SUMMARY
Aminoglycosides, such as gentamycin, are well known ototoxic agents. Toxicity occurs via an activation process involving the formation of an iron-gentamycin complex with free radical production. Antioxidants like Q-ter (a soluble formulation of coenzyme Q10, CoQ10), can limit or prevent cellular ototoxic damage. The present study was designed to investigate the possible protective effects of Q-ter on gentamycin ototoxicity in albino guinea pigs (250-300 g). Animals were divided into five experimental groups: I, a sham control group given an intra-peritoneal (I.P.) injection of 0.5 ml saline (SHAM); II, gentamycin group (GM), treated with an injection of gentamycin (100 mg/ kg); III, gentamycin + Q-ter group (GM+Q-ter), treated with gentamycin (same dose as group II) and an I.P. injection of coenzyme Q10 terclatrate (Q-ter) at 100 mg/kg body weight; IV, injected with gentamycin (100 mg/kg) plus saline; V, treated with Q-ter alone (100 mg/ kg). All animals were treated for 14 consecutive days. Auditory function was evaluated by recording auditory brainstem responses (ABR) at 15 and 30 days from the beginning of treatment. Morphological changes were analyzed by rhodamine-phalloidine staining. Gentamycininduced progressive high-frequency hearing loss of 45-55 dB SPL. Q-ter therapy slowed and attenuated the progression of hearing loss, yielding a threshold shift of 20 dB. The significant loss of outer hair cells (OHCs) in the cochlear medio-basal turn in gentamycin-treated animals was not observed in the cochleae of animals protected with Q-ter. This study supports the hypothesis that Q-ter interferes with gentamycin-induced free radical formation, and suggests that it may be useful in protecting OHC function from aminoglycoside ototoxicity, thus reducing hearing loss.
KEY WORDS: Aminoglycoside antibiotic, Gentamycin, Ototoxicity, Antioxidant therapy, Coenzyme Q10, Outer hair cells
RIASSUNTO
È ben noto che gli antibiotici aminoglicosidi, tra cui la gentamicina, hanno una spiccata ototossicità che si verifica attraverso il legame ferro- gentamicina con l'attivazione di una cascata intracellulare e mitocondriale che porta alla produzione di radicali liberi. Gli antiossidanti come il Q-ter (forma idrosolubile del coenzima Q10), potrebbero limitare o prevenire il danno ototossico. Questo studio ha come obiettivo quello di indagare il possibile ruolo protettivo del Q-ter sull'ototossicità indotta da gentamicina. Come modello sperimentale sono state impiegate cavie albine di peso compreso tra 250-300 g. Gli animali sono stati divisi in modo casuale in cinque gruppi: gruppo I (Sham, controllo), animali trattati con una dose di soluzione fisiologia 0,5 ml somministrata per via intraperitoneale (I.P.): gruppo II GM, animali trattati con gentamicina (GM) somministrata con una iniezione intramuscolare (IM) ad una dose di 100 mg/kg; gruppo III GM+Q-ter, animali trattati con gentamicina (IM100 mg/kg) + Coenzima Q10 Terclatrato (Q-ter) ad una dose di 100 mg/kg I.P.; gruppo IV (controllo farmaco), animali trattati con gentamicina IM+ la soluzione fisiologia I.P.; gruppo V (controllo antiossidante), animali trattati con il solo Q-ter 100 mg/kg I.P. Tutti gli animali sono stati trattati per 14 giorni consecutivi. La funzione uditiva è stata valutata mediante la registrazione dei potenziali evocati uditivi del tronco dell'encefalo (ABR) a 15 e 30 giorni dall'inizio del trattamento. La tecnica di marcatura con Rodamina-Falloidina è stata impiegata per studiare le modificazioni morfologiche delle cellule. La gentamicina induce un innalzamento di soglia uditiva di circa 45-55 dB nel range delle alte frequenze che il trattamento con il Q-ter attenua di circa 20 dB. Inoltre, negli animali trattati con gentamicina si osserva, dopo 30 giorni dal trattamento, una perdita di cellule ciliate esterne che procede dalla prima alla terza fila; la prevenzione farmacologica con Q-ter previene in modo significativo la morte cellulare nel giro mediobasale della coclea. Questo studio dimostra l'ipotesi secondo cui il Q-ter è in grado di prevenire la formazione di radicali liberi indotta da gentamicina proteggendo, in questo modo, le cellule ciliate esterne dall'ototossicità indotta da amminoglicosidici e riducendone così la perdita uditiva.
