Abstract
Using two waves of the National Survey of Midlife Development in the United States, I compare changes in personal growth over a 10-year period among cancer survivors and individuals without cancer. Moreover, I examine joint effects of age and cohort on personal growth after a cancer diagnosis. The theoretical framework of this study integrates impairment, resilience, and thriving perspectives. Findings reveal that, although personal growth declines with age for all individuals regardless of cohort and cancer status, cancer slows the decline in personal growth with age in 1940s, 1950s, and 1960s birth cohorts, yet accelerates the age-related decline in the 1920s cohort. I argue that a sociological perspective can enhance our understanding of the interplay of developmental and socio-cultural influences on psychological adjustment to cancer. Seemingly idiosyncratic psychological reactions to cancer partly reflect macro-level processes represented by cohort differences.
Sixty-seven percent of people diagnosed with cancer in 2000 have survived for at least five years (National Cancer Institute 2008). Because of the increased longevity of cancer survivors and the long-term nature of cancer, the question about the quality of life with cancer has gained prominence in recent years (Stanton, Revenson, and Tennen 2007). Understanding the effects of cancer on psychological well-being is important because successful psychological adjustment is an integral aspect of leading fulfilling and meaningful lives for many years after the diagnosis. Researchers have become increasingly aware that, in addition to psychological distress, cancer can promote positive psychological changes (Stanton et al. 2007). Among positive psychological consequences of cancer, personal growth has been shown particularly important (Cordova and Andrykowski 2003; Cordova et al. 2001; Costanzo, Ryff, and Singer 2009; Widows et al. 2005). Yet, existing research on personal growth among cancer survivors has been psychologically-oriented and, thus, focused on within-individual developmental processes. Personal growth has been viewed as an inherent characteristic of a person, and heterogeneity in psychological adjustment to cancer was assumed to reflect personality differences (Carver 2005), whereas the role of macro-social and cultural factors has not been considered.
In this study, I integrate psychological and sociological approaches to examine personal growth after a cancer diagnosis within a framework that links individual experiences of cancer to the “transformations of the social world” (Ryder 1965: 861). The socio-historical and cultural context is incorporated in the analysis by examining differential experiences of birth cohorts. Age effects represent intra-individual development, whereas cohort effects reflect cultural and social processes through which individuals sharing a birth year move together at a particular life-course stage. I argue that the effect of cancer on personal growth cannot be fully understood without a joint consideration of individual-level developmental processes as well as the macrosocial context of individuals’ lives.
This study compares changes in personal growth with age among cancer survivors and individuals without cancer using the 1994–1995 and 2004–2006 waves of the National Survey of Midlife Development in the United States (MIDUS), a large community-based nationally representative study of men and women aged 25–74 years old at baseline. Moreover, I analyze the ways in which age-related changes in personal growth differ among five birth cohorts. Examining the interplay of age and cohort effects not only adds complexity to the previous psychological models, but also can refine our understanding of the trajectories of personal growth among cancer survivors and their peers without cancer.
Possible Trajectories of Personal Growth: Impairment, Resilience, and Thriving
There are many related faces of personal growth in psychological research, including “posttraumatic growth,” “stress-related growth,” and “benefit finding” (Helgeson, Reynolds, and Tomich 2006). In this study, personal growth is viewed as a sense of developing one’s potential by growing and expanding as a person even in the face of adversity (Ryff 1989). An individual with high levels of personal growth is “continually developing and becoming, rather than achieving a fixed state wherein all problems are solved” (Ryff 1989: 1071). Such an individual is open to new experiences and strives for self-improvement. Conversely, low levels of personal growth are associated with a sense of personal stagnation, lack of improvement over time, feelings of boredom, and a reluctance to develop new attitudes (Ryff 1989). This view of personal growth is particularly relevant to cancer because cancer is a life-shattering experience that threatens the very existence but also involves new challenges and opportunities for growing and developing as a person.
Based on Erikson’s (1950) crisis theory, Turner and Avison (1992) developed a framework representing stressful life events as both hazards and opportunities. Consistent with this dualistic view, cancer is a complex multi-dimensional experience that can have different meanings and consequences depending on individuals’ appraisals of cancer as a threat or a challenge (Lazarus and Folkman 1984). From a life course perspective, cancer can be viewed as a turning point that affects the direction of trajectories of personal growth (Taylor 1983). Psychological research has identified three potential trajectories of adjustment to adversity (Carver 1998; Costanzo et al. 2009). Impairment refers to diminished or impaired functioning after adversity (Carver 1998). Resilience denotes the ability to maintain stable functioning in the face of loss or traumatic events (Bonanno 2008). Thriving reflects not merely maintaining a previous level of functioning but surpassing it in some respect (Carver 1998). Thus, personal growth after a cancer diagnosis can follow impairment, resilience, or thriving trajectories.
An impairment trajectory would be observed if cancer, as a hazard or threat, diminishes or stalls personal growth. A zest for self-improvement and new life experiences may be difficult to maintain in the face of profound acute and chronic stressors associated with cancer. Cancer poses a threat to survival and elevates existential uncertainty (Taylor 1983). Medical treatment is complicated and often causes uncomfortable side effects. Moreover, the stress of cancer can potentially undermine the self-image and raise concerns about mental and physical abilities and appearance (Peleg-Oren, Sherer, and Soskolne 2003). Cancer can elevate financial burden, disrupt employment, and create strains in family and social relationships (Roberts et al. 1997). Such a constellation of chronic and acute stressors as well as stress proliferation may be particularly detrimental to psychological well-being (Pearlin 1999).
Resilience will produce a stable trajectory of personal growth that is unaffected by cancer (Bonanno 2008). Psychological research underscores that resilience is distinct from benefit-finding or enhanced growth (Bonanno, Wortman, and Nesse 2004; Davis, Nolen-Hoeksema, and Larson 1998). Resilient individuals may be less likely to engage in the meaning-making behaviors associated with posttraumatic growth because they do not struggle to the same extent as more traumatized individuals (Westphal and Bonanno 2007). Sociological researchers view resilience in terms of problem-solving. Turner and Avison (1992) suggest that resilience to a stressful event may depend on “its resolution in emotional and practical terms” because crises that have been resolved successfully do not contribute to psychological distress (p. 36). Similarly, Thoits (1994) showed that individuals who solved their problems successfully were similar in terms of distress to individuals in unproblematic situations, whereas persons who failed to solve their problems reported higher levels of distress. Costanzo et al. (2009) found that cancer survivors were resilient with respect to personal growth. Although in their study personal growth decreased over time both for persons with and without cancer, this decline was universal, and cancer survivors were not worse off than individuals without cancer (Costanzo et al. 2009). I will extend their research by examining whether the resilience pattern will hold when we consider heterogeneity by age and cohort.
