Abstract
Objective
To determine whether implementation of free cotrimoxazole (CTX) provision was associated with improved retention among clients ineligible for antiretroviral therapy (ART) enrolled in an HIV treatment program in Kenya.
Design
Data were obtained from a clinical cohort for program evaluation purposes. Twelve-month clinic retention was compared among ART-ineligible clients enrolled in the time period before free CTX versus the time period after.
Methods
Statistical comparisons were made using Kaplan–Meier survival curves, log-rank tests, and multivariate Cox proportional hazards models. To exclude potential temporal program changes that may have influenced retention, ART clients before and after the same cut-off date were compared.
Findings
Among adult clients enrolled between 2005 and 2007, 3234 began ART within 1 year of enrollment, and 1024 of those who did not start treatment were defined as ART-ineligible. ART-ineligible clients enrolled in the period following free CTX provision had higher 12-month retention (84%) than those who enrolled prior to free CTX (63%; P < 0.001). Retention did not change significantly during these periods among ART clients (P = 0.55). In multivariate analysis, ART-ineligible clients enrolled prior to free CTX were more than twice as likely to be lost to follow-up compared to those following free CTX [adjusted hazard ratio (aHR) = 2.64, 95% confidence interval 1.95–3.57, P < 0.001].
Conclusion
Provision of free CTX was associated with significantly improved retention among ART-ineligible clients. Retention and CD4-monitoring of ART-ineligible clients are essential to promptly identify ART eligibility and provide treatment. Implementation of free CTX may improve retention in sub-Saharan Africa and, via increasing timely ART initiation, provide survival benefit.
Keywords: antibiotic, HIV, lost to follow-up, prophylaxis, trimethoprim–sulfamethoxazole combination
Introduction
Scale-up of antiretroviral treatment services in poorer countries has provided 4 million people with access to antiretroviral therapy (ART) [1]. In Kenya, more than 242 000 adults and children accessed ART by the end of 2008. Throughout rapidly expanding programs in Africa, the initial focus for service was ART-eligible clients. Free provision of ART led to rapid enrollment of HIV-infected clients and most programs noted excellent adherence and retention with substantial declines in AIDS-related mortality as a result [2]. Within HIV treatment programs, HIV-infected individuals who were not yet ART-eligible initially received monitoring but were not typically provided with other free medications.
In contrast to the high retention of ART clients, there are emerging data showing that pre-ART and ART-ineligible clients have extremely low retention [3–7]. One-year weighted mean retention rates in ART programs in sub-Saharan Africa between 2000 and 2007 are estimated at 75% [8], but rates among ART-ineligible clients have been documented between 4 and 41% [4,6].
In contrast to US or EU settings, where cotrimoxazole (CTX) prophylaxis is indicated in severe immune suppression, CTX provides benefit to HIV-infected individuals with higher CD4 cell counts in settings with high infectious disease prevalence [9,10]. On the basis of this evidence, in 2006 WHO recommended CTX prophylaxis for individuals at higher CD4 cell counts or to all HIV-infected individuals in these settings [11]. In Kenya, in late 2006, provision of CTX prophylaxis became a routine intervention in many HIV treatment programs under revised Ministry of Health guidelines. Though this policy was not implemented as an intervention for retention, it is plausible that provision of a free medication like CTX may lead to substantive retention benefits in ART-ineligible clients, thus multiplying its benefit. To test this hypothesis, we compared retention among ART-ineligible clients before and after implementation of free CTX for all clients in an HIV treatment program in Kenya.