Introduction
Numerous external agents, including high-intensity noise and various drugs such as aminoglycoside antibiotics, damage hair cells and/or supporting cells of the inner ear 1 2. However, the aminoglycoside antibiotic gentamycin provides benefits that often outweigh the risks: its ototoxicity is a dose-limiting side effect, and the transtympanic injection of gentamycin is widely used for the treatment of Ménière's disease 1. Although the mechanisms underlying gentamycin-induced cytotoxicity have not been understood in detail, drug-induced apoptosis in the cochlea often contributes to ototoxicity 3 4. Despite differences in the initial targeted compartments of the inner ear by the interactions of ototoxic drugs and damaging noise, these agents ultimately seem to produce similar cellular pathologies and likely have overlapping mechanisms of action, perhaps including activation of the same cellular signalling pathways that lead to cell death 5. Exposure to aminoglycosides or acoustic trauma leads to oxidative stress and production of reactive oxygen species (RO S) which trigger an apoptotic signalling pathway 4 6.
Taken together, there is substantial evidence that oxidative damage and mitochondrial dysfunction may play a key role in sensorineural hearing loss caused by aminoglycoside ototoxicity and noise 7 8.
The mitochondrial respiratory chain is a powerful source of RO S, and oxidative stress triggers the opening of the mitochondrial permeability transition pores that causes the collapse of inner mitochondrial membrane potential and release of pro-apoptotic factors. Ubiquinone 50 or coenzyme Q10, (CoQ10) is a mobile electron carrier in the mitochondrial electron transport chain (ETC) that is the major source of ATP in the mitochondria. It participates in the ETC by carrying electrons from complex I (succinateubiquinone oxidoreductase) to complex III (ubiquinonecytochrome C oxidoreductase). Within mitochondria, ubiquinone is reduced by the respiratory chain to its active ubiquinol form, which is an effective antioxidant that prevents lipid peroxidation and mitochondrial damage 9. In a previous report, we demonstrated that idebenone, a synthetic analogue of CoQ10, attenuates hearing loss in a guinea pig model of acoustic trauma by virtue of its antioxidant properties 10. It has been suggested that its efficacy depends on the ability to intercept free radicals in both aqueous phases and lipid-water interfaces 11. In fact, the mobility of the agent in membranes and lipoproteins plays a key role in determining its antioxidant activity. We have further addressed the protective role of CoQ10 in the same acoustic trauma model by comparing the efficacy of the native lypophilic CoQ10 molecule with that of a multi-composite formulation of CoQ10 with high water solubility and oral bioavailability, namely, coenzyme Q10 terclatrate (Q-ter), and we have provided functional and morphological evidence on the potential efficacy of Q-ter in preventing oxidative injuries that result from mitochondrial dysfunction 12. Q-ter consist of an outer case (an inactive pharmaceutical grade exipient), entrapping CoQ10 moieties and an aminoacid that serves as a catalyst to enable the formation of the multicomposite. This formulation has proven to be about 200 times more soluble and approximately 5 times more antioxidant capable than native CoQ10 12.
Considering that CoQ10 serves as an important cofactor in the electron transport chain 13, and serves as an important antioxidant in both mitochondria and lipid membranes, the objective of this study was to assess the efficacy of the enhanced formulation of CoQ10 (Q-ter) in the prevention or slowdown of gentamycin-induced cochlear damage.
Materials and methods
Animals
Adult Hartley albino guinea pigs (3 months old), weighing 250-300 g, with normal Preyer's reflex, were used in the study. All procedures on animal use and care were conducted in accordance with the Laboratory of Animal Care and Use Committee of the Catholic University, School of Medicine of Rome, and of the European Communities Council Directive (86/609/EEC) and were approved by the Italian Department of Health (Ministero della Salute).