In a thriving scenario, cancer can be viewed as a catalyst for personal growth. Carver (1998) suggests that psychological thriving is “growth in response to an adverse event” (Carver 1998: 253). Unlike resilience, thriving involves improved psychological outcomes rather than simply retaining relatively good levels of functioning. Similarly, from the crisis resolution perspective, successfully resolved events can enhance the self and promote personality growth (Turner and Avison 1992). Therefore, personal growth is a particularly relevant psychological outcome for exploring potential thriving after a cancer diagnosis. Thriving is most likely when cancer is appraised as a challenge (Carver 1998). According to Taylor’s (1983) theory of cognitive adaptation, cancer can shatter individuals’ assumptions about the world. As individuals struggle to restore their beliefs, they can find new meanings, gain new strengths, and develop new perspectives (Frank 1995). The realization that one’s existence is transient and insecure leads to discovering new facets in life and re-evaluating one’s priorities. As an existential challenge to one’s taken-for-granted worldview, cancer can promote positive development and uncover strengths about which the person did not know before. Even individuals who have given up making improvements or changes in their lives may be forced by cancer to adopt new attitudes and develop heightened self-knowledge.
In sum, I examine whether change (or continuity) in personal growth among cancer survivors and persons without cancer follows the impairment, resilience, or thriving trajectories. To refine our understanding of psychological adjustment to cancer and extend previous research, I model trajectories of personal growth in the joint context of the interplay of aging and cohort influences.
Age Differences in the Association between Cancer and Personal Growth
Personal growth and cancer follow opposite age trajectories. Cross-sectional and longitudinal studies of nationally representative samples consistently document that personal growth declines with age for every age group beginning in young adulthood (Pudrovska, Hauser, and Springer 2005; Ryff, Keyes, and Hughes 2003). In contrast to the age-related decline in personal growth, the risk of cancer increases with age. Cancer incidence is low in young adulthood, increases in midlife and early old age, and somewhat declines at very advanced ages (National Cancer Institute 2008). Because most cancers are diagnosed among people over 50 years old, cancers that affect younger people — especially persons under 40 years old — can be considered “off-time” compared to similar conditions that develop at later stages of the life course. A stress process view on the life course suggests that “off-time” transitions tend to be more stressful and entail particularly negative psychological outcomes (Pearlin and Skaff 1996). Research shows that older cancer survivors exhibit lower levels of depression and anxiety and better psychological adjustment than younger persons with cancer (Mosher and Danoff-Burg 2005). Yet, there is also evidence that, compared to older patients, younger cancer patients report not only greater vulnerability but also greater positive meaning and higher levels of posttraumatic growth (Bellizzi and Blank 2006; Bower et al. 2005).
Because it is well documented that personal growth declines with age (Pudrovska et al. 2005), I can make specific predictions about different age-related trajectories of personal growth. In the impairment scenario, age-related declines in personal growth will be more pronounced among cancer patients than persons without cancer, with the youngest cancer survivors experiencing the steepest decrease in personal growth. If the resilience mechanism is at work, personal growth will decline with age at a similar rate for cancer survivors and their peers without cancer. In the case of thriving, personal growth will decline slower with age among cancer patients compared to individuals without cancer. To my knowledge, the only study that examined age differences in the association between cancer and personal growth (Costanzo et al. 2009) found similar trajectories of personal growth across age groups. Yet, this study used a cross-sectional measure of age and, thus, confounded age and cohort influences. Therefore, it is not known to what extent the purported age effects reflect developmental aging processes or stable differences among birth cohorts.
Social Context and Experiences of Birth Cohorts
Each cohort has distinctive characteristics reflecting the circumstances of its unique entry in the social world and subsequent age-graded exposure to social conditions and cultural transformations (Ryder 1965). In my analysis, I distinguish five 10-year birth cohorts. Individuals born in the 1920s (Cohort 1) were children and early adolescents during the Great Depression and came of age during World War II. Compared to subsequent cohorts, they experienced more adversity early in life, whereas their peak years of family formation and employment unfolded during the economic growth of the 1950s and 1960s (Elder and Liker 1982). People born in the 1930s (Cohort 2) were children during World War II and entered adulthood in the 1950s, the period of traditional gender roles, early marriage, and high fertility (Henretta 2007). Persons born in the 1940s and 1950s (Cohorts 3 and 4) are mostly Baby Boomers who came of age in the 1960s and 1970s, and assumed work and family roles during women’s and civil rights movements and the resulting social transformations (Pavalko, Gong, and Long 2007). Individuals born in the 1960s (Cohort 5) grew up during the second demographic transition when the values shifted from those favoring family commitment and self-sacrifice to an emphasis on self-actualization and personal freedom (Lesthaeghe 1995).
In addition to distinctive social and cultural imprints, these five cohorts had differential experiences with cancer. Trends in cancer incidence, mortality, and survival in the United States (National Cancer Institute 2008) reveal that earlier cohorts experienced lower cancer incidence than more recent cohorts. In contrast, the 5-year cancer survival rates progressively improved for each successive birth cohort since the 1930s. Mortality rates for most cancer types started declining or leveled off in the 1970s and 1980s after a continuous increase since the 1930s. Thus, compared to older cohorts, younger cohorts witnessed higher cancer incidence but also better survival after a cancer diagnosis. Moreover, older cohorts have been exposed from childhood through most of their adult lives to the predominant cultural discourse of cancer as a “dread disease” (Patterson 1987). Cantor (2006) describes a pervasive image of cancer in the 1950s as a hopeless or incurable condition. In the first half of the 20th century, the fear of cancer was so great that it was common practice to keep cancer diagnosis secret from a patient (Lusk 2005). In contrast, younger cohorts have witnessed a change in the public discourse of cancer from fatalistic acceptance to standing up to a challenge. In U.S. and Canada magazines since the 1970s, individuals with cancer have been described as heroic fighters who never “conceded defeat” (Clarke and Everest 2006: 2597). This new realization that cancer is amenable to treatment and can be conquered was reflected in the shifting perceptions of cancer patients from victims to survivors (Clarke and Everest 2006; Kaiser 2008).
Given pronounced differences among cohorts in population-wide cancer patterns and the shifting socio-cultural meanings of cancer, I hypothesize that the effect of cancer on personal growth depends not only on age-related developmental processes but is also shaped by cohort membership. I expect that among cancer survivors age-related declines in personal growth are steeper in the older cohorts (born before 1950) than younger cohorts (born after 1950).