Methods
The Coptic Hope Center for Infectious Diseases is a comprehensive HIV treatment facility in Nairobi, Kenya that provides basic HIV care and antiretroviral medication free of charge to adults and children. Clients were offered free ART, if ART-eligible based on national criteria, or routine clinical follow-up if ineligible for ART. Clinical protocols are described elsewhere [12]. Based on national guidelines, CTX was offered to all ART-ineligible clients free of charge after September 2006. Patients on ART were scheduled to return to clinic every 1–2 months for a medical follow-up or pharmacy visit. Prior to free CTX provision, ART-ineligible patients were scheduled to return to clinic for medical follow-up every 6 months, though clients with medical issues or clients receiving medications returned more frequently. After CTX provision, all patients were scheduled to return to clinic every 1–2 months for a pharmacy visit.
Patients who missed pharmacy or clinical appointments were contacted through a phone call and urged/assisted to return to clinic. If the client was deceased, a family or household member provided mortality information through a ‘verbal autopsy’ process administered by a trained counselor.
The University of Washington Institutional Review Board and Kenyatta National Hospital Ethical Review Committee approved the use of clinic data for this study.
Clients with a baseline CD4 cell count were included in the analysis. Clients who started ART within 1 year of clinic enrollment were defined as receiving ART. Among those who did not start ART within 1 year of enrollment, ART-naive clients with WHO stage 1 or 2 disease and a CD4 cell count above 250 cells/µl were considered ART-ineligible. Loss to follow-up was defined as not returning to clinic more than 30 days after the next scheduled pharmacy or clinic appointment. Clients who returned to clinic after missing one or more appointments were classified as retained in care. Time in care was defined as the time in months from date of enrollment to date of last visit. There were 66 clients (17 ART-ineligible and 49 on ART) who transferred to another clinic or relocated, and these cases were censored at time of exit or transfer.
Twelve-month clinic retention was compared in ART-ineligible clients enrolled before versus after free CTX provision using Kaplan–Meier survival curves, log-rank test, and multivariate Cox proportional hazards regression models. Potential cofactors for loss to follow-up were evaluated using χ2 analysis and Mann–Whitney U-tests of medians. To determine whether temporal program changes influenced clinic retention, retention of ART clients before versus after the same cut-off date was also compared.
Results
Among 5854 adult clients enrolled at the Hope Center Nairobi clinic between 2005 and 2007, 5175 (88%) had a baseline CD4 cell count. Of these, 3234 clients (62%) started ART during their first year of care. Of the 1941 clients who did not start ART within 1 year of enrollment, 1024 (53%) were ineligible for ART using the definitions above.
Among ART-ineligible clients, 610 (60%) accessed care before free CTX was routinely offered, and 414 (40%) accessed care after free CTX was routinely offered. There were no significant differences in age, sex, tuberculosis (TB) disease, or BMI associated with accessing care before versus after implementation of free CTX. Median age at enrollment was 32 years. Women represented more than 70% of clients and the median BMI was within the normal WHO range in both groups. ART-ineligible clients enrolled after free CTX had similar baseline CD4 cell counts to those enrolled before free CTX (median CD4 cell count 412 cells/µl before free CTX versus 441 cells/µl after free CTX, P = 0.36).
Those lost to follow-up within 1 year were younger (P = 0.03) and BMI was significantly lower (P < 0.001) than those retained in care. Sex, TB status, and baseline CD4 cell counts were similar between retention groups.
