HeLa cell line as a model for human cervical adenocarcinoma. The cells were treated with respective compounds for 48 h and cell viability was assessed through measurements of mitochondrial dehydrogenase activity using MTT method (Table 1).13 It is noteworthy that all potent analogues have a 3-bromo substituent on the aromatic ring at position C4 of the pyranopyridone skeleton (compounds 2–8) and this preference is uniform irrespective of the substitution pattern of this aromatic moiety. The 3-chloro (9) and other variously substituted analogues (10–12) are significantly less potent or are totally inactive. Further, the substitution of the nitrogen in the pyridone ring by oxygen, as in pyranopyranone 13, abolishes the activity as well. Moderate potency of the N-(β-arylethyl)pyridone 14, synthesized in a manner analogous to the rest of the library, warrants further investigation of compounds having a bulky moiety on the pyridone nitrogen. Efforts to prepare a library of such compounds are underway in our laboratories.
Since many clinically used anticancer agents induce apoptosis in cancer cells, we tested the pyranopyridone analogues for their ability to induce apoptosis in Jurkat cells using a flow cytometric annexin-V/propidium iodide assay (Figure 4). Compounds 2–8, exhibiting submicromolar or low micromolar potencies for the inhibition of proliferation of HeLa cells, were found to be strong inducers of apoptosis in Jurkats at 5 μM concentrations. The magnitude of apoptosis induction (50–60 % after 36 h treatment) is comparable to the known antimitotic agent colchicine used at the same concentration. In contrast, compounds 9, 11, 14, which are much less potent or totally inactive in the HeLa MTT assay, show no apoptosis induction of Jurkats at this concentration.
Figure 4.

Induction of apoptosis in Jurkat cells treated for 36 h with DMSO control, colchicine (5 μM) and selected pyridone library analogues (5 μM) in flow cytometric annexin-V/propidium iodide assay. Error bars represent data from two experiments.
For comparison we selected some of the most potent chromene C (Figure 2) analogues on the basis of the literature data (e.g. R5, R6, R8 = H; R7 = NMe2; X = 3,4,5-tri-OMe or X = 3-Br-4,5-di-OMe),7 synthesized and evaluated them in our assays. While the chromenes are significantly more cytotoxic to HeLa cells (IC50 = 1–10 nM), the magnitude of apoptosis induction in Jurkats is similar to compounds 2–8 (50–55% at 5 μM).
The images of Jurkat cells after 48 h treatment are shown in Figure 5. Cells treated with an inactive compound 11 (Figure 5B) look similar to the DMSO treated counterparts (Figure 5A). In contrast, extensive deformation and fragmentation are observed with cells treated with a potent analogue 3 (Figure 5C).
Figure 5.
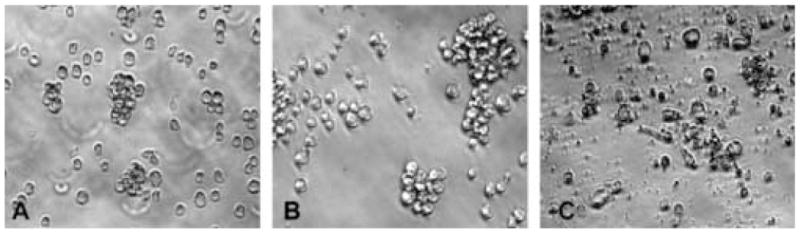
Jurkat cells after 48 h treatment with DMSO control (A), inactive compound 11 (B) and potent analogue 3 (C).
Lastly, the flow cytometric cell cycle analysis, performed with pyranopyridones 2 and 3 using Jurkat cell line, shows pronounced cell cycle arrest in the G2/M phase (Table 2). This effect is characteristic of antimitotic agents disrupting microtubule assembly, and is also observed with chromenes C that bind to or near the colchicine binding site on β-tubulin.7d This observation is indicative of the antitubulin mechanism for pyranopyridones B similar to the one established for chromenes C.
Table 2.
Flow cytometric cell cycle analysis of Jurkat cells.
| Compound | % Relative DNA content ± SD after 15 h treatment as assessed with Vybrant Orange staining | ||
|---|---|---|---|
|
| |||
| G0/G1 | S | G2/M | |
| DMSO | 49.4 ± 2.7 | 26.6 ± 0.1 | 23.9 ± 2.4 |
| 2 (5 μM) | 14.4 ± 1.7 | 18.1 ± 0.6 | 67.5 ± 2.3 |
| 3 (5 μM) | 12.0 ± 3.5 | 19.0 ± 2.0 | 68.5 ± 4.7 |
| colchicine (25 μM) | 19.0 ± 0.2 | 23.1 ± 0.1 | 57.9 ± 0.1 |
Further optimization of the pyranopyridione library with the aim of identifying more potent analogues as well as more detailed mechanistic studies are underway and will be reported in due course.
