Abstract
Objective
To define systemic release kinetics of a panel of cytokines and heat shock proteins (HSP) in porcine polytrauma/hemorrhage models and to evaluate whether they could be useful as early trauma biomarkers.
Design and Setting
Prospective study in a research laboratory.
Subjects
Twenty-one Yorkshire pigs.
Measurements and Main Results
Pigs underwent polytrauma (femur fractures/lung contusion, P), hemorrhage (mean arterial pressure 25-30mmHg, H), polytrauma plus hemorrhage (P/H) or sham procedure (S). Plasma was obtained at baseline, in 5-15min intervals during a 60min shock period without intervention and in 60-120min intervals during fluid resuscitation for up to 300min. Plasma was assayed for IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/IL-23p40, IL-13, IL-17, IL-18, IFNγ, TGFβ, TNFα, HSP40, HSP70 and HSP90 by ELISA. All animals after S, P and H survived (n=5/group). Three of six animals after P/H died. IL-10 increased during shock after P and this increase was attenuated after H. TNFα increased during the shock period after P, H and also after S. P/H abolished the systemic IL-10 and TNFα release and resulted in 20-30% increased levels of IL-6 during shock. As fluid resuscitation was initiated TNFα and IL-10 levels decreased after P, H and P/H, HSP 70 increased after P, IL-6 levels remained elevated after P/H and also increased after P and S.
Conclusions
Differential regulation of the systemic cytokine release after polytrauma and/or hemorrhage, in combination with the effects of resuscitation, can explain the variability and inconsistent association of systemic cytokine/HSP levels with clinical variables in trauma patients. Insults of major severity (P/H) partially suppress the systemic inflammatory response. The plasma concentrations of the measured cytokines/HSPs do not reflect injury severity or physiological changes in porcine trauma models and are unlikely to be able to serve as useful trauma biomarkers in patients.
Keywords: biomarker, resuscitation, inflammation, injury severity, shock, mortality
Introduction
Severe trauma has been documented to induce dysfunction of various immune cells (1-6). Their production of mediators that induce and determine the magnitude of the systemic inflammatory response syndrome (SIRS) is regarded as a key contributing factor for patient morbidity and mortality (7-9). Accordingly, systemic levels of various markers of inflammation, such as cytokines or heat shock proteins (HSP), have been used to document inflammation and have been associated with disease severity and outcomes (10-21). However, robust correlations between systemic levels of inflammation markers with injury severity and clinically relevant outcome variables after trauma in patients are missing. Many reasons exist to explain the inability of systemic inflammation markers to serve as clinically useful trauma biomarkers. Trauma patient populations are inherently heterogeneous. Besides the diversity of the individual organ and tissue injuries, various degrees of injury-associated hemorrhage complicate the disease pathology. Furthermore, the mediator response to trauma shows large inter-individual variability, the systemic half-life of cytokines is usually short and their release kinetics in patients are poorly defined (22-26). Therefore, blood sampling intervals that have been used in previous observational studies in patients could have missed a useful diagnostic or prognostic window, and subsequent therapeutic interventions may have further confounded relevant associations (3, 27).
It is obvious that a detailed characterization of the early release kinetics of inflammation markers after trauma in patients is problematic. Thus, trauma models are required to define their release kinetics, to establish possible associations with injury severity, organ dysfunction and outcome, and to provide a rational framework for the design of future clinical observational studies.
In the majority of trauma models, the mechanical trauma component, such as isolated extremity fracture or laparotomy, is minor and hemorrhage is superimposed to produce shock and enhance the physiological and immunological responses to injury (28-31). Accordingly, it was suggested that hemorrhage-associated ischemia and reperfusion injury is probably the predominant pathophysiological effector in trauma models that use a combination of minor injury and hemorrhage (29). Thus, little information is currently available about the influence of major trauma, hemorrhage and their combination on the systemic release of inflammation markers. In order to understand their association with clinically relevant variables, a detailed characterization of their release kinetics after major trauma, hemorrhage, and major trauma plus hemorrhage is needed. To fill this gap, we sought to define the systemic release patterns of a broad panel of cytokines and heat shock proteins (HSPs) in a porcine model of polytrauma. In this model, we aimed to evaluate the effects of an additional hemorrhage component on those markers of inflammation, which increase in the systemic circulation after polytrauma alone. We hypothesized that the addition of a hemorrhagic component would increase the overall injury severity which will be manifested by a further increase in systemic concentrations of cytokines and/or HSPs.
Material and Methods
General instrumentation and data collection
All procedures were performed according to National Institutes of Health Guidelines for Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Twenty-one male and female Yorkshire pigs (30-40 kg of body weight) were fasted overnight. Anesthesia was induced with 10 mg/kg ketamine and 1 mg/kg xylazine intramuscularly. Peripheral intravenous (i.v.) access was obtained for continuous infusion of 10 mg/kg/h ketamine, 0.25 mg/kg/h xylazine and 50 μg/kg/h fentanyl for maintenance of anesthesia throughout the experiment. Orotracheal intubation was performed using 5.0-6.0 mm internal diameter cuffed endotracheal tubes (Teleflex Medical) under direct laryngoscopy. Animals were mechanically ventilated (Evita XL, Draeger Medical) with intermittent mandatory ventilation adjusted to tidal volumes of 12 mL/kg at 12-18 breaths per minute to maintain a partial pressure of CO2 (pCO2) between 35-45 mmHg. The fraction of inspired oxygen (FiO2) was 0.4 and positive end expiratory pressure (PEEP) 5 mmHg, except where otherwise noted. Core body temperature was maintained using conductive warming blankets (Gaymar T/Pump 500 T/Pad). A central venous catheter (Triple-Lumen 7Fr. AGB+ Arrow Catheter, Teleflex Medical) was placed in the external jugular vein for administration of fluids, anesthesia and continuous monitoring of central venous pressure (CVP). The ipsilateral common carotid artery was cannulated for continuous measurement of mean arterial pressure (MAP). Electrocardiography, pulse oximetry, capnography (Evita XL Capnography module, Draeger Medical) and body temperature were measured and monitored continuously. Arterial blood was sampled every 30 min for measurements of pH, pCO2, pO2, hemoglobin, sodium, potassium, chloride, glucose and lactate using a blood gas analyzer (Stat Profile pHOx Plus L, Nova Biomedical). Venous blood was obtained at baseline (two samples per animal in 20 min intervals) and every five minutes for the first 30 minutes after injury, followed by increasing intervals (15 – 120 min) for the remaining 270 minutes. Venous blood was collected in lithium heparin tubes (APP Pharmaceuticals), centrifuged at 1000 × g for 30 min, plasma separated, aliquoted and stored at −80°C until further analyses of total protein content, cytokines, HSPs and creatine kinase (CK) levels. Plasma protein was measured using the NanoDrop micro-volume methodology (Thermo Scientific) and CK plasma levels were determined in the routine veterinary laboratory of the Loyola University Chicago comparative medicine facility.