METHODS
The data for this analysis come from the two waves of the National Survey of Midlife Development in the United States (MIDUS). The first wave was conducted in 1994–1995. The main sample included 4,242 noninstitutionalized English-speaking adults aged 25 to 74. In addition, interviews were conducted with 951 siblings of the main participants and 1,996 twins identified in the Twin Screening Project. The response rate for the MIDUS I telephone interview was 70% in the main sample. Among the telephone participants, 86.3% completed self-administered questionnaires. A longitudinal follow-up of the original MIDUS study was conducted in 2004–2006. The longitudinal retention rate for the entire sample was 70%. The main sample in MIDUS II contained 2,257 participants, the sibling sample included 733 siblings of the main participants, and the twin sample included 1,484 twins. Self-administered questionnaires in MIDUS II were completed by 1,805 main participants (80% of phone participants), 637 siblings (87% of the phone participants), and 1,204 twins (81% of the phone participants). My analysis is based on the pooled longitudinal sample of main participants, siblings, and twins who participated in the two waves of the MIDUS study and completed both phone interviews and mail questionnaires. The analytic sample in this study comprises 1,748 main participants, 621 siblings, and 1,175 twins.
Sample attrition
Attrition related to unobserved residual changes in the response variable may produce biased estimates. If personal growth increases the probability of attrition, the coefficients in my analysis may be biased. To address this possibility, I conducted logistic regression analysis that revealed no effect of personal growth at baseline on the probability of participating in the follow-up (OR = .991, p = .360). Although there is no evidence of outcome-dependent attrition bias, I adjust for the hazard of attrition in all models as an additional precaution.
Further, I conducted a detailed analysis of patterns of sample attrition among cancer patients (available upon request). Using propensity score matching, I estimated the likelihood of being retained in the sample by comparing persons who were similar on a wide variety of characteristics at baseline but differed with respect to their cancer status. Cancer patients were significantly more likely than non-cancer controls to drop out of the study due to death, yet the likelihood of nonparticipation due to reasons other than death was lower among cancer survivors compared to controls. In other words, persons who had cancer at baseline and survived to the follow-up were significantly more likely to participate in the study than individuals without cancer. In addition, ANOVA comparisons show that persons who had cancer in Wave 1 and participated in Wave 2 were similar in terms of baseline personal growth to individuals with cancer who were deceased by Wave 2 (the mean levels of personal growth are 17.81 versus 17.79, respectively, F = .001, p = .946). Similarly, no difference in personal growth is observed between two groups of participants who were deceased by Wave 2: persons with cancer at baseline and persons without cancer at baseline (17.82 versus 17.52, respectively, F = .51, p = .475). Thus, there is no evidence that sample attrition and, especially, selective mortality among cancer patients may significantly bias my findings.
Measures
Each time-varying variable in the analysis includes both baseline (Wave 1) and follow-up (Wave 2) values. To assess personal growth, participants were asked about the extent of agreement or disagreement with the following statements: “It is important to have new experiences that challenge how I think about myself and the world.” “I gave up trying to make big improvements or changes in my life a long time ago” (reverse coded). “For me, life has been a continuous process of learning, changing, and growth.” These items were selected for the inclusion in the national survey from the original 20-item scale developed by Ryff (1989). Rather than choosing these items to maximize the internal consistency, the emphasis was on representing the multifactorial structure of the parent scale. As such, the three-item scale of personal growth has a reasonably good reliability of α = .55 (Ryff et al. 2003).
The focal predictor variable is the presence or absence of a cancer diagnosis. At each wave, it was coded 1 if a person has ever been diagnosed with cancer and 0 for people without a cancer diagnosis. Further, a dummy indicator of multiple cancers reflects 34 persons who reported diagnoses of two different cancers, and 4 persons who had three cancers. Treatment was coded 1 for persons who were undergoing treatment for cancer at the time of the interview. Age at cancer diagnosis is included as a linear and squared term. Time since diagnosis is measured as a continuous variable in years and, alternatively, represented with four mutually exclusive dummy variables: 0–2 years, 2–4 years, 4–8 years, and over 8 years.
Age and cohort
I categorized participants into five 10-year birth cohorts shown in Table 1: individuals born in the 1920s (n = 355), in the 1930s (n = 654), in the 1940s (n = 933), in the 1950s (n = 946), and in the 1960s (n = 656). Cohort is included in all models as an ordinal variable with five categories (0 = the oldest cohort and 4 = the youngest cohort). Age is coded in years. Age at baseline ranged from 25 to 74 years old, and the participants aged on average nine years by the follow-up.
Table 1.
Cancer Prevalence by Age and Cohort: MIDUS, 1995–2005
| Cohort | N | Birth year | Age at Time 1 | Age at Time 2 | n (%) ever diagnosed with cancer | n (%) never diagnosed with cancer | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Time 1 | Time 2 | Time 1 | Time 2 | |||||
| 1 | 355 | 1920–1929 | 66–75 | 76–85 | 63 (24.05%) | 114 (22.94%) | 292 (8.90%) | 241 (7.91%) |
| 2 | 654 | 1930–1939 | 56–65 | 66–75 | 82 (31.30%) | 146 (29.38%) | 572 (17.43%) | 508 (16.67%) |
| 3 | 933 | 1940–1949 | 46–55 | 56–65 | 72 (27.48%) | 133 (26.76%) | 861 (26.23%) | 800 (26.26%) |
| 4 | 946 | 1950–1959 | 36–45 | 46–55 | 34 (12.98%) | 71 (14.29%) | 912 (27.79%) | 875 (28.72%) |
| 5 | 656 | 1960–1970 | 25–35 | 35–45 | 11 (4.20%) | 33 (6.64%) | 645 (19.65%) | 623 (20.45%) |
| Total | 3,544 | 262 (100%) | 497 (100%) | 3,282 (100%) | 3,047 (100%) | |||
Physical characteristics
Comorbidity is assessed as the number of chronic illnesses other than cancer diagnosed by a physician in the past 12 months. The measure of functional limitations reflects the extent to which participants’ health limited activities of daily living (ADLs), such as lifting or carrying groceries, bathing or dressing oneself, climbing several flights of stairs, bending, kneeling or stooping, and walking more than one mile. In addition, I include an indicator of physical activity limited because of health (1 = “not limited at all”, 2 = “limited a little”, 3 = “limited a lot”). Finally, pain is represented with three mutually exclusive categories: no pain, pain that did not interfere with activities, and pain that interfered with activities.
Sociodemographic characteristics
All models include participants’ gender and race. Gender is coded 1 for women and 0 for men. Race is represented with three mutually exclusive dummy variables: White (reference category), Black, and other race. The categories of education include less than high school, high school or GED (reference category), some college, bachelor’s degree, and graduate or professional degree. The measure of income is a natural log of the respondent’s total household income. Employment status is coded 1 if a participant was working for pay at the time of the interview and 0 otherwise. Occupational education is a natural log of the proportion of persons in the respondent’s occupation that completed at least some college as of 1990, whereas occupational income is a natural log of the proportion of persons earning at least $14.30 per hour in 1990. Five mutually exclusive categories represent marital status: married (reference category), cohabiting, divorced/separated, widowed, and never married. Parental status is assessed with the total number of children (0 for nonparents) and the presence of at least one child under 18.