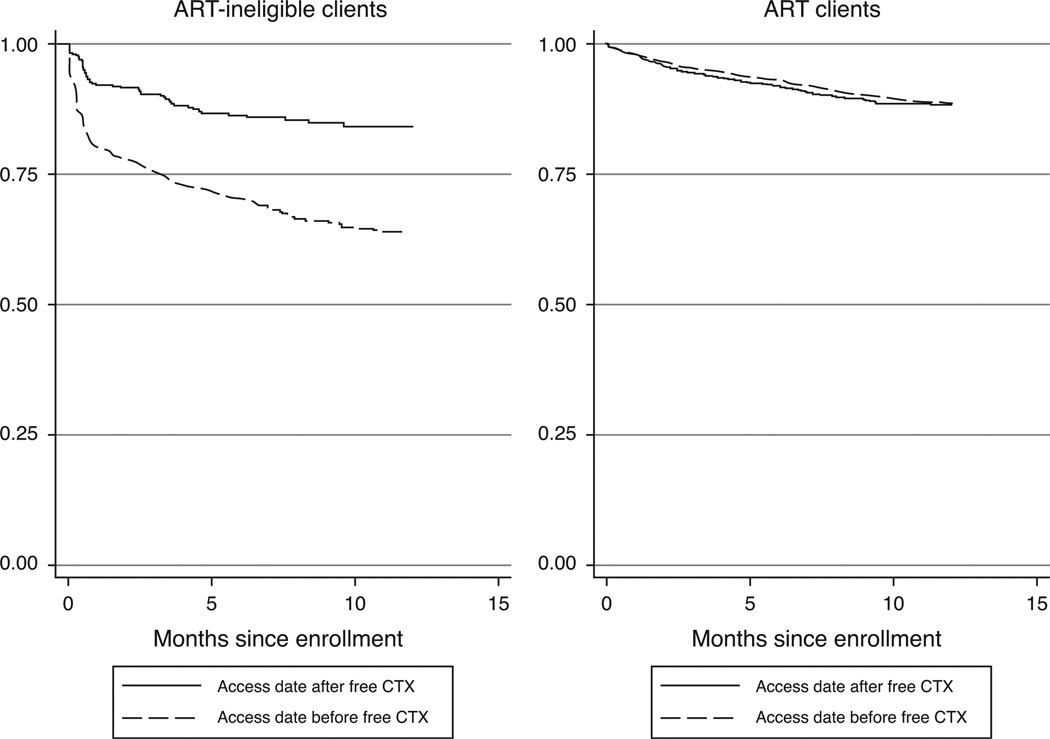
ART-ineligible clients enrolled in the period following free CTX provision had significantly higher 12-month retention (84%) than those who enrolled in the period prior to free CTX availability (63%; P < 0.001; Fig. 1). Among clients on ART, there was not a significant difference in retention in comparisons of these periods (89 and 88% respectively, P = 0.55).
Fig. 1.
Kaplan–Meier survival estimates of 1-year retention before and after implementation of free cotrimoxazole among antiretroviral therapy-ineligible and antiretroviral therapy-treated clients in Nairobi, Kenya. ART, antiretroviral therapy; CTX, cotrimoxazole.
In multivariate analysis, adjusted for age, sex, and CD4 cell count, ART-ineligible clients enrolled prior to free CTX provision were more than twice as likely to be lost to follow-up as those enrolled after free CTX provision (adjusted hazard ratio = 2.64, 95% confidence interval 1.95–3.57, P < 0.001). Similar results were found when combining mortality and lost to follow-up as an outcome. In a sub-analysis stratifying those with baseline CD4 cell counts between 251 and 350 cells/µl and those with baseline CD4 cell counts above 350 cells/µl, improved immunologic status made no difference in retention during each time period. Retention rates were 63 and 64% prior to free CTX, and 86 and 83% after free CTX, for those with 251–350 cells/µl and those with more than 350 cells/µl, respectively.
Discussion
In this study, we found that free CTX provision was associated with significantly higher retention in ART-ineligible clients, increasing from 63 to 84% during the period after free CTX. During the same comparison periods, retention among ART clients was stable and high.
Late presentation into HIV care has been associated with early mortality and poor response to treatment [13–16]. Interventions are not routinely offered to clients not yet eligible for ART, thus retention among this group is often low. Many of these clients may not return to HIV care until symptomatic, missing an opportunity for preventive care and timely initiation of ART.
In the few studies observing retention among those ineligible for ART, rates are consistently low. In Malawi, 6-month retention rates were 4% among clients with stage 1 or 2 WHO disease compared to 90% among those who started ART [4]. A South African program reported that among adults with a CD4 cell count greater than 350, only 26% returned within 1 year, and among adults with a CD4 cell count between 251 and 350, only 41% returned within 1 year [6].