Polytrauma and hemorrhage
Polytrauma consisted of bilateral open femur fractures and lung contusion, modified as described previously (32, 33). Injuries were produced with a captive bolt gun (Karl Schermer), modified with exchangeable mushroom shaped metal heads (1 and 2.5 inches in diameter). In brief, the bolt gun with the small metal head was placed vertically against the femur and fired while the animal was in supine position and the leg extended. The metal head perforated the skin and produced a 2nd degree complex open femur fracture without injury of major vessels. After both femurs were fractured, the small metal head was exchanged for the large metal head and the bolt gun was fired against the right chest wall in the midaxillary line at the level of the fourth intercostal space with a 45° cephalad trajectory. As confirmed by necropsy, this resulted in lung contusion involving approximately 20-30% of the right lung without producing hemo/pneumothoraces or rib fractures. All injuries were produced within 5 min. Based on an Abbreviated Injury Score (AIS) of 3 for the extremity trauma and 3 - 4 for the chest trauma, the estimated Injury Severity Score was 18 – 25 (34). In the experimental groups with hemorrhage, blood was rapidly withdrawn from the arterial catheter to maintain a MAP of 25-30 mmHg until t=60 min, as described previously (32, 35).
Experimental groups and treatment protocol
After achieving stable baseline conditions (at least 30 min after instrumentation) animals were randomized to one of the following four experimental groups: 1. Polytrauma only (n = 5): Animals were subjected to bilateral femur fractures plus lung contusion. 2. Hemorrhage only (n = 5): Animals were hemorrhaged to a MAP of 25 - 30 mmHg for 60 min. 3. Polytrauma plus hemorrhage (n = 6): Animals were subjected to bilateral femur fractures plus lung contusion followed by hemorrhage to a MAP of 25 - 30 mmHg for 60 min. 4. Sham control (n = 5): No injury or hemorrhage.
To simulate a 60 min period of shock after trauma, animals were ventilated with FiO2 0.21, PEEP 0 mmHg and no resuscitation was allowed other than the minimum amount of lactated Ringer’s solution required for delivery of anesthesia. At t=60 min, ventilation was adjusted to FIO2 0.4, PEEP 5 mmHg and resuscitation to a MAP of 70 mmHg was performed with warmed lactated Ringer’s solution. Between t=60 - 120 min, lactated Ringer’s solution was administered in i.v. boluses of 500 mL until the MAP reached 70 mmHg to simulate typical human resuscitation regimens during the pre-hospital phase. From 120 min until the end of the experiment, resuscitation was performed continuously to maintain a MAP of at least 70 mmHg to simulate in-hospital resuscitation. At the conclusion of the experiment (t=300 min), a mixture of saturated potassium chloride was infused via the central venous catheter for euthanasia while the animal was under general anesthesia.
Cytokines and HSPs
Plasma levels of cytokines and HSPs were measured utilizing the following commercially available porcine enzyme linked immunosorbent assays (ELISAs): Interleukin (IL)-1β (R&D Systems; lower detection limit (LDL): 10 pg/mL), IL-4 (Invitrogen; LDL: 2 pg/mL); IL-5 (USCN Life Science; LDL: 3.9 pg/mL), IL-6 (R&D Systems; LDL: 9 pg/mL), IL-8 (R&D Systems; LDL: 4.6 pg/mL), IL-10 (Alpco Diagnostics; LDL: 3 pg/mL), IL-12/IL-23 p40 (R&D Systems; LDL: 9 pg/mL), IL-13 (USCN Life Science; LDL: 7.8 pg/mL), IL-17 (USCN Life Science; LDL: 7.8 pg/mL), IL-18 (Invitrogen; LDL: 21.3 pg/mL), Interferon (IFN)γ (R&D Systems; LDL: 6.1 pg/mL), Transforming Growth Factor (TGF)β1 (R&D Systems; LDL: 4.6 pg/mL), Tumor Necrosis Factor (TNF)α (R&D Systems; LDL: 3.7 pg/mL), HSP40 (USCN Life Science; LDL: 78 pg/mL), HSP70 (USCN Life Science; LDL: 78 pg/mL), HSP90 (USCN Life Science; LDL: 78 pg/mL). All assays were performed according to the manufacturers’ protocols using plasma that had not been thawed previously. Measurements were performed after all animal experiments had been completed.
Algorithm for cytokine/HSP measurements, data analysis and statistics
The following algorithm was used to limit the number of cytokine/HSP measurements: All cytokine/HSPs were measured in plasma from animals after polytrauma only (group 1). This was done because hemorrhage without significant tissue damage is unlikely to occur after severe blunt trauma in patients. Only cytokines/HSPs which were consistently detectable (≥ 50% of all specimens after polytrauma with plasma levels above the detection limit of the ELISA) and which showed significant increases of their plasma concentrations from baseline after polytrauma alone were then measured in the specimens obtained from groups 2-4. Data are described as mean ± standard deviation (SD) or median with range (in parenthesis), as appropriate. Normal distribution was assessed with the Komolgorov-Smirnov test. Most of the cytokine/HSP plasma concentrations did not pass the normality test (alpha = 0.05). Therefore, Kruskal-Wallis H test with Dunn’s post hoc correction to control for multiple testing were used to compare differences between the experimental phases (baseline, shock, resuscitation) and groups. The average plasma level during each of the experimental phases was calculated for each animal and used for statistical analyses. Because physiological and clinical parameters were normally distributed, these data were analyzed with Student’s t-test or one-way analysis of variance with Tukey post-hoc test to correct for multiple testing. Chi-square test was used for dichotomous categorical variables. Data analyses were calculated with the GraphPad Prism program (GraphPad Software). A two-tailed p<0.05 was considered significant.