Analytic Approach
I estimate a three-level random-coefficient model: Level-1 units (measurements for a given individual at two time points) are nested within Level-2 units (individuals), and individuals are nested within Level-3 units (families, i.e. sibling groups). This model can be represented by the following reduced-form equation:
| (1) |
In the Equation 1, Yijv is personal growth measured at occasion i for individual j in family v. The fixed part of the model contains the fixed intercept β0, the fixed slopes for the main effects of cancer, age, and cohort (β1, β2, and β3, respectively), and the fixed slope β4 for the interactive effects of cancer, age, and cohort. The slope β4 is the focal coefficient in this model because my purpose is to explore how the effect of cancer on personal growth depends on age and cohort. In addition, βk are coefficients for individual-specific explanatory variables and all two-way interactions among cancer, age, and cohort.
The random part of the model is represented by random effects at each of the three levels. ζ0jv is the random intercept for individual j in family v (Level 2), or the between-individual intercept. ζ0v is the random intercept for family v (Level 3), or the between-family intercept. The between-individual (Level-2) random intercept ζ0jv ~ N (0, ψ22) specifies deviations of individual-specific intercepts from the average intercept β0. The between-family (Level-3) random intercept ζ0v ~ N (0, ψ11) specifies deviations of family-specific intercepts from the average (fixed) intercept β0. Additionally, εijv ~ N (0, θij) is the within-individual (Level-1) error term. Further, ζ1j is a random individual-specific slope for the effects of cancer with variance ψ33. It represents the deviation of individual j’s slope from the mean slope β1.
ζ0v, ζ0jv, ζ1j and εijv are random parameters whose variances ψ11, ψ22, ψ33, and θij – not the parameters themselves – are estimated in the model. All four random terms are assumed to be uncorrelated with the explanatory variables in the model. The residual error term is uncorrelated with ζ0v, ζ0jv, and ζ1j. Both intercepts ζ0v and ζ0jv and slopes ζ1j are assumed to be independent across families and individuals, and the Level-1 residuals εij are independent across families, individuals, and occasions (Rabe-Hesketh and Skrondal 2008).
The model in Equation 1 is specified to compare two categories: all persons with cancer to all persons without cancer. In a preliminary analysis, I also estimated an alternative “three-category” specification comparing persons who have never been diagnosed with cancer to two groups of cancer survivors: persons who had cancer at baseline and persons who did not have cancer at baseline but were diagnosed by the follow-up. Because the patterns of personal growth in the two cancer groups were very similar, I decided to report results from the two-category specification. The model that has only two comparison groups (cancer versus non-cancer) is more parsimonious. More importantly, cancer is rare in the general population, so there are relatively few cancer survivors in this community-based sample. Subdividing cancer survivors into two groups reduces the statistical power substantially, especially given that I test three-way interactions among cancer, age, and cohort. Along with the gain in statistical power, however, combining all persons with cancer in one group could potentially present a problem of reverse causation. Longitudinal mixed models link contemporaneous information about time-varying predictors and outcomes (Singer and Willett 2003). Because cancer and personal growth are both time-varying, the model specified in Equation 1 could become problematic for my argument if personal growth causes cancer, and not vice versa. Yet, it is reassuring that personal growth at Wave 1 does not predict cancer at Wave 2 (OR = 1.004; p = .560); therefore, endogeneity is unlikely to be a problem in this analysis. Another potential caveat is that the fixed-effects specification would be more appropriate than the random-effects specification in Equation 1 because my analysis is based on only two waves of data. I examined the cancer × age × cohort interaction in a fixed-effects model (available upon request), and the findings were very similar to the random-effects model.
RESULTS
As shown in Table 1, among people who participated in both waves, 262 had cancer at baseline and 235 developed new cancer between the MIDUS waves; thus, the total number of cancer patients in MIDUS II was 497. About 30% of cancer patients are in the 66–75 age group (age in Wave 2), about 27% in the 56–65 age group, and 23–24% are in the oldest age group. Finally, 20% of cancer patients belonged to the two youngest age groups in Wave 2.
Among persons that did not have cancer at baseline, 8.7% reported a cancer diagnosis by the follow-up. A comparison of these persons to persons without cancer (not shown) reveals that individuals diagnosed with cancer between the waves were older (OR = 1.65, p < .001), reported worse self-rated health (OR = .79, p < .05), were more likely to smoke (OR = 1.32, p < .05) and less likely to exercise (OR = .86, p < .05) at baseline, i.e. before the cancer diagnosis. In contrast, there were no significant differences with respect to gender and other baseline variables, including socioeconomic characteristics, family characteristics, depression, personal growth, physical symptoms, the number of chronic illnesses, body mass index, and alcohol consumption.
The joint effects of cancer, age, and cohort on personal growth are shown in Table 2. Model 1 in Table 2 indicates that cancer has no effect on personal growth net of age, cohort, and a wide array of sociodemographic and socioeconomic characteristics. Age is related negatively and linearly to personal growth. The negative coefficient for cohort in Model 1 suggests that younger cohorts have lower levels of personal growth than older cohorts net of age.
Table 2.
Three-Level Random-Coefficient Models of the Associations among Cancer, Age, Cohort, and Personal Growth: MIDUS, 1995–2005 (N = 3,544)
| Variable | (1) | (2) | (3) |
|---|---|---|---|
| Fixed Part: | |||
| Constant | 17.838*** | 17.591*** | 19.097*** |
| Cancer = 1 | .073 (.127) | .345 (.532) | .473 (.539) |
| Age (mean-centered)f | −.090*** (.006) | −.069*** (.009) | −.068*** (.009) |
| Cohort (0 = 1920–1929) | −.796*** (.067) | −.787*** (.069) | −.880*** (.070) |
| Interactions: | |||
| Cohort × Age | −.009*** (.003) | −.009*** (.003) | |
| Cancer × Age | −.037 (.023) | −.035 (.023) | |
| Cancer × Cohort | .004 (.217) | −.013 (.215) | |
| Cancer × Age × Cohort | .030*** (.007) | .028*** (.007) | |
| Sociodemographic Characteristics: | |||
| Female = 1 | .602*** (.099) | .597*** (.100) | .725*** (.099) |
| White (reference group) | |||
| Black = 1 | .421 (.239) | .426 (.239) | .439 (.235) |
| Other race = 1 | −.126 (.294) | −.121 (.294) | −.041 (.289) |
| Physical Characteristics: | |||
| Currently in treatment for cancer = 1 | .175 (.306) | ||
| Multiple cancers = 1 | −.089 (.149) | ||
| Number of chronic illnesses | −.093*** (.016) | ||
| Limited ADLs | −.365*** (.092) | ||
| Limited physical activity | −.089 (.065) | ||
| No pain (reference group) | |||
| Pain but not interferes | .129 (.173) | ||
| Pain interferes | .042 (.110) | ||
| Random Part: | |||
| Level-three random intercept variance (between-family) ψ11 | 1.236 (.212) | 1.231 (.212) | 1.190 (.203) |
| Level-two random effects: | |||
| Random intercept variance (between- individual) ψ22 | 2.899 (.236) | 2.910 (.237) | 2.716 (.227) |
| Random slope variance (cancer) ψ33 | .467 (.469) | .408 (.466) | .389 (.455) |
| Level-one variance (within-individual) θij | 5.061 (.122) | 5.041 (.122) | 5.028 (.121) |
| AIC | 35,083 | 35,070 | 34,944 |
| BIC | 35,263 | 35,248 | 35,184 |
| Log likelihood | −17,517 | −17,507 | −17,437 |
Note:
p < .05.
p < .01.
p < .001 (two-tailed).