Eighteen guinea pigs were randomly separated into five groups: I) sham control group undergoing intra-peritoneal (I.P.) injection with 0.5 ml saline (SHAM; n = 4); II) gentamycin group (GM; n = 4), treated with an injection of gentamycin (100 mg/kg body weight [b.w]); III) gentamycin + Q-ter group (GM+Q-ter; n = 4), treated with gentamycin (same dose as group II ) and with I.P. injection of coenzyme Q10 terclatrate (Q-ter) (100 mg/kg b.w); IV) control group injected with gentamycin plus saline (n = 3) at the same dose as group II ; V) control animals treated with Q-ter alone (n = 3) at the same dose of group III . All animals were treated daily for 14 consecutive days, the administration of Q-ter was given 1 hour before gentamycin. The animals were housed in separate cages in temperaturecontrolled rooms, with a 12-hour light-dark cycle, and had free access to commercial food and water.
Drug preparation
Gentamycin was used as a sulphate salt dissolved in saline at a dose of 127 mg per 2 ml corresponding to 40 mg/ml of gentamycin (Gentalyn® Schering Plough SpA, Milan, Italy). Q-ter is a terclatrate substance, obtained by mechano- physical activation: a solid-state procedure that brings different substances into supramolecular contact through the administration of energy, and turning a simple mixture into a multi-composite material (Applicant, Asoltech Srl, Trieste, Italy). Q-ter consists of an outer case (an inactive pharmaceutical grade excipient) that entraps CoQ10
moieties (10% w/w) and an aminoacid that serves as a catalyst to enable the formation of the multicomposite. Qter was provided by Scharper Therapeutics, Milan, Italy, and was manufactured using an industrially available native CoQ10 (Kaneka Pharma Europe, Brussels, Belgium). For the purposes of treatment, Q-ter was dissolved in saline and injected intraperitoneally at a dose of 100 mg/ kg body weight, corresponding to 10 mg/kg CoQ10. The Q-ter saline solution was prepared fresh daily as previously described 12.
Electrophysiological measurements of auditory function
Hearing function was evaluated in all animals by recording auditory brainstem responses (ABR) at low (2, 4 kHz), mid (6, 8, 12 kHz) and high (16, 20 kHz) frequencies. ABRs were measured before the first drug administration and 15 and 30 days afterwards. Animals were mildly anaesthetized (ketamine hydrochloride 12.5 mg/kg, xylazine 2.5 mg/kg and acepromazine maleate 0.75 mg/kg body weight) and placed in a sound-proof room. Three electrodes were subcutaneously inserted into the right mastoid (active), vertex (reference) and left mastoid (ground). A PC-controlled TDT System 3 (Tucker-Davis Technologies, Alachua, Florida, USA) data acquisition system with real time digital signal processing was used to generate the auditory stimulus and ABR recording. Tone bursts from 2 to 20 kHz (1 msec rise/fall time, 10 msec total duration, 20/sec repetition rate) were presented under free field conditions. Responses were filtered (0.3-3 kHz), digitized and averaged across 500 discrete samples at each frequency- level combination. Thresholds were determined by increasing the intensity of the 5 dB tone, starting at 0 dB and moving up to 100 dB, until ABR response was detected. Next, the stimulus intensity was decreased in 5 dB steps until the latency-appropriate response disappeared. The threshold value was defined as the lowest intensity able to evoke an appropriate ABR response 12.