Results
Physiological responses to trauma and hemorrhage
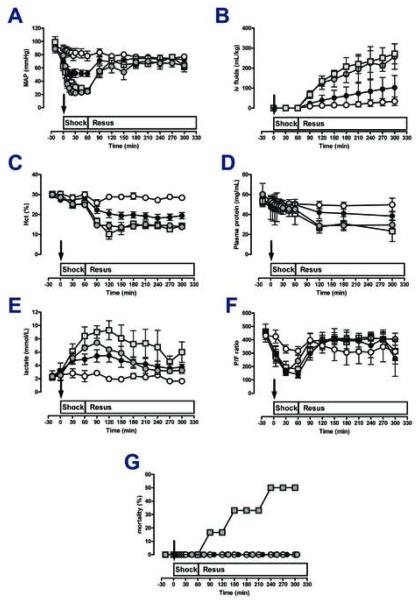
There were no statistically significant differences between physiological variables among the four experimental groups at baseline (p>0.05 for all measurements). CK plasma levels increased from 449 ± 154 U/mL at baseline to 2000 ± 674 U/mL at 300 min after polytrauma alone (p<0.05). The MAP (Fig. 1A) decreased to 50-55 mmHg during the shock period after polytrauma alone. The hemorrhage volume to achieve the MAP target value of 25-30 mmHg during the shock period in the group with hemorrhage only was 37 ± 7 mL/kg and 24 ± 8 mL/kg in the group with polytrauma plus hemorrhage (p<0.05 vs. hemorrhage only). Animals in the control group required 34 ± 20 mL/kg of i.v. fluids to maintain MAP of 70 mmHg during the observation period (Fig. 1B). The fluid requirements to maintain the target MAP increased to 103 ± 60 mL/kg after polytrauma alone (p<0.05 vs. control) and to 257 ± 63 mL and 271 ± 50 mL/kg after hemorrhage alone and hemorrhage plus polytrauma (both groups: p<0.05 vs. sham control and polytrauma alone), respectively. Hematocrit values (Fig. 1C) remained constant in the sham control group (30%), decreased in all other groups and remained constant during the resuscitation period at 18-20% after polytrauma alone and at 13-15% in the groups with hemorrhage. Plasma protein concentrations paralleled changes in hematocrit values (Fig. 1D). Lactate levels increased in all trauma and/or hemorrhage groups (p<0.05 vs. control) and reached peak levels of 5.5 ± 2.2 mmol/L, 6.5 ± 0.5 mmol/L and 9.2 ± 3.2 mmol/L between 60-120 min after polytrauma only, hemorrhage only and polytrauma plus hemorrhage (p>0.05 between groups), respectively (Fig. 1E). In all trauma/hemorrhage groups the ratio of arterial oxygen concentration to the fraction of inspired oxygen (P/F) decreased during the shock period below 200 and recovered during the resuscitation period when ventilated with PEEP of 5 mmHg, FiO2 0.4 (Fig. 1F). Mortality was 50% after polytrauma plus hemorrhage and 0% in all other groups (p<0.05; Fig. 1G).
Figure 1. Physiological responses to trauma and hemorrhage.
Arrows indicate the time point of injury/hemorrhage. Resus: Resuscitation period. Data are mean ± SD. Open circles: sham control, n=5. Black circles: Polytrauma only, n=5. Grey circles: Hemorrhage only, n=5. Grey squares: Polytrauma plus hemorrhage, n=3-6. A. Mean arterial pressure (MAP, mmHg). B. I.v. fluids: Intravenous fluid requirements (mL/kg body weight). C. Hematocrit (%). D. Plasma protein concentrations (mg/mL). E. Plasma lactate concentrations (mmol/L). F. P/F ratio: ratio of arterial oxygen concentration to the fraction of inspired oxygen. G. Mortality (%).
Plasma levels of cytokines and HSPs
The plasma concentrations of the various cytokines and HSPs during experimental baseline conditions, shock and resuscitation are summarized in Tab. 1. IL-1β, IL-18 and IFNγ were not detectable. IL-4, IL-8, IL-17 and HSP40 were detectable in only few plasma specimens and did not show significant changes between the experimental periods. IL-5, IL-12/IL-23 p40, IL-13, TGFβ and HSP90 were detectable in all plasma specimens. However, their plasma concentrations did not show significant changes. In contrast, IL-6, IL-10, TNFα and HSP70 were detectable in the majority of plasma specimens and their concentrations increased after polytrauma.
Table 1.
Plasma levels of cytokines/heat shock proteins after polytrauma.
| Marker (pg/mL) |
Baseline | Shock (t=1-60 min) |
Resuscitation (t=61-300 min) |
|---|---|---|---|
|
| |||
| IL-1β | |||
| Mean ± SD | 0 | 0 | 0 |
| Median (Min – Max) | 0 (0) | 0 (0) | 0 (0) |
|
| |||
| IL-4 | |||
| Mean ± SD | 0.1 ± 0.13 | 0.08 ± 0.05 | 0.05 ± 0.09 |
| Median (Min – Max) | 0.05 (0 – 0.31) | 0.11 (0.02 – 0.13) | 0 (0 – 0.24) |
|
| |||
| IL-5 | |||
| Mean ± SD | 39.3 ± 12.5 | 49.2 ± 9.7 | 41.6 ± 13.3 |
| Median (Min – Max) | 37.8 (24 – 58) | 48.7 (35 – 60) | 50.1 (23 – 52) |
|
| |||
| IL-6 | |||
| Mean ± SD | 134 ± 7 | 141 ± 8 | 188 ± 15a |
| Median (Min – Max) | 131 (128 – 145) | 142 (131 – 152) | 184 (170 – 206) |
|
| |||
| IL-8 | |||
| Mean ± SD | 0 | 12 ± 29 | 2.2 ± 4.2 |
| Median (Min – Max) | 0 (0) | 0 (0 – 71) | 0 (0 – 10.5) |
|
| |||
| IL-10 | |||
| Mean ± SD | 15.3 ± 14.2 | 80.9 ± 63.6 | 90.7 ± 56.4a |
| Median (Min – Max) | 8.9 (0 – 31) | 76.3 (15 – 174) | 92.9 (39 – 175) |
|
| |||
| IL-12/IL-23 p40 | |||
| Mean ± SD | 739 ± 268 | 791 ± 257 | 900 ± 294 |
| Median (Min – Max) | 654 (582 – 1213) | 681 (628 – 1245) | 857 (621 – 1389) |
|
| |||
| IL-13 | |||
| Mean ± SD | 6.5 ± 3.4 | 9.6 ± 3.1 | 7.2 ± 2.3 |
| Median (Min – Max) | 6.2 (2.5 – 11.1) | 10 (5.4 – 13.6) | 66.5 (5.7 – 11.2) |
|
| |||
| IL-17 | |||
| Mean ± SD | 0 | 0.5 ± 1.1 | 0.4 ± 0.9 |
| Median (Min – Max) | 0 (0) | 0 (0 – 2.5) | 0 (0 – 1.9) |
|
| |||
| IL-18 | |||
| Mean ± SD | 0 | 0 | 0 |
| Median (Min – Max) | 0 (0) | 0 (0) | 0 (0) |
|
| |||
| IFNγ | |||
| Mean ± SD | 0 | 0 | 0 |
| Median (Min – Max) | 0 (0) | 0 (0) | 0 (0) |
|
| |||
| TNFα | |||
| Mean ± SD | 133 ± 69 | 261 ± 186 | 249 ± 60a |
| Median (Min – Max) | 122 (44 – 265) | 199 (54 – 574) | 249 (153 – 327) |
|
| |||
| TGFβ | |||
| Mean ± SD | 926 ± 407 | 1006 ± 508 | 1084 ± 861 |
| Median (Min – Max) | 1019 (173 – 1289) | 1033 (395 – 1731) | 866 (422 – 2770) |
|
| |||
| HSP40 | |||
| Mean ± SD | 0.41 ± 0.5 | 0.04 ± 0.06 | 0 |
| Median (Min – Max) | 0.25 (0 – 1.2) | 0 (0 – 0.16) | 0 (0) |
|
| |||
| HSP70 | |||
| Mean ± SD | 144 ± 48 | 155 ± 29 | 193 ± 43b |
| Median (Min – Max) | 169 (79 – 189) | 168 (122 – 184) | 216 (145 – 234) |
|
| |||
| HSP90 | |||
| Mean ± SD | 3.4 ± 1.0 | 3.4 ± 0.6 | 3.8 ± 0.9 |
| Median (Min – Max) | 3.4 (1.9 – 4.8) | 3.5 (2.6 – 4.2) | 3.3 (3.3 – 5.4) |
Average plasma concentrations at baseline, during shock and resuscitation (n = 5 per time period).