Consistent with these studies, in our analysis retention in ART-ineligible clients was initially substantially lower than those on ART. However, after implementation of free CTX, 1-year retention rates among those who were ART-ineligible were similar to estimated mean retention rate for clients receiving ART in sub-Saharan African ART programs [8,17,18].
Our finding that free CTX substantially improves retention in ART-ineligible clients is also consistent with studies showing that free treatment improves retention in HIV care [19,20]. In another ART treatment program in Kenya, free ART was associated with a 57% risk reduction in loss to follow-up compared to clients who paid for treatment [19]. ART-ineligible clients may have perceived more benefit from clinic attendance due to CTX provision. In previous studies of clients in ART programs, individual perception of treatment, in addition to service factors of cost or quality, is an important factor in the decision to remain in care [21,22].
CTX has been demonstrated to be one of the most cost-effective interventions in HIV treatment [23–25] and has been associated with improved personal and family health outcomes [26,27]. Our study suggests that the cost of CTX is likely to be outweighed by benefit, not only in terms of improved prevention of infections, but also as an incentive to maintain clinical monitoring that can ensure timely initiation of ART.
Study strengths include the ability to adjust for disease status and CD4 cell count, and a comparison group of ART clients in a parallel analysis in which retention was unchanged.
Limitations of this study are consistent with a historical design. These include possible misclassification of enrollment during pre-CTX or post-CTX periods, as free CTX was implemented over time, and inability to measure socio-demographic factors or programmatic changes that may have influenced retention. The lack of change in retention among ART clients during an identical comparison period makes temporal changes in the program a less likely explanation.
The improvement in retention following free CTX may have been due in part to increased monitoring of clients as part of CTX delivery. Although the frequency of clinical visits did not increase after implementation of free CTX, clients returned monthly to the pharmacy for CTX pick-up. Missing a pharmacy pick-up triggered a follow-up phone call. Thus, the programmatic change in implementation of CTX included both free CTX and more frequent monitoring for return.
Although some of the differences we observed in retention may be attributable to improved health status, it is unlikely that the differences were due to reductions in mortality. Studies have noted improved survival among clients on CTX in the setting of severe immunosuppression [9,10,26]. Among clients with early HIV infection without immune suppression, CTX decreases morbidity but has not been associated with survival benefit [9,26]. Our analysis was restricted to clients with baseline counts greater than 250 cells/µl and stratified analyses showed comparable benefit in increasing retention in both those with 251–350 cells/µl and more than 350 cells/µl.
In summary, we found that provision of free CTX was associated with significantly improved retention among ART-ineligible clients. Retention and CD4-monitoring of pre-ART clients are essential to promptly identify ART eligibility and initiate treatment. Thus, implementation of free CTX, in addition to anti-infective benefits, may improve HIV survival by enhancing clinic retention, and free provision should be considered for ART-ineligible clients in resource poor settings. Additional research is also necessary to determine the role of increased monitoring in retention in care.
Acknowledgements
P.K. was responsible for study design, data analysis, and article preparation. J.T. assisted with data analysis. M.C., C.M., S.B.N., J.T., and G.J.S. contributed to study design and article preparation.
The present publication was supported by Cooperative Agreement Number U62/CCU024512 from Centers for Disease Control and Prevention (CDC) and PEPFAR. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC. G.C.J-S. is supported by NIH grant K24 HD054314. M.H.C. is supported by NIH grant K23 AI065222. C.J.M. is supported by the National Center for Research Resources, a component of NIH (TL1RR025016). Field site support is also provided by the University of Washington Center for AIDS Research International Core, an NIH funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM).
Footnotes
Conflicts of interest
There are no conflicts of interest.
This work was presented at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, 27 February to 2 March 2011.