Quantitative assessment of hair cell survival and cochleogram
After ABR testing all animals were killed by a lethal injection of anaesthetic (ketamine hydrochloride 25 mg/kg, xylazine 5 mg/kg and acepromazine maleate 1.5 mg/kg body weight). Cochleae were processed for the quantitative assessment of hair cell survival and cochleogram. The right cochleae were quickly removed from the skull and fixed in 10% buffered formalin for 4 hours. Cochleae were then dissected in 0.1 M PBS, and the organs of Corti were incubated with a solution containing 0.5% Triton X-100 and rhodamine-conjugated phalloidin (1:100 dilution; R-415, Molecular Probes) for 1 hour at room temperature and protected from light. At the end of incubation, all specimens were washed twice in PBS. Next, the specimens were mounted on slides containing an antifade medium (ProLong Gold, Invitrogen P36930). Quantification of the remaining number of hair cells and calculation of hair cell loss was done with the aid of the Leica confocal microscope (TCS-SP2; Leica Microsystem, GmbH, Wezlar, Germany). Hair cells were considered missing if the stereocilia bundles were absent in rhodamine-phalloidin- stained and/or the profiles of outer hair cells (OHCs) or inner hair cells (IHCs) were not detectable. The results were expressed as a percentage of remaining hair cells in IHC layer and the three rows of OHCs over the entire length of the cochlear duct.
Data analysis
Statistical significance of ABR and cochleogram data was calculated by analysis of variance (ABR: group × frequency × day, three-way ANOVA with repeated measures; cochleogram: group × H Cs type × cells survived along 20 mm, three-way ANOVA with repeated measures). When significant differences were found with the overall analysis, post-hoc comparisons were assessed with Tukey's test (Statistica, Statsoft, Tulsa, USA). A p value < 0.05 was considered significant.
Results
Auditory function evaluation
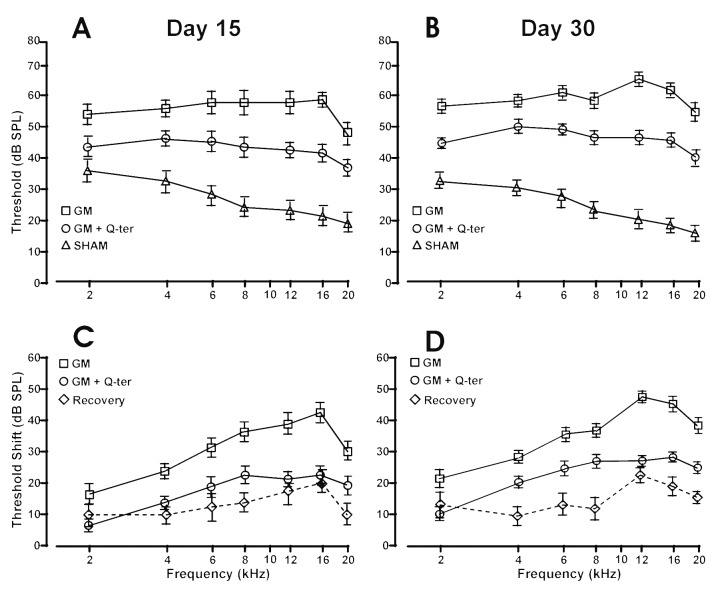
ABRs were recorded in each animal before, and at 15 and 30 days from the beginning of treatment (Fig. 1). Pretreatment baseline ABR thresholds did not differ among the five groups and were consistent with data previously obtained in our laboratory 12. The administration of Q-ter alone did not modify auditory threshold at each time point compared to group I . No differences were observed between group II and the group of animals treated with gentamycin plus saline (data not shown), and threshold values remained stable throughout the course of treatment. Consistent with data obtained previously by recording compound action potentials 14 15, gentamycin treatment in our guinea pig model (group II , GM) determined, at day 15 upon the end of treatment, the greatest hearing loss at frequencies of 6-16 kHz. The average hearing threshold shifts with standard deviation, are shown in Figure 1C. In gentamycin-treated animals, at the end of treatment (day 15), the threshold shift was about 15-25 dB at 2 and 4 kHz, 35-45 dB at 6-16 kHz and 25-30 dB at 20 kHz (Fig. 1C). At day 30, the average threshold shift further increased by about 5-10 dB at low frequencies, 5-10 dB at 6-16 kHz and 10 dB at 20 kHz (Fig. 1D).
Fig. 1.
Functional data measured with ABR. Panels A-B: ABR threshold values in dB (mean ± standard error) corresponding to the differences between the recordings measured in sham group (triangles), gentamycin group (squares) and gentamycin + Q-ter group (circles) at 15 and 30 days from the beginning treatment respectively. Panels C-D: The average hearing threshold shifts (mean±standard error) in GM group (squares) and GM+Q-ter group (circles), the dotted line shows the recovery among group GM and group GM+Q-ter at days 15 and 30 respectively.