p<0.05 vs. baseline;
p<0.05 vs. shock. The average plasma concentration for each animal was calculated as the mean of two, eight and three plasma levels that were determined at baseline, during shock and resuscitation, respectively.
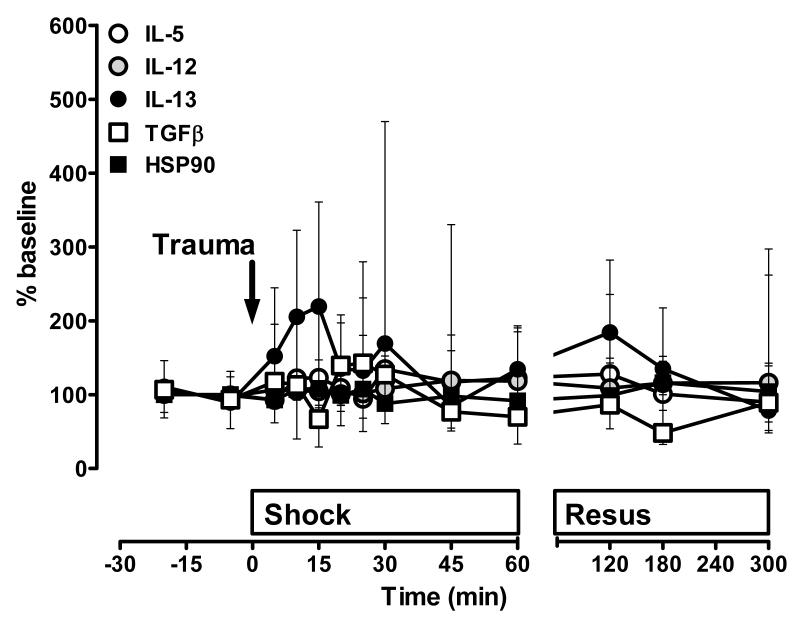
The time course of the plasma levels of the individual cytokines and HSPs that were consistently detectable after polytrauma are shown in Fig. 2-6. Due to the large differences among the individual molecules (Tab. 1), plasma levels are shown as percent of baseline to permit direct comparison of relative changes after polytrauma. In agreement with their average concentrations during the experimental periods (Tab. 1), plasma levels of IL-5, IL-12, IL-13, TGFβ and HSP90 were unchanged throughout the entire observation period (Fig. 2).
Figure 2. Plasma concentrations of cytokines/heat shock proteins without significant changes after polytrauma alone.
Data are expressed as % of the individual baseline concentrations and plotted as median with interquartile range. n=5 per time point. Open circles: IL-5. Grey circles: IL-12. Black circles: IL-13. Open squares: TGFβ. Black squares: HSP90. The arrow indicates the time point of injury. Resus: Resuscitation period.
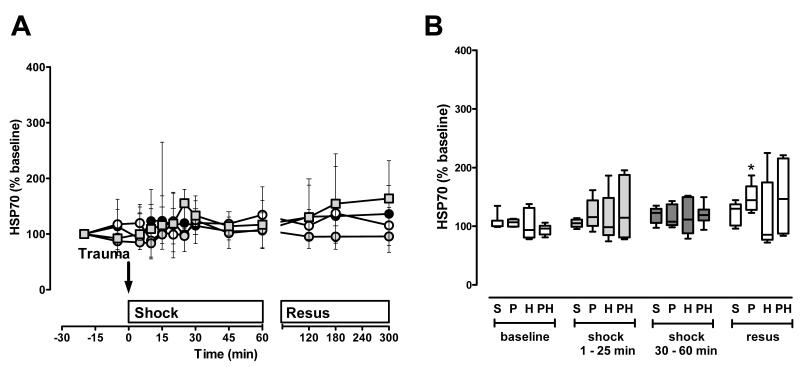
Figure 6. HSP70 plasma concentrations after polytrauma and hemorrhage.
A. Time course. Data are expressed as % of the individual baseline concentrations and plotted as median with interquartile range. Open circles: Sham control, n=5. Black circles: Polytrauma only, n=5. Grey circles: Hemorrhage only, n=5. Grey squares: Polytrauma plus hemorrhage, n=3-6. The arrow indicates the time point of injury/hemorrhage. Resus: Resuscitation period. B. Average HSP70 plasma levels during the individual experimental phases (baseline, early shock phase (t=1-25 min), late shock phase (30-60 min), resuscitation phase (resus)). S: Sham control. Average plasma levels for each animal were calculated as the mean of all plasma levels which were determined during each time period; data from A. P: Polytrauma only. H: Hemorrhage only. PH: Polytrauma plus hemorrhage. Boxes extend from the 25th to 75th percentile; the horizontal line shows the median. Error bars show the 10th and 90th percentile. Groups not sharing the same letter are significantly different (p<0.05) during each individual experimental phase. *: p<0.05 vs. baseline. $: p<0.05 vs. early shock period (1-25 min). #: p<0.05 vs. late shock period (30-60 min).