References
- 1.World Health Organization. Geneva: WHO; 2009. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. [Google Scholar]
- 2.UNAIDS. AIDS epidemic update 2009. Geneva: UNAIDS; 2009. [Google Scholar]
- 3.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, et al. The ‘ART’ of linkage: pretreatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tayler-Smith K, Zachariah R, Massaquoi M, Manzi M, Pasulani O, van den Akker T, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104:313–319. doi: 10.1016/j.trstmh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Raguenaud ME, Isaakidis P, Zachariah R, Te V, Soeung S, Akao K, et al. Excellent outcomes among HIV+ children on ART, but unacceptably high pre-ART mortality and losses to follow-up: a cohort study from Cambodia. BMC Pediatr. 2009;9:54. doi: 10.1186/1471-2431-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson B, Brennan A, McNamara L, Long L, Rosen S, Sanne I, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(Suppl 1):43–47. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglaret X, Chêne G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 10.Wiktor S, Sassan-Morokro M, Grant A, Abouya L, Karon J, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva: World Health Organization; 2006. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach. [Google Scholar]
- 12.Chung M, Drake A, Richardson B, Reddy A, Thiga J, Sakr S, et al. Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Curr HIV Res. 2009;7:441–446. doi: 10.2174/157016209788680552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn S, Myer L, Harling G, Orrell C, Bekker L, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, May M, Chêne G, Phillips A, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 15.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabin C, Smith C, Youle M, Lampe F, Bell D, Puradiredja D, et al. Deaths in the era of HAART: contribution of late presentation, treatment exposure, resistance and abnormal laboratory markers. AIDS. 2006;20:67–71. doi: 10.1097/01.aids.0000196178.73174.24. [DOI] [PubMed] [Google Scholar]
- 17.Fox M, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health 2010. 2007;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassie J, Baijal P, Vitoria M, Alisalad A, Crowley S, Souteyrand Y. Trends in retention on antiretroviral therapy in national programs in low-income and middle-income countries. J Acquir Immune Defic Syndr. 2010;54:437–441. doi: 10.1097/QAI.0b013e3181d73e1b. [DOI] [PubMed] [Google Scholar]
- 19.Zachariah R, Van Engelgem I, Massaquoi M, Kocholla L, Manzi M, Suleh A, et al. Payment for antiretroviral drugs is associated with a higher rate of patients lost to follow-up than those offered free-of-charge therapy in Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2008;102:288–293. doi: 10.1016/j.trstmh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller C, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Health. 2010;15(Suppl 1):48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roura M, Busza J, Wringe A, Mbata D, Urassa M, Zaba B. Barriers to sustaining antiretroviral treatment in Kisesa, Tanzania: a follow-up study to understand attrition from the antiretroviral program. AIDS Patient Care STDS. 2009;23:203–210. doi: 10.1089/apc.2008.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldie S, Yazdanpanah Y, Losina E, Weinstein M, Anglaret X, Walensky R, et al. Cost-effectiveness of HIV treatment in resource-poor settings: the case of Côte d’Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 24.Pitter C, Kahn J, Marseille E, Lule J, McFarland D, Ekwaru J, et al. Cost-effectiveness of cotrimoxazole prophylaxis among persons with HIV in Uganda. J Acquir Immune Defic Syndr. 2007;44:336–343. doi: 10.1097/QAI.0b013e31802f12b5. [DOI] [PubMed] [Google Scholar]
- 25.Ryan M, Griffin S, Chitah B, Walker A, Mulenga V, Kalolo D, et al. The cost-effectiveness of cotrimoxazole prophylaxis in HIV-infected children in Zambia. AIDS. 2008;22:749–757. doi: 10.1097/QAD.0b013e3282f43519. [DOI] [PubMed] [Google Scholar]
- 26.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 27.Mermin J, Lule J, Ekwaru JP, Downing R, Hughes P, Bunnell R, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. AIDS. 2005;19:1035–1042. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]