In the animals treated with gentamycin plus Q-ter (group III ), the gentamycin-induced hearing loss was greatly attenuated. Namely, the threshold shift was about 5-15 dB at low frequencies and 15-20 at mid and high frequencies (Fig. 1C). At day 30, the trend of threshold shift increase was similar to group II (GM). However, in group III (GM+ Q-ter) the threshold shift increased by only about 5 dB for all frequencies (Fig. 1D).
A comparison between group GM and group GM+ Q-ter (Figs. 1C-D) showed that the ABR threshold shift was significantly attenuated at all frequencies in the Q-ter group (post hoc analysis p < 0.03) compared to the gentamycin group at days 15 and 30. Specifically, at day 15 (Fig. 1C) there was a difference of about 10 dB at low frequencies (2-6 kHz), 14 dB at 8 kHz, 17-20 dB at 12-16 kHz and 10 dB at 20 kHz. At day 30 (Fig. 1D), the difference between the two groups was about 8-10 dB at 2-8 kHz, 20 dB at 12-16 kHz and 15 dB at 20 kHz.
Taken together, the frequencies most affected were 12 and 16 kHz, and the ability of Q-ter to protect from gentamycin ototoxic injury reached, at day 15, about 55% of protection at 12 kHz (21.25 dB vs. 38.75 dB) and about 53% of protection at 16 kHz (22.50 dB vs. 42.50 dB). A further 6% of protection was detected at day 30 at 16 kHz, leading to a total of 59% protection (23.75 dB vs. 47.50 dB). Remarkably, Q-ter administered in parallel with the ototoxic gentamycin diminished hearing loss by about 50%; the protection was maintained for 15 days and more pronounced attenuation was observed at 16 kHz.
Hair cell loss
The functional data were paralleled by histological findings. Normal cochleograms were observed in untreated animals (control animals, data not shown) 15, and no significant differences were observed between the gentamycin group and the one treated with vehicle, suggesting that saline did not interfere with cochlear damage and protection. Missing hair cells were counted in rhodamine-phalloidin labelled surface preparations, and the percentage loss of IHC and OHC was quantitatively evaluated and expressed as the mean for each treatment group (Fig. 2).
Fig. 2.
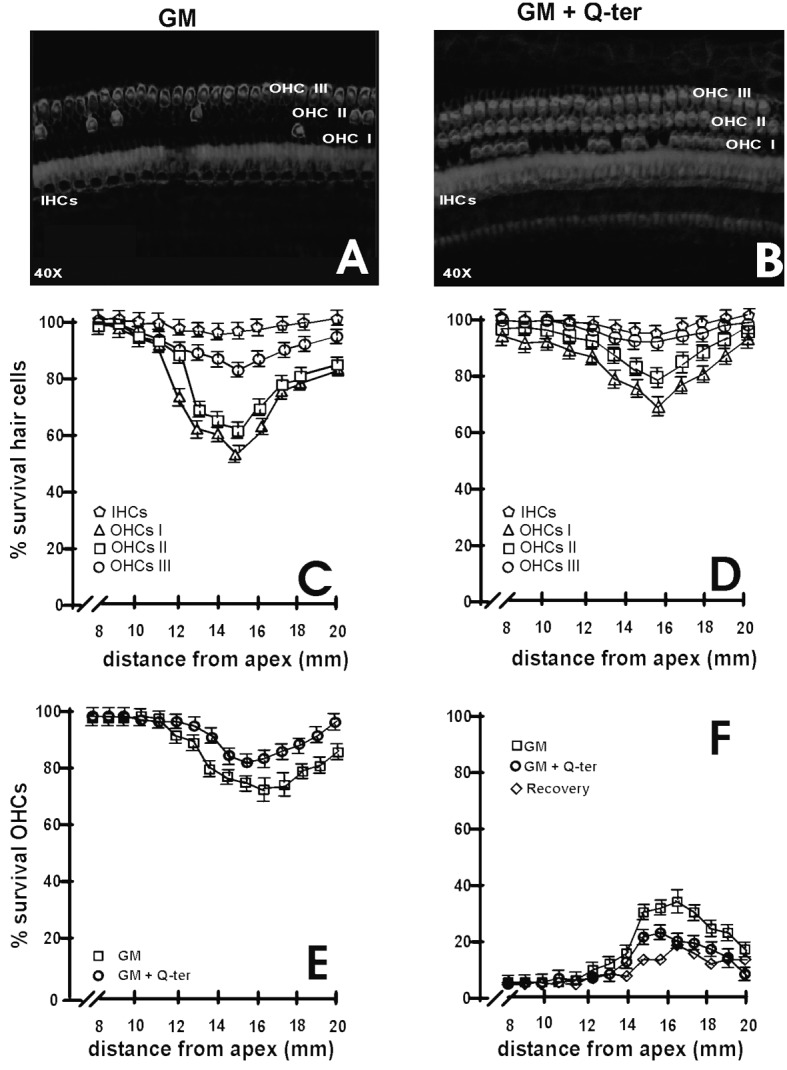
Rhodamine-phalloidin staining and cochleograms. Panels A-B: Confocal images of surface preparations of a damaged area from gentamycin and gentamycin+treatment for rhodamine-phalloidin staining. Panel A shows massive OHC loss and minor IHC disappearance, detected in the medio-basal turn of the cochlea in the group. Panel B shows moderate hair cell loss for OHCs and IHCs in gentamycin plus Q-ter treated animals. Panels