All models adjust for education, income, employment status, occupational education, occupational income, marital status, the number of children, the presence of children 18 or younger, and the hazard of attrition.
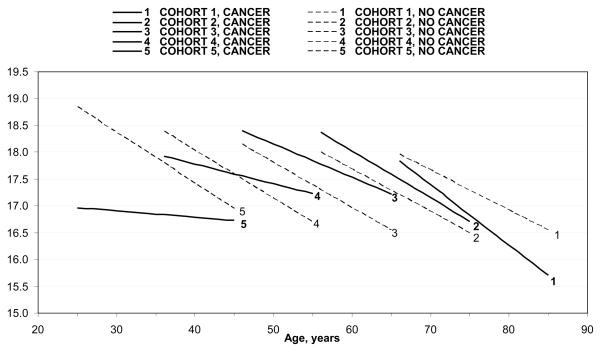
Model 2 in Table 2 is the central model in my analysis. It shows that the effect of cancer on personal growth is contingent on the interaction of age and cohort influences. Figure 1 illustrates the cancer × age × cohort interaction term significant at the .001 level (b = .030, SE = .007). Figure 1 indicates that personal growth declines linearly with age within each cohort of cancer survivors and individuals without cancer, yet there are pronounced differences in the level and rate of change in personal growth among cohorts.
Figure 1.
Interactive Effects of Cancer, Age, and Cohort on Personal Growth
First, in the three youngest cohorts (denoted 5, 4, and 3 in Figure 1), the age-related decline in personal growth is slower among cancer patients compared to their peers without cancer. Among people born in the 1930s (Cohort 2), personal growth decreases with age at a roughly similar pace for cancer survivors and individuals without cancer. Finally, in the oldest cohort (Cohort 1), the levels of personal growth are lower among persons with cancer than without cancer; moreover, personal growth declines faster with age for cancer patients. Second, the levels of personal growth differ across cohorts. In Cohorts 2 and 3 (people aged between 56 and 75 in Wave 2), personal growth is higher among cancer survivors than individuals without cancer. In Cohort 4 aged 46–55 in Wave 2, personal growth declines steeper among persons without cancer, and by mid-40s, cancer patients exhibit higher levels of personal growth than their peers without cancer. Conversely, among the youngest and the oldest cohorts (Cohorts 1 and 5), individuals without cancer have higher levels of personal growth than cancer patients, although personal growth declines very slowly among the youngest cancer survivors and very steeply in the oldest group of cancer survivors. It is noteworthy that the baseline level of personal growth is the lowest among cancer survivors in Cohort 5, yet this is the only group that shows virtually no decline in personal growth with age. This low level of personal growth is driven by the youngest cancer survivors who already reported a cancer diagnosis at baseline. I elaborate on this interesting pattern in the discussion. In sum, the effect of cancer on personal growth depends on both age and cohort. Cancer slows the age-related decline in personal growth in the three youngest cohorts such that cancer survivors experience significantly smaller decreases in personal growth than individuals without cancer. In addition, cohort patterns are reflected in the fact that cancer survivors in Cohorts 2, 3, and 4 have higher levels of personal growth than their peers without cancer.
Further, I conducted additional analyses to incorporate other components into these complex age and cohort patterns. I examined gender differences in the effects of cancer on personal growth and in the interactive effects between cancer and age as well as cancer and cohort. None of the gender interaction terms were significant; therefore, I conclude that age and cohort differences in the association between cancer and personal growth are similar for men and women. Moreover, somewhat surprisingly, age at cancer diagnosis and duration since diagnosis do not affect the association between cancer and personal growth. I conducted extensive analyses using linear terms, quadratic terms, and dummy variables. Neither main nor interactive effects were significant, and age at diagnosis and duration since diagnosis do not explain the effects of current age and cohort membership on the association between cancer and personal growth.
Finally, Model 3 includes physical characteristics that may confound the effect of age because comorbidity and functional limitations are more prevalent among older adults. Yet, the coefficient for the focal three-way interaction term remains virtually unchanged after adjustment for cancer treatment, comorbidity, pain, and functional limitations.
DISCUSSION
Using data from the 1994–1995 and 2004–2006 waves of the National Survey of Midlife Development in the United States, I examine whether change (or continuity) in personal growth among cancer survivors and persons without cancer follows the impairment, resilience, or thriving trajectories. My findings reveal that the effect of cancer on personal growth strongly depends on the interplay of age and cohort influences.
Age Effects on the Association between Cancer and Personal Growth
Personal growth declines with age for all individuals regardless of cohort membership and cancer status. Although personal growth does not increase among persons with cancer, there is clear evidence that cancer is still protective for personal growth. Specifically, consistent with the thriving perspective, cancer slows the decline in personal growth with age in the three youngest cohorts. In other words, among the 1940s, 1950s, and 1960s birth cohorts, personal growth decreases at a slower rate over time for cancer survivors than for individuals without cancer. Conversely, in the 1930s cohort, personal growth declines at the same rate for cancer survivors and their peers without cancer, which is consistent with the resilience mechanism. Finally, consistent with the impairment prediction, the age-related decline in personal growth is steeper for cancer patients than individuals without cancer in the oldest cohort born in the 1920s. Extensive additional analyses reveal that these patterns are driven specifically by age as a life-course stage, and not age at the cancer diagnosis or duration since diagnosis. Moreover, age differences in the association between cancer and personal growth are similar for men and women.
It is consistently documented in cross-sectional and longitudinal studies that older persons exhibit the lowest levels of personal growth even in the absence of life-threatening chronic illnesses (Pudrovska et al. 2005; Ryff and Keyes 1995). In addition to this developmental decline in personal growth, older cancer patients who cope with disease symptoms, complex treatment regimens and their side effects, functional limitations, and other ensuing stressors may have less energy and vigor left to seek opportunities for growing and expanding as a person. In late life, surviving cancer and dealing with cancer-related daily hassles one step at a time may be a challenge in itself. Moreover, personal growth may be less adaptive for older cancer patients than maintaining continuity and stability to achieve ego integrity (Erikson 1950). This stability can reflect maturational accommodative processes, such as emotional regulation. Compared to younger people, older adults have a greater ability to regulate their emotions (Lawton et al. 1992) and exhibit less variability in emotional responses to environmental challenges (Mroczek and Kolarz 1998).
