Abstract
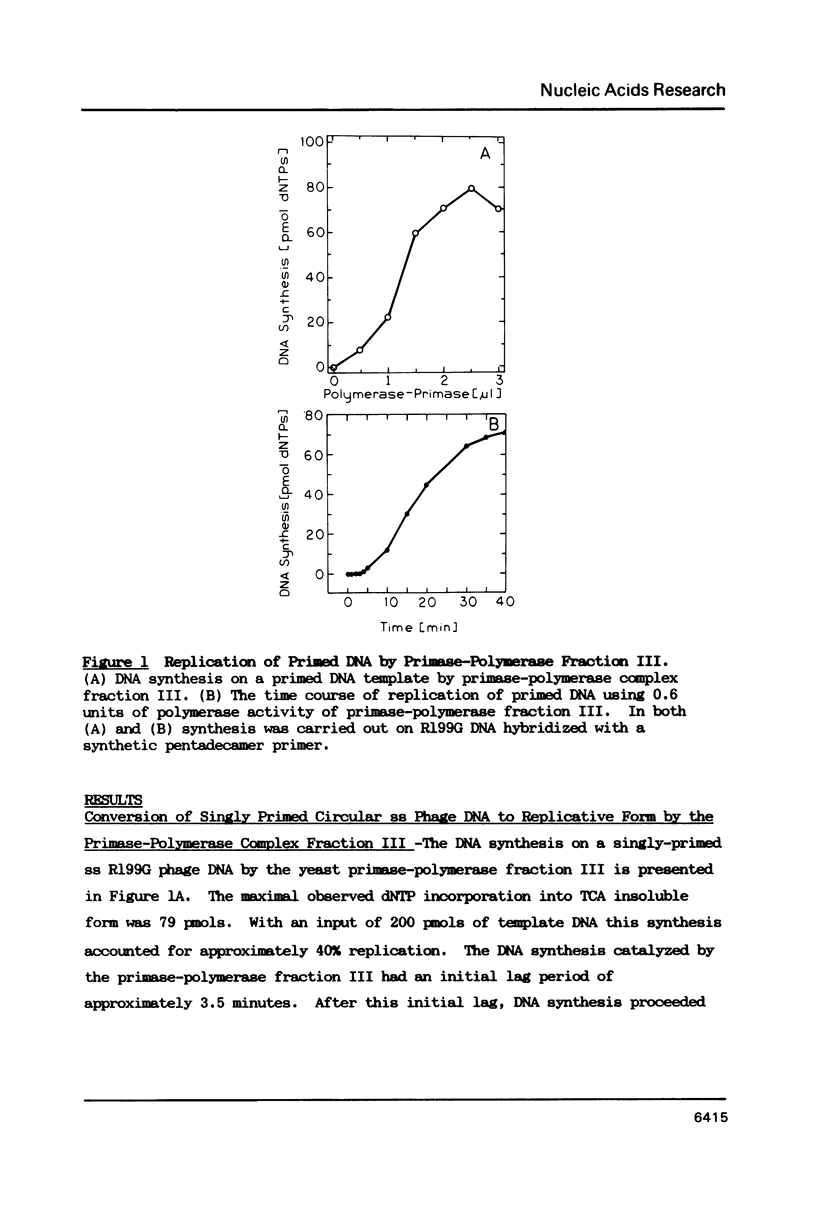
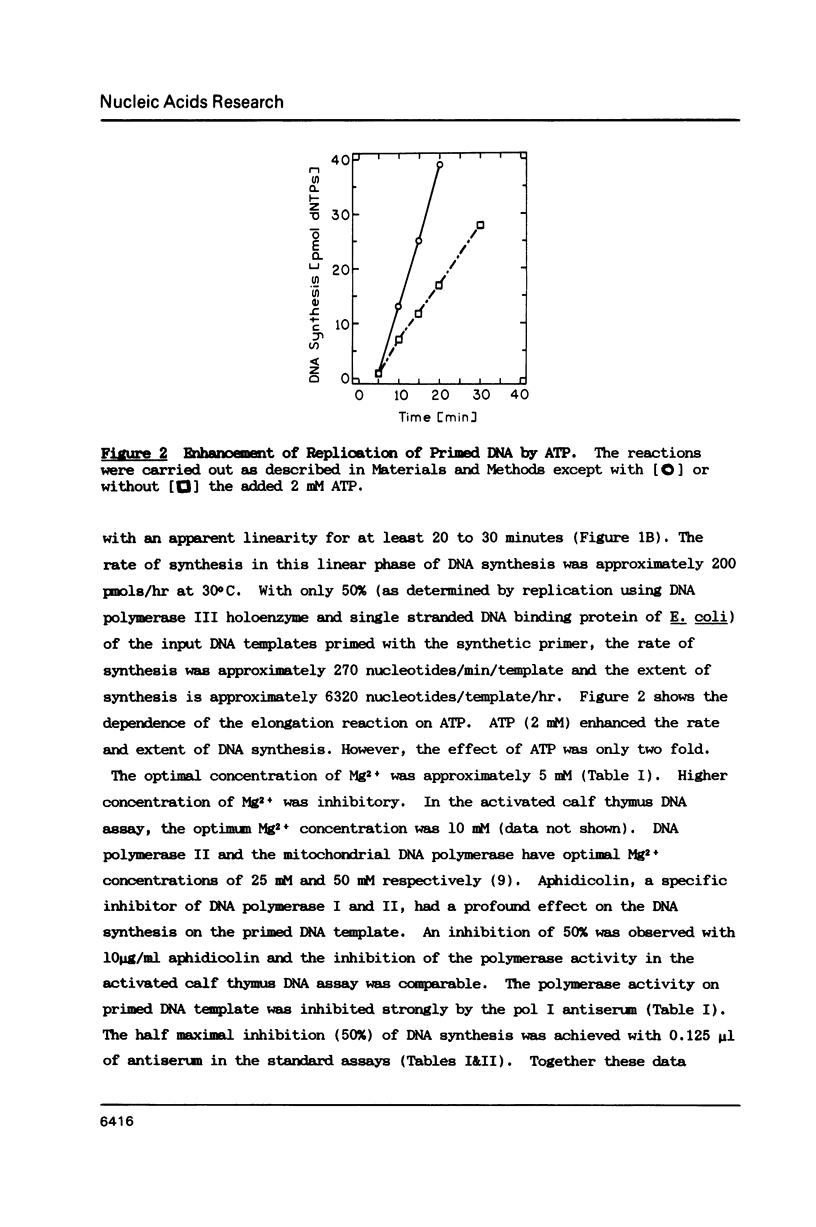
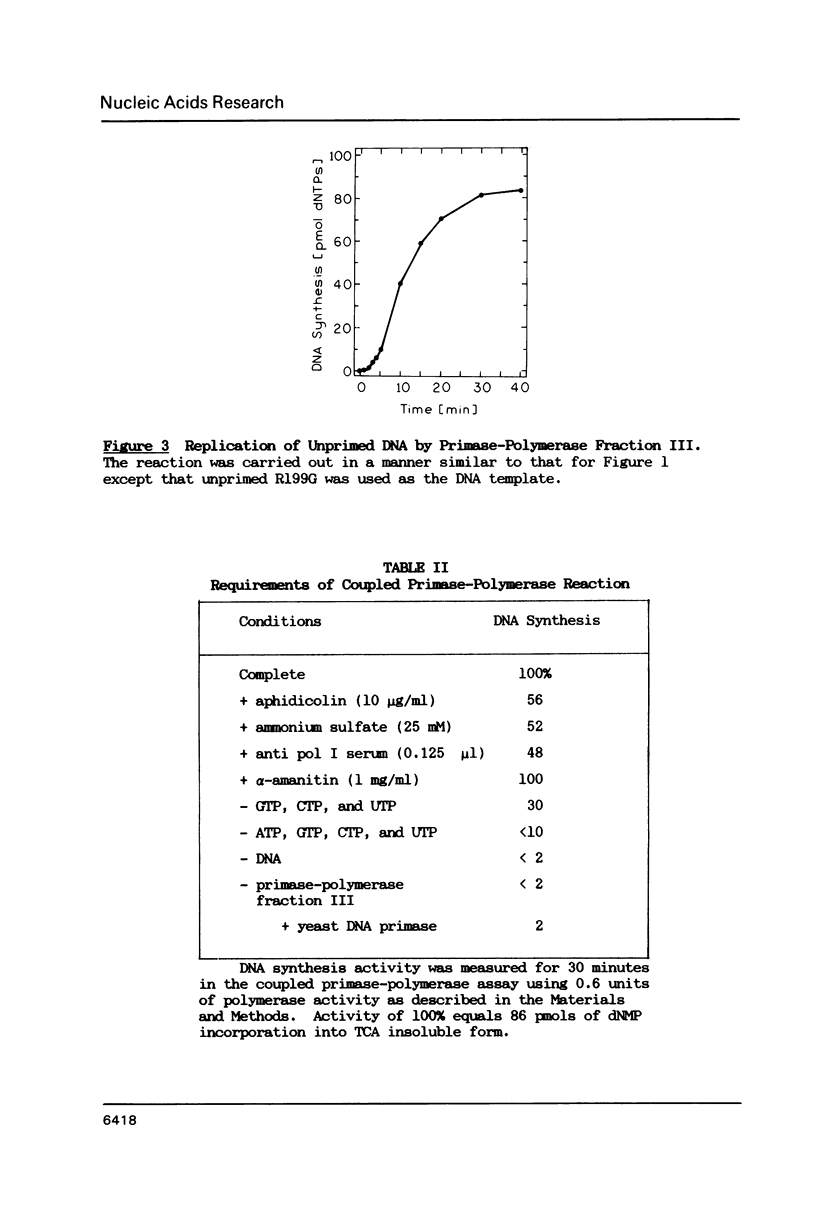
A partially purified primase-polymerase complex from the yeast, Saccharomyces cerevisiae, was capable of replicating a single stranded circular phage DNA into a replicative form with high efficiency. The primase-polymerase complex exhibited primase activity and polymerase activity on singly primed circular ssDNA as well as on gapped DNA. In addition, it was able to replicate an unprimed, single-stranded, circular phage DNA through a coupled primase-polymerase action. On Biogel A-O.5m filtration the primase-polymerase activities appeared in the void volume, demonstrating a mass of greater than 500 kilodaltons. Primase and various primase-polymerase complexes synthesized unique primers on single stranded DNA templates and the size distribution of primers was dependent on the structure of the DNA and the nature of the primase-polymerase assembly.