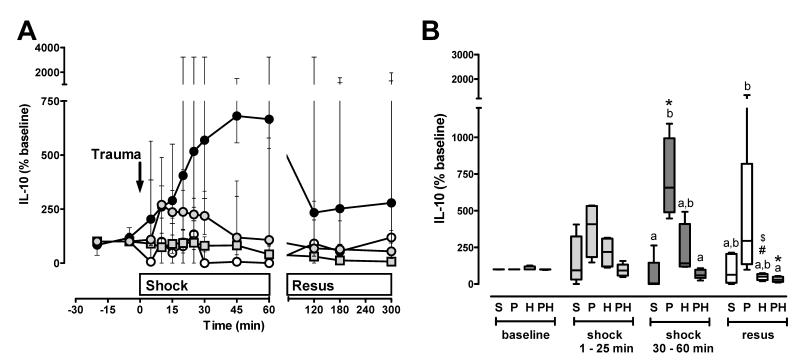
IL-10 (Fig. 3A/B) plasma concentrations increased significantly during the shock period after polytrauma and remained unchanged in sham control animals. When compared with polytrauma alone, the increase in IL-10 plasma levels during the shock phase was attenuated in magnitude and duration after hemorrhage. The combined insult of polytrauma plus hemorrhage did not result in increased IL-10 plasma levels during the shock phase (Fig. 3A/B).
Figure 3. IL-10 plasma concentrations after polytrauma and hemorrhage.
A. Time course. Data are expressed as % of the individual baseline concentrations and plotted as median with interquartile range. Open circles: Sham control, n=5. Black circles: Polytrauma only, n=5. Grey circles: Hemorrhage only, n=5. Grey squares: Polytrauma plus hemorrhage, n=3-6. The arrow indicates the time point of injury/hemorrhage. Resus: Resuscitation period. B. Average IL-10 plasma levels during the individual experimental phases (baseline, early shock phase (t=1-25 min), late shock phase (30-60 min), resuscitation phase (resus)). S: Sham control. Average plasma levels for each animal were calculated as the mean of all plasma levels which were determined during each time period; data from A. P: Polytrauma only. H: Hemorrhage only. PH: Polytrauma plus hemorrhage. Boxes extend from the 25th to 75th percentile; the horizontal line shows the median. Error bars show the 10th and 90th percentile. Groups not sharing the same letter are significantly different (p<0.05) during each individual experimental phase. *: p<0.05 vs. baseline. $: p<0.05 vs. early shock period (1-25 min). #: p<0.05 vs. late shock period (30-60 min).
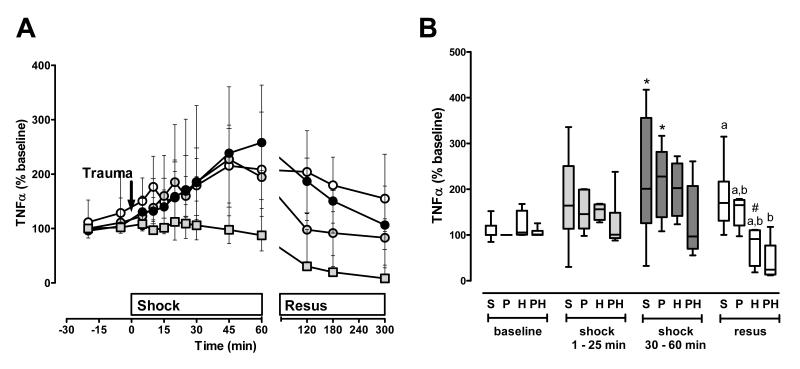
In contrast to IL-10, TNFα plasma levels increased during the shock period after polytrauma alone, hemorrhage alone and also after sham procedure (Fig. 4A/B). Similar to IL-10, TNFα plasma levels did not increase during the shock period after polytrauma with superimposed hemorrhage. As resuscitation was initiated, IL-10 and TNFα plasma concentrations decreased in the trauma/hemorrhage groups (Fig. 3B, Fig. 4B). These effects were also detectable when IL-10 and TNFα plasma levels were normalized to plasma protein content (not shown).
Figure 4. TNFα plasma concentrations after polytrauma and hemorrhage.
A. Time course. Data are expressed as % of the individual baseline concentrations and plotted as median with interquartile range. Open circles: Sham control, n=5. Black circles: Polytrauma only, n=5. Grey circles: Hemorrhage only, n=5. Grey squares: Polytrauma plus hemorrhage, n=3-6. The arrow indicates the time point of injury/hemorrhage. Resus: Resuscitation period. B. Average TNFα plasma levels during the individual experimental phases (baseline, early shock phase (t=1-25 min), late shock phase (30-60 min), resuscitation phase (resus)). S: Sham control. Average plasma levels for each animal were calculated as the mean of all plasma levels which were determined during each time period; data from A. P: Polytrauma only. H: Hemorrhage only. PH: Polytrauma plus hemorrhage. Boxes extend from the 25th to 75th percentile; the horizontal line shows the median. Error bars show the 10th and 90th percentile. Groups not sharing the same letter are significantly different (p<0.05) during each individual experimental phase. *: p<0.05 vs. baseline. $: p<0.05 vs. early shock period (1-25 min). #: p<0.05 vs. late shock period (30-60 min).
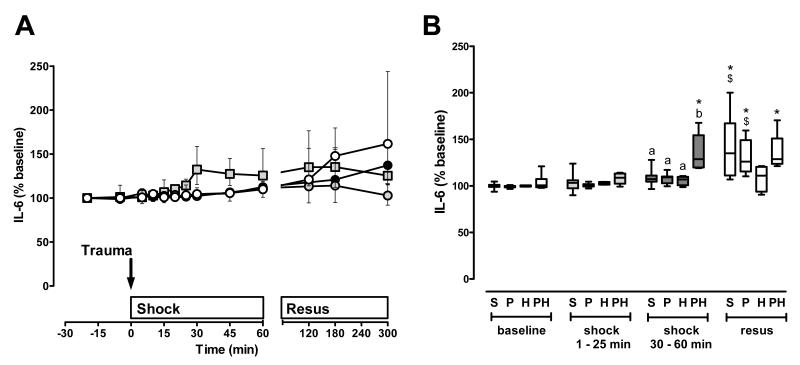
After polytrauma alone, IL-6 plasma levels were unchanged during the shock phase and increased during resuscitation (Fig. 5A/B). However, this effect was also detectable in sham control animals. Although hemorrhage alone was not associated with increases in IL-6 plasma levels, IL-6 levels increased during the shock period and remained constant during resuscitation after polytrauma plus hemorrhage.
Figure 5. IL-6 plasma concentrations after polytrauma and hemorrhage.