In contrast to the oldest cohort, cancer slows the age-related decline in personal growth among the three youngest cohorts. The nature of Ryff’s construct of personal growth is central to understanding this mechanism of thriving. Personal growth as viewed in this study emphasizes the plasticity of individuals and their potential for self-improvement as a result of dealing with new experiences (Ryff et al. 2003). This view of personal growth is particularly relevant to cancer and, broadly, to other life-threatening transitions. Cancer is a psychosocial transition (Parkes 1971) that inevitably causes people to question their assumptions about the familiar world that used to be taken for granted. After the diagnosis, individuals are likely to find many aspects of their assumptive world to be discrepant with their newly changed situation and, thus, may be forced to redefine their life goals and priorities (Cordova et al. 2001). In addition, cancer may promote new insights about the self in the context of adversity, such as the knowledge of personal strengths, limitations, and coping skills (Taylor 1983). These processes of accommodation to the new reality appear to be a particularly fruitful soil for personal growth.
Cohort Experiences and the Changing Discourse of Cancer
For an adequate understanding of psychological adjustment to cancer, aging processes should be considered in the context of cohort experiences. In the oldest cohort, individuals with cancer report lower levels of personal growth than their peers without cancer. In contrast, cancer survivors in the 1930s, 1940s, and 1950s birth cohorts have higher levels of personal growth than persons without cancer. In the youngest cohort born in the 1960s, the level of personal growth at baseline is lower among individuals with cancer, yet personal growth declines at a much slower pace among cancer survivors than persons without cancer.
People born in the 1920s – the oldest cohort in this study – were exposed to the predominant cultural messages of cancer’s invincibility and patients’ powerlessness for most of their adult lives. Black (1995) observes that the views of cancer among women aged 75–84 in 1995 were remarkably consistent with what women’s magazines wrote about cancer in 1929–1949, such as beliefs that cancer is contagious, that all cancers are incurable, and that the treatment is worse than the disease. In the educational films created by the American Cancer Society in the 1920s–1950s, patients were depicted as ignorant, prejudiced, and scared, whereas male physicians as enlightened, authoritative, and possessing the power and knowledge (Cantor 2007). In sum, this view of patients as powerless victims that are better off not knowing the dreaded diagnosis was incompatible with the idea that cancer can be an opportunity for growth and self-improvement. These discourses promoted fatalistic acceptance of cancer rather than realization of one’s potential in the face of an existential challenge.
In contrast to the oldest cohort, the effect of cancer on personal growth in younger cohorts is largely consistent with the resilience and thriving mechanisms. In the 1930s, 1940s, and 1950s birth cohorts, cancer survivors report higher levels of personal growth than their peers without cancer. In the 1960s cohort, cancer survivors exhibit lower levels of personal growth but also a remarkably slower decline over time compared to people without cancer. These cohort patterns may reflect a gradual change in the public discourse of cancer from fatalistic acceptance to putting up a good fight. In the beginning, public figures played an important role in changing attitudes about cancer. Cancer forcefully entered the U.S. public domain in the 1970s when several well-known women, including Betty Ford, shared their experiences of breast cancer (Kolker 2004). Celebrities who suffered but gained new insights through suffering became new role models. They suggested to other people that cancer can be a challenge “with the promise of a bright future” (Clarke 1999:122). Moreover, recent proliferation of highly visible cancer fundraising events and cancer products may have also contributed to the view of cancer as an opportunity for personal growth. For example, King (2006) shows how breast cancer was transformed from a stigmatized disease into a market-driven industry of survivorship. In addition, mortality rates for most cancer types started declining or leveled off in the 1970s and 1980s after a continuous increase since the 1930s (National Cancer Institute 2008). This new realization that cancer is amenable to treatment and can be conquered was reflected in the proliferation of war and battle metaphors (Clarke and Everest 2006; Kaiser 2008). Cancer has become a challenge, a battle, and people grow stronger in the process of fighting this battle because they discover new strengths, new territories, and new perspectives on life. The victory or even a mere participation in this battle can contribute to personal growth.
Personal growth starts and remains low for the youngest cancer survivors over the 10-year period, yet this is the only group that shows virtually no decline in personal growth with age. This combination of a remarkably low level of personal growth that is nonetheless resistant to decline over time reflects both the impairment and thriving mechanisms. Cancer at a young age is a profoundly “off-time” stressor and, thus, particularly disruptive, life-shattering, and unforeseen (Pearlin and Skaff 1996). Yet, personal growth declined very little over time among the youngest cancer survivors – a much slower decline compared to same-aged individuals without cancer. The youngest cohort grew up and came of age in the decades when the images of battle and survivorship were particularly prominent in the cancer discourse; therefore, cancer appears to be a catalyst for personal growth in this cohort even despite its “off-time” and disorderly nature.
Implications for Social Research and Clinical Practice
This study suggests that a sociological perspective can enhance our understanding of psychological adjustment to cancer because mental health trajectories of cancer survivors unfold within socio-historical and cultural contexts. Psychological reactions to a chronic and life-threatening illness may appear highly individualized and intimate as they are typically viewed in clinical research, yet psychological adjustment at the individual level is socially and culturally patterned. It is important to consider psychological consequences of cancer not only in the context of adult development and aging, but also in the context of macro-level influences represented by cohort differences. It is well documented that cohorts share life chances affecting marriage timing, family size, and labor force participation (Easterlin 1987; Pavalko et al. 2007). My findings also suggest that deeply personal and seemingly idiosyncratic psychological processes can be influenced by shared experiences within a birth cohort.
With respect to clinical implications, this study reveals that personal growth declines with age for all individuals regardless of cohort membership and the presence or absence of cancer. Therefore, clinical interventions for cancer patients should not overstate a potential for cancer-related personal growth. At the same time, psychosocial interventions directed at positive reframing of cancer as an opportunity to negotiate new challenges, reevaluate life goals and priorities, and enhance self-knowledge may be particularly relevant and effective, especially for younger survivors.
Limitations and Future Directions
Although MIDUS is one of the longitudinal social surveys with the most detailed measures of physical and mental health over time, information on certain cancer characteristics is not available, including a stage of cancer at diagnosis and cancer recurrence. Moreover, because there are few people with specific cancer types, I could not analyze age and cohort differences in the effects of cancers at different sites. An important direction for future research would be collecting data that contain large numbers of individuals of different ages with one cancer type.