Acknowledgments
Russian Foundation for Basic Research (grant 07-03-00577 to I.V.M) and US National Institutes of Health (grants CA-99957 and RR-16480 to A.K. and S.R) are gratefully acknowledged for financial support of this work. We thank Professors Scott T. Shors and Patrick S. Mariano for helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.See Gorobets NYu, Yousefi BH, Belaj F, Kappe CO. Tetrahedron. 2004;60:8633. and references cited therein.
- 2.Pettit GR, Du J, Pettit RK, Richert LA, Hogan F, Mukku JVRV, Hoard MS. J Nat Prod. 2006;69:804. doi: 10.1021/np058087v. [DOI] [PubMed] [Google Scholar]
- 3.Saag MS, Emini EA, Laskin OL, Douglas J, Lapidus WI, Schleif WA, Whitley RJ, Hildebrand C, Byrnes VW, Kappes JC, Anderson KW, Massari FE, Shaw GM. N Engl J Med. 1993;329:1065. doi: 10.1056/NEJM199310073291502. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa GR. Clin Pharmacy. 1986;5:201. [PubMed] [Google Scholar]
- 5.(a) Choudhari BP, Mulwad VV. Indian J Chem Sect B-Org Chem Incl Med Chem. 2003;42:2080. [Google Scholar]; (b) Balasubramanian C, Sekar M, Mohan PS. Indian J Chem Sect B-Org Chem Incl Med Chem. 1996;35:1225. [Google Scholar]
- 6.(a) Elagamey AGAE, Sawellim SZ, El-Taweel FMA, Elnagdi MH. Coll Czech Chem Commun. 1988;53:1534. [Google Scholar]; (b) El-Taweel FMA, Sofan MA, Mashaly MA, Hanna MA, Elagamey AA. Pharmazie. 1990;45:671. [Google Scholar]; (c) Sowellim SZA, El-Taweel FMAA, Elagamey AGA. Bull Soc Chim Fr. 1996;133:229. [Google Scholar]; (d) El-Nabi HAA. Pharmazie. 1997;52:28. [Google Scholar]; (e) Mekheimer RA, Mohamed NH, Sadek KU. Bull Chem Soc Jpn. 1997;70:1625. [Google Scholar]; (f) Dodia N, Shah A. Indian J Heterocycl Chem. 2000;10:155. [Google Scholar]; (g) Stoyanov EV, Ivanov IC, Heber D. Molecules. 2000;5:19. [Google Scholar]; (h) Dodia N, Shah A. Heterocycl Commun. 2001;7:289. [Google Scholar]; (i) Stadlbauer W, Badawey ES, Hojas G, Roschger P, Kappe T. Molecules. 2001;6:338. [Google Scholar]; (j) Mulwad VV, Lohar MV. Indian J Heterocycl Chem. 2002;12:57. [Google Scholar]; (k) Rodinovskaya LA, Shestopalov AM, Gromova AV. Russ Chem Bull, Int Ed. 2003;52:2185. [Google Scholar]; (l) Rodinovskaya LA, Shestopalov AM, Shestopalov AA, Litvinov VP. Russ Chem Bull, Int Ed. 2005;54:992. [Google Scholar]; (m) El-Taweel FMAA. J Heterocyclic Chem. 2005;42:943. [Google Scholar]; (n) Rodinovskaya LA, Shestopalov AM, Gromova AV, Shestopalov AA. Synthesis. 2006:2357. [Google Scholar]
- 7.(a) Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Zhao J, Jia S, Xu L, Crogan-Grundy C, Denis R, Barriault N, Vaillancourt L, Charron S, Dodd J, Attardo G, Labrecque D, Lamothe S, Gourdeau H, Tseng B, Drewe J, Cai SX. Bioorg Med Chem Lett. 2005;15:4745. doi: 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]; (b) Gourdeau H, Leblond L, Hamelin B, Desputeau C, Dong K, Kianicka I, Custeau D, Boudreau C, Geerts L, Cai S-X, Drewe J, Labrecque D, Kasibhatla S, Tseng B. Mol Cancer Ther. 2004;3:1375. [PubMed] [Google Scholar]; (c) Kasibhatla S, Gourdeau H, Meerovitch K, Drewe J, Reddy S, Qiu L, Zhang H, Bergeron F, Bouffard D, Yang Q, Herich J, Lamothe S, Cai SX, Tseng B. Mol Cancer Ther. 2004;3:1365. [PubMed] [Google Scholar]; (d) Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, Jia S, Herich J, Labreque D, Storer R, Meerovitch K, Bouffard D, Rej R, Denis R, Blais C, Lamothe S, Attardo G, Gourdeau H, Tseng B, Kasibhatla S, Cai SX. J Med Chem. 2004;47:6299. doi: 10.1021/jm049640t. [DOI] [PubMed] [Google Scholar]; (e) Cai SX, Jiang S, Kemnitzer WE, Zhang H, Attardo G, Denis R. 2003097806. 20031127. PCT Int Appl WO. 2004:A2.; (f) Cai SX, Jiang S, Attardo G, Denis R, Storer R, Rej R. 2003096982. 20031127. PCT Int Appl WO. 2003:A2.; (g) Cai SX, Zhang H, Jiang S, Storer R. 2002092594. 20021121. PCT Int Appl WO. 2002:A1.; (h) Cai SX, Xu L, Storer R, Attardo G. 2002092083. 20021121. PCT Int Appl WO. 2002:A1.; (i) Cai SX, Zhang H, Kemmitzer WE, Jiang S, Drewe JA, Storer R. 2002092076. 20021121. PCT Int Appl WO. 2002:A1.; (j) Drewe JA, Cai SX, Wang Y. 2001034591. 20010517. PCT Int Appl WO. 2001:A2.