A. Time course. Data are expressed as % of the individual baseline concentrations and plotted as median with interquartile range. Open circles: Sham control, n=5. Black circles: Polytrauma only, n=5. Grey circles: Hemorrhage only, n=5. Grey squares: Polytrauma plus hemorrhage, n=3-6. The arrow indicates the time point of injury/hemorrhage. Resus: Resuscitation period. B. Average IL-6 plasma levels during the individual experimental phases (baseline, early shock phase (t=1-25 min), late shock phase (30-60 min), resuscitation phase (resus)). S: Sham control. Average plasma levels for each animal were calculated as the mean of all plasma levels which were determined during each time period; data from A. P: Polytrauma only. H: Hemorrhage only. PH: Polytrauma plus hemorrhage. Boxes extend from the 25th to 75th percentile; the horizontal line shows the median. Error bars show the 10th and 90th percentile. Groups not sharing the same letter are significantly different (p<0.05) during each individual experimental phase. *: p<0.05 vs. baseline. $: p<0.05 vs. early shock period (1-25 min). #: p<0.05 vs. late shock period (30-60 min).
HSP70 plasma levels did not show significant changes during the shock period (Fig. 6A/B). During resuscitation, HSP70 levels increased after polytrauma alone, but did not show significant changes in any other group.
Discussion
In the present study, we provide the first detailed characterization of the immediate systemic release kinetics of a broad panel of inflammation markers in porcine models of polytrauma and hemorrhage. The injuries in our model created a pathophysiological condition that closely resembled the typical clinical characteristics of severely injured blunt trauma patients. The lactate levels after the shock period underscored the injury severity (36-39) and the increase in CK plasma concentrations after polytrauma documented significant soft tissue damage. Hemorrhagic shock alone produced physiological responses comparable with polytrauma alone. As expected, the combination of polytrauma plus hemorrhage resulted in further increases in fluid dependency and lactate levels, and produced mortality during the early resuscitation period. This demonstrates that the overall severity of the insult was highest after polytrauma plus hemorrhage.
Although we expected that plasma levels of several pro- and anti-inflammatory cytokines would increase after polytrauma alone, and that the combined insult of polytrauma plus hemorrhage would further exaggerate their systemic concentrations, only TNFα and IL-10 levels increased during the shock period after polytrauma alone, and the combined insult abolished their release into the systemic circulation. Despite the similarity of the physiological responses to polytrauma alone and hemorrhage alone, comparison of the systemic release kinetics of TNFα and IL-10 documented distinct plasma profiles. This implies differential regulation of the systemic cytokine release after major tissue injury and after hemorrhage, and advises caution in the interpretation of findings from trauma models that rely on a hemorrhage component to evoke physiological changes.
Only IL-6 plasma levels showed a small (25-30%) but statistically significant increase during the shock period after the combination of polytrauma plus hemorrhage, when compared with hemorrhage or polytrauma alone. Our measurements in short intervals during the shock phase excluded transient peak levels of the other molecules during a simulated pre-hospital phase. These findings make it unlikely that transient peak levels of these molecules during the pre-hospital phase have been overlooked in previous clinical observational studies, and also that measurements of cytokines or HSPs at intervals closer to the traumatic insult may not provide a diagnostic or prognostic advantage.
Although the time dependent and monophasic increase in IL-10 plasma levels during the shock period could be attributed to the inflammatory response induced by blunt trauma, measurements of TNFα after polytrauma alone, hemorrhage alone and sham procedure revealed that the plasma concentrations during the shock phase were identical in regards to magnitude and time progression. This suggests that minor interventions, such as adjustment of PEEP and FiO2, modulate the systemic TNFα release to the same degree as severe tissue injury or blood loss and demonstrates that TNFα levels are unable to discriminate the degree of tissue injury. The TNFα and IL-10 plasma levels measured after polytrauma plus hemorrhage also show that their systemic release kinetics do not reflect the magnitude of the physiological response or the severity of the overall insult. Our results indicate that the combined effects of polytrauma and hemorrhage on systemic IL-10 and TNFα plasma concentrations are neither synergistic nor additive. On the contrary, polytrauma plus hemorrhage resulted in significant mortality but did not induce the systemic release of these cytokines. These findings are in agreement with previous observations, which indicated that mortality from trauma in patients and from thoracotomy plus hemorrhage in pigs is associated with a blunted systemic TNFα release (40). Thus, our findings support the concept that major trauma in patients or the combination of polytrauma plus hemorrhage in the experimental setting is associated with the inability to mount an appropriate cytokine response in the systemic circulation (3, 40). As an additional hemorrhage component appears to attenuate systemic release of TNFα and IL-10 after polytrauma, these observations may further reflect that the combined insult leads to the induction of a state of immune paralysis (41).
The unchanged plasma levels of IL-6 during the shock period after polytrauma and hemorrhage alone and the slightly increased levels after a combined insult are consistent with plasma concentrations of IL-6 in trauma patients when blood was collected on the scene of the accident (42, 43). Although IL-6 was the only molecule that showed increased plasma levels with higher injury severity during the shock period, this increase was small and variable.
The changes in plasma levels of cytokines that we detected during the resuscitation period after polytrauma alone are consistent with changes that have been observed in severely injured trauma patients on hospital admission (4, 43-50), which confirms the clinical relevance of our model. However, standard fluid resuscitation resulted in distinct and differential effects on plasma cytokine/HSP concentrations in each experimental scenario. Our findings that TNFα and IL-10 levels also decreased when plasma concentrations were normalized to total plasma protein content, and that all other detectable cytokines and HSPs showed increased or unchanged plasma levels during resuscitation, document that this decrease cannot be attributed to hemodilution. Resuscitation fluids have been shown to influence immune responses after trauma (51-53). Thus, it appears more likely that the multiple changes of TNFα, IL-10, IL-6 and HSP70 levels that were observed during resuscitation in sham control and injured animals are related to the immune modulatory effects of the resuscitation fluid, which further hampers interpretation of findings on systemic cytokine levels from observational studies in trauma models or in patients.
An obvious limitation of the present study is that we did not measure all cytokines/HSPs in all experimental groups. Although such data may provide valuable information from an experimental standpoint, the resources required to perform these analyses would be extensive. As a controlled hemorrhage only model is of limited clinical relevance and the expected gain in clinically relevant information would be minimal, we refrained from these measurements.