Further, whereas multicohort longitudinal studies are particularly well suited for disentangling the influences of age and cohort, they are less able to distinguish between cohort and period effects (Pavalko et al. 2007). It is reassuring, however, that the two MIDUS waves are nine years apart, and there is no evidence that the cultural discourse of cancer has changed substantially over this period (Kaiser 2008). Therefore, I assume that cohort differences in psychological adjustment to cancer are likely to be more important than period variation, and that any unmeasured period effects operate to affect individuals through cohort membership (Lynch 2006).
Human psychological functioning is multi-dimensional (Ryff 1989), and personal growth is only one of its dimensions. I have started related projects to examine implications of cancer for other positive and negative psychological outcomes. Comparing similarities and, more importantly, differences across outcomes will enable me to elucidate mechanisms through which cancer affects psychological well-being. Finally, few studies have examined the influence of race and ethnicity on adjustment to cancer. Although I include race in all models, the MIDUS sample contains very few non-White cancer survivors to allow detailed comparisons of age and cohort differences by race. Moreover, grouping non-White participants in one category might obscure important racial and ethnic differences in psychological adjustment to cancer. Therefore, an important direction for future research is to compare age and cohort patterns of cancer-related personal growth among racial and ethnic groups. Despite these limitations, this study shows that sociological perspectives can make an important contribution to research on cancer survivors’ mental health and expand our understanding of the interplay of developmental and socio-cultural influences on psychological adjustment to chronic illness.
Acknowledgments
The author thanks Mark Hayward, Robert Hummer, and Yang Yang for providing helpful and insightful comments at various stages of this project. The National Survey of Midlife Development in the United States (MIDUS) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by a grant from the National Institute on Aging (P01 AG020166). The data are publicly available at http://www.icpsr.umich.edu/access/index.html.
Biography
Tetyana Pudrovska is an assistant professor in the Department of Sociology and a faculty research associate in the Population Research Center at the University of Texas-Austin. As a demographer of health and aging, she studies how social, biological, and psychological factors interact over the life course to affect physical and mental health of middle-aged and older adults. Among her ongoing research projects are psychological implications of cancer and a life-course analysis of social etiology of breast and prostate cancers.
References
- Bellizzi Keith M, Blank Thomas O. Predicting Posttraumatic Growth in Breast Cancer Survivors. Health Psychology. 2006;25:47–56. doi: 10.1037/0278-6133.25.1.47. [DOI] [PubMed] [Google Scholar]
- Black Margaret E. What Did Popular Women’s Magazines from 1929 to 1949 Say About Breast Cancer? Cancer Nursing. 1995;18:270–277. [PubMed] [Google Scholar]
- Bonanno George A. Loss, Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive After Extremely Aversive Events? Psychological Trauma: Theory, Research, Practice, and Policy. 2008;1:101–113. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bonanno George A, Wortman Camille B, Nesse Randolph M. Prospective Patterns of Resilience and Maladjustment During Widowhood. Psychology and Aging. 2004;19:260–271. doi: 10.1037/0882-7974.19.2.260. [DOI] [PubMed] [Google Scholar]
- Bower Julienne E, Meyerowitz Beth E, Desmond Katherine A, Bernaards Coen A, Rowland Julia H, Ganz Patricia A. Perceptions of Positive Meaning and Vulnerability Following Breast Cancer: Predictors and Outcomes Among Long-Term Breast Cancer Survivors. Annals of Behavioral Medicine. 2005;29:236–245. doi: 10.1207/s15324796abm2903_10. [DOI] [PubMed] [Google Scholar]
- Cantor David. Cancer, Quackery and the Vernacular Meanings of Hope in 1950s America. Journal of the History of Medicine and Allied Sciences. 2006;61:324–368. doi: 10.1093/jhmas/jrj048. [DOI] [PubMed] [Google Scholar]
- Cantor Uncertain Enthusiasm: The American Cancer Society, Public Education, and the Problems of the Movie, 1921–1960. Bulletin of the History of Medicine. 2007;81:39–69. doi: 10.1353/bhm.2007.0002. [DOI] [PubMed] [Google Scholar]
- Carver Charles S. Resilience and Thriving: Issues, Models, and Linkages. Journal of Social Issues. 1998;54:245–265. [Google Scholar]
- Carver Enhancing Adaptation During Treatment and the Role of Individual Differences. Cancer. 104(supplement):2602–2607. doi: 10.1002/cncr.21247. [DOI] [PubMed] [Google Scholar]
- Clarke Juanne N. Breast Cancer in Mass Circulating Magazines in the U.S.A. and Canada, 1974–1995. Women and Health. 1999;28:113–130. doi: 10.1300/J013v28n04_07. [DOI] [PubMed] [Google Scholar]
- Clarke Juanne N, Everest Michelle M. Cancer in the Mass Print Media: Fear, Uncertainty and the Medical Model. Social Science and Medicine. 2006;62:2591–2600. doi: 10.1016/j.socscimed.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Cordova Matthew J, Cunningham Lauren LC, Carlson Charles R, Andrykowski Michael A. Posttraumatic Growth Following Breast Cancer: A Controlled Comparison Study. Health Psychology. 2001;20:176–185. [PubMed] [Google Scholar]
- Cordova Matthew J, Andrykowski Michael A. Responses to Cancer Diagnosis and Treatment: Posttraumatic Stress and Posttraumatic Growth. Seminars in Clinical Neuropsychiatry. 2003;8:286–296. [PubMed] [Google Scholar]
- Costanzo Erin S, Ryff Carol D, Singer Burton H. Psychosocial Adjustment Among Cancer Survivors: Findings From a National Survey of Health and Well-Being. Health Psychology. 2009;28:147–156. doi: 10.1037/a0013221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Christopher G, Nolen-Hoeksema Susan, Larson Judith. Making Sense of Loss and Growing from the Experience: Two Construals of Meaning. Journal of Personality and Social Psychology. 1998;75:561–574. doi: 10.1037//0022-3514.75.2.561. [DOI] [PubMed] [Google Scholar]
- Easterlin Richard A. Birth and Fortune: The Impact of Numbers on Personal Welfare. Chicago, IL: University of Chicago Press; 1987. [Google Scholar]
- Elder Glen H, Jr, Liker Jeffrey K. Hard Times in Women’s Lives: Historical Influences Across Forty Years. American Journal of Sociology. 1982;88:241–269. [Google Scholar]