C-F: cochleograms were plotted as percentage with standard deviation of survival IHCs and OHCs for each row against distance from the apex. A gradient of OHC loss was observed for gentamycin treatment (panel C) and gentamycin+Q-ter treatment (panel D). Quantitative analysis showed that gentamycin treatment caused a more consistent OHC loss in a narrow region of the cochlea from 11-19 mm from the apex. Q-ter treated animals had significantly less OHC loss than gentamycintreated animals in a narrower area of about 13-19 mm from the apex. Panel E: average survival of cells in the GM (square) and GM+Q-ter (circles) groups. Panel F shows average cell loss and recovery of damage by concomitant Q-ter treatment in group GM (squares) and group GM+Q-ter (circles) and recovery (diamonds).
In group II (GM) (Fig. 2A), massive OHC loss and minor IHC disappearance were detected in the medio-basal turn of the cochlea. In fact, the damaged region extended from approximately 11-19 mm from the apex with a decreasing pattern in the transitional area and a typical grading from the first (inner row) to the third row of OHCs. In contrast, animals receiving Q-ter treatment group III (GM+Q-ter) (Fig. 2B) had only moderate hair cell loss and narrower gentamycin-induced lesions, with cell damage visible between 13 and 19 mm from the apex. Specifically, in the first row of the basal turn of the gentamycin group (II, GM) and gentamycin plus Q-ter group (III, GM+Q-ter), the surviving stereocilia bundles were 59% and 74%, respectively; in the second row, OHCs were missing only in the gentamycin group, whereas only a few cells were lost in gentamycin plus Q-ter group (survival values of 62.5% and 80%, respectively). OHCs of the third row were occasionally affected in both groups (survival values of 84% for gentamycin vs. 95% for gentamycin plus Qter) (Figs. 2C-D). Concerning IHCs, the damage showed a trend similar to OHC loss (Figs. 2C-D). In the gentamycin- treated group, 73% of OHCs survived in the basal turn, whereas 87% of OHCs remained in the Q-ter treated group (Fig. 2E). IHC survival was no significantly different between Sham and Q-ter treated group. The recovery of damage by concomitant Q-ter treatment is illustrated in Figure 2F, where it can be seen that the area of greatest cell loss is around 16 mm from the apex in both groups.
Taken together, OHCs and IHCs in the basal turn of the cochlea were mostly affected in the gentamycin group, whereas hair cell loss extension and width decreased with concomitant administration of the antioxidant Q-ter (post hoc analysis p < 0.005).