Full text
PDF












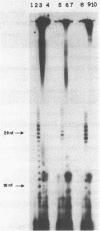
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharya P., Basu S. Probable involvement of a glycoconjugate in IMR-32 DNA synthesis: decrease of DNA polymerase alpha 2 activity after tunicamycin treatment. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1488–1491. doi: 10.1073/pnas.79.5.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas E. E., Joseph P. E., Biswas S. B. Yeast DNA primase is encoded by a 59-kilodalton polypeptide: purification and immunochemical characterization. Biochemistry. 1987 Aug 25;26(17):5377–5382. doi: 10.1021/bi00391a024. [DOI] [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Chang L. M., Rafter E., Augl C., Bollum F. J. Purification of a DNA polymerase-DNA primase complex from calf thymus glands. J Biol Chem. 1984 Dec 10;259(23):14679–14687. [PubMed] [Google Scholar]
- Cotterill S., Chui G., Lehman I. R. DNA polymerase-primase from embryos of Drosophila melanogaster. DNA primase subunits. J Biol Chem. 1987 Nov 25;262(33):16105–16108. [PubMed] [Google Scholar]
- Hübscher U., Gerschwiler P., McMaster G. K. A mammalian DNA polymerase alpha holoenzyme functioning on defined in vivo-like templates. EMBO J. 1982;1(12):1513–1519. doi: 10.1002/j.1460-2075.1982.tb01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. A DNA primase from yeast. Purification and partial characterization. J Biol Chem. 1985 Apr 25;260(8):4995–5002. [PubMed] [Google Scholar]
- Lamothe P., Baril B., Chi A., Lee L., Baril E. Accessory proteins for DNA polymerase alpha activity with single-strand DNA templates. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4723–4727. doi: 10.1073/pnas.78.8.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Novak B., Baril E. F. HeLa DNA polymerase alpha activity in vitro: specific stimulation by a non-enzymic protein factor. Nucleic Acids Res. 1978 Jan;5(1):221–239. doi: 10.1093/nar/5.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevani P., Foiani M., Valsasnini P., Badaracco G., Cheriathundam E., Chang L. M. Polypeptide structure of DNA primase from a yeast DNA polymerase-primase complex. J Biol Chem. 1985 Jun 10;260(11):7102–7107. [PubMed] [Google Scholar]
- Pritchard C. G., DePamphilis M. L. Preparation of DNA polymerase alpha X C1C2 by reconstituting DNA polymerase alpha with its specific stimulatory cofactors, C1C2. J Biol Chem. 1983 Aug 25;258(16):9801–9809. [PubMed] [Google Scholar]
- Pritchard C. G., Weaver D. T., Baril E. F., DePamphilis M. L. DNA polymerase alpha cofactors C1C2 function as primer recognition proteins. J Biol Chem. 1983 Aug 25;258(16):9810–9819. [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Singh H., Brooke R. G., Pausch M. H., Williams G. T., Trainor C., Dumas L. B. Yeast DNA primase and DNA polymerase activities. An analysis of RNA priming and its coupling to DNA synthesis. J Biol Chem. 1986 Jun 25;261(18):8564–8569. [PubMed] [Google Scholar]
- Stayton M. M., Kornberg A. Complexes of Escherichia coli primase with the replication origin of G4 phage DNA. J Biol Chem. 1983 Nov 10;258(21):13205–13212. [PubMed] [Google Scholar]
- Vishwanatha J. K., Baril E. F. Resolution and purification of free primase activity from the DNA primase-polymerase alpha complex of HeLa cells. Nucleic Acids Res. 1986 Nov 11;14(21):8467–8487. doi: 10.1093/nar/14.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanatha J. K., Coughlin S. A., Wesolowski-Owen M., Baril E. F. A multiprotein form of DNA polymerase alpha from HeLa cells. Resolution of its associated catalytic activities. J Biol Chem. 1986 May 15;261(14):6619–6628. [PubMed] [Google Scholar]
- Wahl A. F., Kowalski S. P., Harwell L. W., Lord E. M., Bambara R. A. Immunoaffinity purification and properties of a high molecular weight calf thymus DNA alpha-polymerase. Biochemistry. 1984 Apr 24;23(9):1895–1899. doi: 10.1021/bi00304a001. [DOI] [PubMed] [Google Scholar]
- Wilson F. E., Sugino A. Purification of a DNA primase activity from the yeast Saccharomyces cerevisiae. Primase can be separated from DNA polymerase I. J Biol Chem. 1985 Jul 5;260(13):8173–8181. [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. Studies on deoxyribonucleic acid polymerases from yeast. 2. Partial purification and characterization of mitochondrial DNA polymerase from wild type and respiration-deficient yeast cells. Eur J Biochem. 1970 Mar 1;13(1):20–27. doi: 10.1111/j.1432-1033.1970.tb00894.x. [DOI] [PubMed] [Google Scholar]