- Erikson Erik. Childhood and Society. New York: W. W. Norton; 1950. [Google Scholar]
- Frank Arthur W. The Wounded Storyteller: Body, Illness, and Ethics. The University of Chicago Press; 1995. [Google Scholar]
- Helgeson Vicki S, Reynolds Kerry A, Tomich Patricia L. A Meta-Analytic Review of Benefit Finding and Growth. Journal of Consulting and Clinical Psychology. 2006;74:797–816. doi: 10.1037/0022-006X.74.5.797. [DOI] [PubMed] [Google Scholar]
- Henretta John C. Early Childbearing, Marital Status, and Women’s Health and Mortality after Age 50. Journal of Health and Social Behavior. 2007;48:254–266. doi: 10.1177/002214650704800304. [DOI] [PubMed] [Google Scholar]
- Kaiser Karen. The Meaning of the Survivor Identity for Women with Breast Cancer. Social Science and Medicine. 2008;67:79–87. doi: 10.1016/j.socscimed.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King Samantha. Pink Ribbons, Inc: Breast Cancer and the Politics of Philanthropy. University of Minnesota Press; 2006. [Google Scholar]
- Kolker Emily S. Framing as a Cultural Resource in Health Social Movements: Funding Activism and the Breast Cancer Movement in the US, 1990–1993. Sociology of Health and Illness. 2004;26:820–844. doi: 10.1111/j.0141-9889.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- Lawton M Powell, Kleban Morton H, Rajagopal Doris, Dean Jennifer. Dimensions of Affective Experience in Three Age Groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Lazarus Richard, Folkman Susan. Stress, Appraisal, and Coping. Springer Publishing Company; 1984. [Google Scholar]
- Lesthaeghe Ronald. The Second Demographic Transition in Western Countries: An Interpretation. In: Mason KO, Jensen A-M, editors. Gender and Family Change in Industrialized Countries. Oxford: Oxford University Press; 1995. pp. 17–62. [Google Scholar]
- Lusk Brigid. Prelude to Specialization: US Cancer Nursing, 1920–50. Nursing Inquiry. 2005;12:269–277. doi: 10.1111/j.1440-1800.2005.00296.x. [DOI] [PubMed] [Google Scholar]
- Lynch Scott M. Explaining Life Course and Cohort Variation in the Relationship between Education and Health: The Role of Income. Journal of Health and Social Behavior. 2006;47:324–338. doi: 10.1177/002214650604700402. [DOI] [PubMed] [Google Scholar]
- Mosher Catherine, Danoff-Burg Sharon. A Review of Age Differences in Psychological Adjustment to Breast Cancer. Journal of Psychosocial Oncology. 2005;23:101–114. doi: 10.1300/j077v23n02_07. [DOI] [PubMed] [Google Scholar]
- Mroczek Daniel K, Kolarz Christian M. The Effect of Age on Positive and Negative Affect: A Developmental Perspective on Happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: 2008. [Google Scholar]
- Parkes Colin M. Psychosocial Transitions: A Field for Study. Social Science and Medicine. 1971;5:101–115. doi: 10.1016/0037-7856(71)90091-6. [DOI] [PubMed] [Google Scholar]
- Patterson James T. The Dread Disease: Cancer and Modern American Culture. Harvard University Press; 1987. [Google Scholar]
- Pavalko Eliza K, Gong Fang, Scott Long J. Women’s Work, Cohort Change, and Health. Journal of Health and Social Behavior. 2007;48:352–368. doi: 10.1177/002214650704800402. [DOI] [PubMed] [Google Scholar]
- Pearlin Leonard I. The Stress Process Revisited: Reflections on Concepts and Their Interrelationships. In: Aneshensel Carol S, Phelan Jo C., editors. Handbook of the Sociology of Mental Health. New York: Springer; 1999. pp. 395–416. [Google Scholar]
- Pearlin Leonard I, Skaff Marilyn M. Stress and the Life Course: A Paradigmatic Alliance. Gerontologist. 1996;36:239–247. doi: 10.1093/geront/36.2.239. [DOI] [PubMed] [Google Scholar]
- Peleg-Oren Neta, Sherer Moshe, Soskolne Varda. Effect of Gender on the Social and Psychological Adjustment of Cancer Patients. Social Work in Health Care. 2003;37:17–34. doi: 10.1300/J010v37n03_02. [DOI] [PubMed] [Google Scholar]
- Pudrovska Tetyana, Hauser Robert M, Springer Kristen W. Does Psychological Well-being Change with Age?. Paper presented at the 58th Annual Meeting of the Gerontological Society of America; Orlando, FL. November 2005. [Google Scholar]
- Rabe-Hesketh Sophia, Skrondal Anders. Multilevel and Longitudinal Modeling Using Stata. 2. College Station, TX: Stata Press; 2008. [Google Scholar]
- Roberts Cleora S, Severinsen Cindy, Carraway Christina, Clark D’Aun, Freeman Marguerite, Daniel Paulette. Life Changes and Problems Experienced by Young Adults with Cancer. Journal of Psychosocial Oncology. 1997;15:15–25. [Google Scholar]
- Ryder Norman B. The Cohort as a Concept in the Study of Social Change. American Sociological Review. 1965;30:843–861. [PubMed] [Google Scholar]
- Ryff Carol D. Happiness is Everything, or Is It? Explorations on the Meaning of Psychological Well-Being. Journal of Personality and Social Psychology. 1989;57:1069–1081. [Google Scholar]
- Ryff Carol D, Keyes Corey LM. The Structure of Psychological Well-Being Revisited. Journal of Personality and Social Psychology. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Ryff Carol D, Keyes Corey LM, Hughes Diane L. Status Inequalities, Perceived Discrimination, and Eudaimonic Well-Being: Do the Challenges of Minority Life Hone Purpose and Growth? Journal of Health and Social Behavior. 2003;44:275–291. [PubMed] [Google Scholar]
- Stanton Annette, Revenson Tracey, Tennen Howard. Health Psychology: Psychological Adjustment to Chronic Disease. Annual Review of Psychology. 2007;58:565–92. doi: 10.1146/annurev.psych.58.110405.085615. [DOI] [PubMed] [Google Scholar]
- Taylor Shelley E. Adjustment to Threatening Events: A Theory of Cognitive Adaptation. American Psychologist. 1983;38:1161–1173. [Google Scholar]
- Turner R Jay, Avison William R. Innovations in the Measurement of Life Stress: Crisis Theory and the Significance of Event Resolution. Journal of Health and Social Behavior. 1992;33:36–50. [PubMed] [Google Scholar]
- Thoits Peggy A. Stressors and Problem-Solving: The Individual as Psychological Activist. Journal of Health and Social Behavior. 1994;35:143–160. [PubMed] [Google Scholar]
- Westphal Maren, Bonanno George A. Posttraumatic Growth and Resilience to Trauma: Different Sides of the Same Coin or Different Coins? Applied Psychology. 2007;56:417–427. [Google Scholar]
- Widows Michelle R, Jacobsen Paul B, Booth-Jones Margaret, Fields Karen. Predictors of Posttraumatic Growth Following Bone Marrow Transplantation for Cancer. Health Psychology. 2005;24:266–273. doi: 10.1037/0278-6133.24.3.266. [DOI] [PubMed] [Google Scholar]