The present study was designed as an observational study to assess the association of numerous inflammation markers with physiological variables and injury severity. Therefore, our data do not permit conclusions on the molecular mechanisms regulating the systemic release of cytokines or on their possible biological roles in the systemic circulation or at local sites after trauma. Nevertheless, it might be speculated that the differentially regulated systemic release kinetics of TNFα, IL-10 and IL-6 in our models are due to distinct patterns of alarmins (damage associated molecular pattern molecules, DAMPs) that are released in response to the specific modes of injury (54, 55). Irrespective of the underlying mechanisms, our findings make it unlikely that these molecules could be useful as a prognostic or diagnostic tool in the clinical setting. The differential effects of polytrauma alone, hemorrhage alone and polytrauma plus hemorrhage, in combination with the influence of fluid resuscitation, are able to explain the large variability and inconsistent association of systemic cytokine levels with clinical variables and outcomes in severely injured patients (13, 14, 45, 56). Because an injury severity that is associated with significant mortality appears to suppress the systemic inflammatory response, at least for TNFα and IL-10, proteins or other molecules that are actively secreted from immunological competent cells in response to trauma are unlikely to be able to serve as useful trauma biomarkers early after injury.
Acknowledgement
We thank Farshid Azarafrooz and Richard Duff for technical support during the animal experiments and Jim Keaton for modifying the captive bolt gun.
This work was supported by NIH T32 GM008750 and the U.S. Department of the Army under Award Number W81XWH-05-1-0585. The U.S. Army Medical Research Acquisition Activity (820 Chandler Street, Fort Detrick MD 21702-5014) is the awarding and administering acquisition office. Information contained in this article does not necessarily reflect the position or the policy of the government, and no official endorsement is inferred.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keel M, Schregenberger N, Steckholzer U, et al. Endotoxin tolerance after severe injury and its regulatory mechanisms. J Trauma. 1996;41(3):430–437. doi: 10.1097/00005373-199609000-00008. discussion 437-438. [DOI] [PubMed] [Google Scholar]
- 2.Majetschak M, Flach R, Heukamp T, et al. Regulation of whole blood tumor necrosis factor production upon endotoxin stimulation after severe blunt trauma. J Trauma. 1997;43(6):880–887. doi: 10.1097/00005373-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Majetschak M, Flach R, Kreuzfelder E, et al. The extent of traumatic damage determines a graded depression of the endotoxin responsiveness of peripheral blood mononuclear cells from patients with blunt injuries. Crit Care Med. 1999;27(2):313–318. doi: 10.1097/00003246-199902000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Majetschak M, Borgermann J, Waydhas C, et al. Whole blood tumor necrosis factor-alpha production and its relation to systemic concentrations of interleukin 4, interleukin 10, and transforming growth factor-beta1 in multiply injured blunt trauma victims. Crit Care Med. 2000;28(6):1847–1853. doi: 10.1097/00003246-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Ditschkowski M, Kreuzfelder E, Majetschak M, et al. Reduced B cell HLA-DR expression and natural killer cell counts in patients prone to sepsis after injury. Eur J Surg. 1999;165(12):1129–1133. doi: 10.1080/110241599750007630. [DOI] [PubMed] [Google Scholar]
- 6.Ditschkowski M, Kreuzfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229(2):246–254. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ertel W, Keel M, Marty D, et al. Significance of systemic inflammation in 1,278 trauma patients. Unfallchirurg. 1998;101(7):520–526. doi: 10.1007/s001130050304. [DOI] [PubMed] [Google Scholar]
- 8.Flohe SB, Flohe S, Schade FU. Invited review: deterioration of the immune system after trauma: signals and cellular mechanisms. Innate Immun. 2008;14(6):333–344. doi: 10.1177/1753425908100016. [DOI] [PubMed] [Google Scholar]
- 9.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12(4):218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218(6):769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier B, Lefering R, Lehnert M, et al. Early versus late onset of multiple organ failure is associated with differing patterns of plasma cytokine biomarker expression and outcome after severe trauma. Shock. 2007;28(6):668–674. [PubMed] [Google Scholar]
- 12.Martin C, Boisson C, Haccoun M, et al. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25(11):1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Svoboda P, Kantorova I, Ochmann J. Dynamics of interleukin 1, 2, and 6 and tumor necrosis factor alpha in multiple trauma patients. J Trauma. 1994;36(3):336–340. doi: 10.1097/00005373-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Hoch RC, Rodriguez R, Manning T, et al. Effects of accidental trauma on cytokine and endotoxin production. Crit Care Med. 1993;21(6):839–845. doi: 10.1097/00003246-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Adib-Conquy M, Cavaillon JM. Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Lett. 2007;581(19):3723–3733. doi: 10.1016/j.febslet.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 16.Pespeni M, Mackersie RC, Lee H, et al. Serum levels of Hsp60 correlate with the development of acute lung injury after trauma. J Surg Res. 2005;126(1):41–47. doi: 10.1016/j.jss.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Pittet JF, Lee H, Morabito D, et al. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52(4):611–617. doi: 10.1097/00005373-200204000-00001. discussion 617. [DOI] [PubMed] [Google Scholar]
- 18.Jastrow KM, 3rd, Gonzalez EA, McGuire MF, et al. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209(3):320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Majetschak M, Obertacke U, Waydhas C, et al. Mediator modulation in infection and multiple organ failure. Unfallchirurg. 2000;103(10):903–907. doi: 10.1007/s001130050639. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler DS, Fisher LE, Jr., Catravas JD, et al. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6(3):308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler TR, Ogden LG, Singleton KD, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive care medicine. 2005;31(8):1079–1086. doi: 10.1007/s00134-005-2690-5. [DOI] [PubMed] [Google Scholar]
- 22.Majetschak M, Flohe S, Obertacke U, et al. Relation of a TNF gene polymorphism to severe sepsis in trauma patients. Ann Surg. 1999;230(2):207–214. doi: 10.1097/00000658-199908000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majetschak M, Krehmeier U, Ostroverkh L, et al. Alterations in leukocyte function following surgical trauma: differentiation of distinct reaction types and association with tumor necrosis factor gene polymorphisms. Clin Diagn Lab Immunol. 2005;12(2):296–303. doi: 10.1128/CDLI.12.2.296-303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamada H, Tsutsumi Y, Yamamoto Y, et al. Antitumor activity of tumor necrosis factor-alpha conjugated with polyvinylpyrrolidone on solid tumors in mice. Cancer Res. 2000;60(22):6416–6420. [PubMed] [Google Scholar]