- 8.The calculated log P values are 1.5–2 units lower for pyranopyridones B (log P ~ 2–2.5) compared with the previously reported (Ref. 7d) 7-Me2N-substituted (R5, R6, R8 = H; R7 = NMe2) chromenes C (log P ~ 3.5–4.5).
- 9.Castillo S, Ouadahi H, Herault V. Bull Soc Chim Fr. 1982:257. [Google Scholar]
- 10.To our knowledge this is the first example of a multicomponent reaction used for the preparation of pyrano-[3,2-c]-pyridones. However, such process has been described for the synthesis of the corresponding quinolones utilizing KF-Al2O3 as a catalyst: Wang XS, Zeng ZS, Shi DQ, Wei XY, Zong ZM. Synth Commun. 2004;34:3021.
- 11.General procedure for the synthesis pyrano-[3,2-c]-pyridones 2–12: A mixture of 4-hydroxy-1,6-dimethylpyridin-2(1H)-one (0.8 mmol), malononitrile (0.8 mmol), triethylamine (0.05 mL) and a corresponding aldehyde (0.8 mmol) in EtOH (96% aqueous solution, 3 mL) was refluxed for 50 minutes. The reaction mixture was allowed to cool to room temperatrure, the precipitated product was collected by filtration and washed with EtOH (5 mL). In all cases the product was > 98% pure as judged by 1H NMR analysis.
- 12.Selected Characterization Data: Compound 2: 83%; mp 248–250 °C (EtOH); 1H NMR (DMSO-d6) δ 7.27 – 7.08 (m, 5H), 6.06 (s, 1H), 4.28 (s, 1H), 3.33 (s, 3H), 2.66 (s, 6H), 2.33 (s, 3H); 13C NMR (DMSO-d6) δ 161.7, 159.8, 155.4, 153.7, 148.8, 145.0, 143.1, 122.9, 112.7, 105.5, 97.4, 60.5, 56.6, 39.3, 37.2, 31.2, 20.8; HRMS m/z (ESI) calcd for C19H19BrN4O2 (M+Na+) 437.0589, found 437.0580. Compound 4: 88%; mp 258–260 °C (EtOH); 1H NMR (DMSO-d6) δ 7.09 (s, 2H), 6.90 (s, 1H), 6.79 (s, 1H), 6.07 (d, J = 2.7 Hz, 1H), 4.33 (d, J = 3.0 Hz, 1H), 3.92 (q, J = 3.0 Hz, 2H), 3.76 (s, 3H), 3.33 (s, 3H), 2.33 (s, 3H), 1.27 (t, J = 3.0 Hz. 3H); 13C NMR (DMSO-d6) δ 161.6, 159.8, 155.4, 153.8, 148.6, 144.3, 142.7, 122.9, 117.5, 112.6, 105.5, 97.42, 68.9, 57.8, 56.6, 37.1, 31.1, 20.8, 16.1; HRMS m/z (ESI) calcd for C20H20BrN3O4 (M+Na+) 468.0535, found 468.0540. Compound 5: 75%; mp 246–247 °C (EtOH); 1 H NMR (DMSO-d6) δ 9.32 (s, 1H), 7.04 (s, 2H), 6.82 – 6.71 (m, 2H), 6.06 (s, 1H), 4.28 (s, 1H), 3.77 (s, 3H), 3.32 (s, 3H), 2.34 (s, 3H); 13C NMR (DMSO-d6) δ 161.6, 159.6, 155.1, 148.5, 148.4, 143.0, 137.5, 123.1, 111.5, 109.6, 105.9, 97.4, 58.0, 56.7, 37.0, 31.1, 20.8;; HRMS m/z (ESI) calcd for C18H16BrN3O4 (M+Na+) 440.0222, found 440.0223.
- 13.Mosmann T. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]