- 25.Castell JV, Geiger T, Gross V, et al. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988;177(2):357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- 26.Kampschmidt RF, Upchurch HF. Rate of clearance of circulating leukocytic endogenous mediator in the rat. Proc Soc Exp Biol Med. 1980;164(4):537–539. doi: 10.3181/00379727-164-40912. [DOI] [PubMed] [Google Scholar]
- 27.Flohe S, Lendemans S, Schade FU, et al. Influence of surgical intervention in the immune response of severely injured patients. Intensive Care Med. 2004;30(1):96–102. doi: 10.1007/s00134-003-2041-3. [DOI] [PubMed] [Google Scholar]
- 28.Gryglewski A, Marcinkiewicz J. Influence of anatomic localization and extent of surgical trauma on immune responses in mice. Arch Immunol Ther Exp (Warsz) 1985;33(3):489–492. [PubMed] [Google Scholar]
- 29.Schmitz D, Bangen JM, Herborn CU, et al. Isolated closed minor-muscle injury of the lower leg did not cause an obvious systemic immune response. Inflamm Res. 2010;59(2):141–149. doi: 10.1007/s00011-009-0081-z. [DOI] [PubMed] [Google Scholar]
- 30.Proctor KG. Gender differences in trauma theory vs. practice: Comments on “Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent upregulation of HO-1” by Hsu JT et al. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1822–1824. doi: 10.1152/ajpregu.90301.2008. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki T, Choudhry MA, Schwacha MG, et al. Effect of interleukin-15 on depressed splenic dendritic cell functions following trauma-hemorrhage. Am J Physiol Cell Physiol. 2009;296(1):C124–130. doi: 10.1152/ajpcell.00447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majetschak M, Cohn SM, Obertacke U, et al. Therapeutic potential of exogenous ubiquitin during resuscitation from severe trauma. J Trauma. 2004;56(5):991–999. doi: 10.1097/01.ta.0000127770.29009.5a. discussion 999-1000. [DOI] [PubMed] [Google Scholar]
- 33.Dudkiewicz M, Harpaul TA, Proctor KG. Hemoglobin-based oxygen carrying compound-201 as salvage therapy for severe neuro- and polytrauma (Injury Severity Score = 27-41) Crit Care Med. 2008;36(10):2838–2848. doi: 10.1097/CCM.0b013e318186f6b3. [DOI] [PubMed] [Google Scholar]
- 34.Greenspan L, McLellan BA, Greig H. Abbreviated Injury Scale and Injury Severity Score: a scoring chart. J Trauma. 1985;25(1):60–64. doi: 10.1097/00005373-198501000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Earle SA, Proctor KG, Patel MB, et al. Ubiquitin reduces fluid shifts after traumatic brain injury. Surgery. 2005;138(3):431–438. doi: 10.1016/j.surg.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Moomey CB, Jr., Melton SM, Croce MA, et al. Prognostic value of blood lactate, base deficit, and oxygen-derived variables in an LD50 model of penetrating trauma. Crit Care Med. 1999;27(1):154–161. doi: 10.1097/00003246-199901000-00044. [DOI] [PubMed] [Google Scholar]
- 37.Cerovic O, Golubovic V, Spec-Marn A, et al. Relationship between injury severity and lactate levels in severely injured patients. Intensive Care Med. 2003;29(8):1300–1305. doi: 10.1007/s00134-003-1753-8. [DOI] [PubMed] [Google Scholar]
- 38.Abramson D, Scalea TM, Hitchcock R, et al. Lactate clearance and survival following injury. J Trauma. 1993;35(4):584–588. doi: 10.1097/00005373-199310000-00014. discussion 588-589. [DOI] [PubMed] [Google Scholar]
- 39.Manikis P, Jankowski S, Zhang H, et al. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med. 1995;13(6):619–622. doi: 10.1016/0735-6757(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 40.Namas R, Ghuma A, Torres A, et al. An adequately robust early TNF-alpha response is a hallmark of survival following trauma/hemorrhage. PLoS One. 2009;4(12):e8406. doi: 10.1371/journal.pone.0008406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 42.Gebhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135(3):291–295. doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 43.Arand M, Melzner H, Kinzl L, et al. Early inflammatory mediator response following isolated traumatic brain injury and other major trauma in humans. Langenbecks Arch Surg. 2001;386(4):241–248. doi: 10.1007/s004230100204. [DOI] [PubMed] [Google Scholar]
- 44.Yadav K, Zehtabchi S, Nemes PC, et al. Early immunologic responses to trauma in the emergency department patients with major injuries. Resuscitation. 2009;80(1):83–88. doi: 10.1016/j.resuscitation.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Cinat M, Waxman K, Vaziri ND, et al. Soluble cytokine receptors and receptor antagonists are sequentially released after trauma. J Trauma. 1995;39(1):112–118. doi: 10.1097/00005373-199507000-00015. discussion 118-120. [DOI] [PubMed] [Google Scholar]
- 46.Liener UC, Bruckner UB, Knoferl MW, et al. Chemokine activation within 24 hours after blunt accident trauma. Shock. 2002;17(3):169–172. doi: 10.1097/00024382-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Hensler T, Sauerland S, Riess P, et al. The effect of additional brain injury on systemic interleukin (IL)-10 and IL-13 levels in trauma patients. Inflamm Res. 2000;49(10):524–528. doi: 10.1007/s000110050626. [DOI] [PubMed] [Google Scholar]
- 48.Liu JQ, He ZJ, Lin HY, et al. Dynamic change in interleukin-18 and its relationships with multiple organ dysfunction syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16(2):70–72. [PubMed] [Google Scholar]
- 49.Roetman B, Schinkel C, Wick M, et al. Elevated systemic interleukin-18 in multiple injured patients is not related to clinical outcome. J Interferon Cytokine Res. 2008;28(12):741–747. doi: 10.1089/jir.2008.0029. [DOI] [PubMed] [Google Scholar]
- 50.Frangen TM, Bogdanski D, Schinkel C, et al. Systemic IL-17 after severe injuries. Shock. 2008;29(4):462–467. doi: 10.1097/shk.0b013e3181598a9d. [DOI] [PubMed] [Google Scholar]
- 51.Watters JM, Tieu BH, Todd SR, et al. Fluid resuscitation increases inflammatory gene transcription after traumatic injury. J Trauma. 2006;61(2):300–308. doi: 10.1097/01.ta.0000224211.36154.44. discussion 308-309. [DOI] [PubMed] [Google Scholar]
- 52.Rizoli SB, Rhind SG, Shek PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006;243(1):47–57. doi: 10.1097/01.sla.0000193608.93127.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bulger EM, Cuschieri J, Warner K, et al. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann Surg. 2007;245(4):635–641. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De AK, Kodys KM, Yeh BS, et al. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165(7):3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- 55.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 56.Spielmann S, Kerner T, Ahlers O, et al. Early detection of increased tumour necrosis factor alpha (TNFalpha) and soluble TNF receptor protein plasma levels after trauma reveals associations with the clinical course. Acta Anaesthesiol Scand. 2001;45(3):364–370. doi: 10.1034/j.1399-6576.2001.045003364.x. [DOI] [PubMed] [Google Scholar]