Discussion
In this study, we investigated the protective effects of a CoQ10 analogue, namely Q-ter, against gentamycininduced ototoxicity. Our results showed that the concomitant administration of Q-ter reduced the extent of gentamycin-induced auditory impairment. The cochlear histopathological findings correlated with the functional results obtained by ABR recordings. In gentamycin- treated animals, the increase in ABR threshold shift at high and medium frequencies was consistent with a high degree (60%) of OH C loss in the basal and middle turns, as shown by the rhodamine-phalloidin hair cell count along the organ of Corti. The inner row of OHCs was mostly affected and damage to IHCs was also detected. Both functional and morphological results are consistent with our previous investigations on gentamycin toxicity 14 15 and literature data (see for review 2). In the Q-ter protected group, the reduced ABR threshold shift in the high and medium frequencies was coupled with a small degree (20%) of OH C and IH C loss in the basal and middle turns. Taken together, Qter reduces hearing loss and the extent and distribution of hair cell damage.
A first question to be discussed concerns the distribution of gentamycin toxicity in the basal-middle cochlear turns and in the OHC inner row and IHCs. The cochlea OHCs are substantially more vulnerable to insult than IHCs, whether the insult is from aminoglycoside ototoxicity 16, excessive noise 17 or presbycusis 18. Similarly, basal turn OHCs are more vulnerable than more apically located OHCs to the
same insults 19. However, by comparing gentamycin damage with noise damage, a difference in the order of affected OHC rows can be observed. Noise first damages the outermost row with grading towards the inner row; gentamycin affects the inner row with grading to the second and third outer rows 17 19 20. This histopathological data may provide an indication on the distribution of systemic gentamycin to OHCs. Recent experimental studies suggest that, in vivo, systemically administered aminoglycosides enter cochlear hair cells from the endolymph prior to inducing auditory dysfunction 21 22. As detailed by other authors 22, there are two routes by which systemically-administered aminoglycosides could enter the endolymph: first by a trans-strial trafficking route from strial capillaries to marginal cells, followed by clearance into the endolymph, and secondly by crossing the blood-labyrinth barrier into the perilymph and then into the endolymph via (a) transcytosis across the epithelial perilymph/endolymph barrier or (b) trafficking of aminoglycosides from the perilymph domain via fibrocytes to the stria vascularis, to marginal cells and clearance into
endolymph (Fig. 3, white arrow heads) 23. It is also suggested that through the endolynph, the positive endolymphatic potential drives aminoglycosides into hair cells via open mechano-transduction channels 24 25. Alternatively, aminoglycosides in the scala tympani could pass through the basilar membrane into extracellular fluids within the organ of Corti, and from there enter hair cells by traversing their basolateral membranes (Fig. 3, red arrow head). The distribution of damage, consistent with previous investigations and literature data 14 15 19 may indirectly favours this last hypothesis. However, the mechanism by which gentamycin reaches the endolymph and damages mostly the inner OHC row remains undetermined and needs further specific experimental approaches.
Fig. 3.
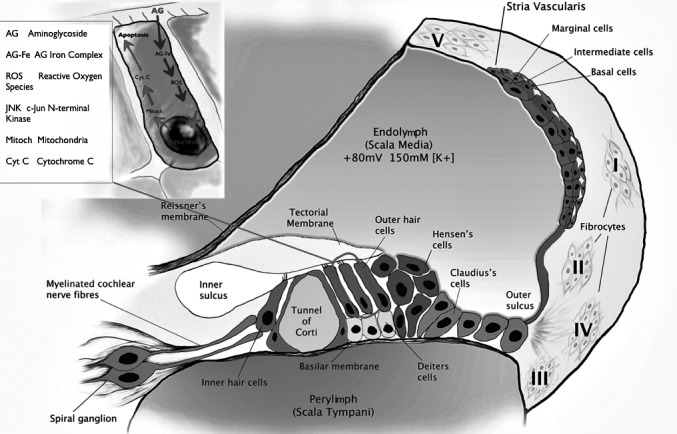
Suggested mechanisms of intracochlear uptake of gentamycin and intracellular pathways of damage. There are two routes by which systemically-administered aminoglycosides could enter the endolymph: first by a trans-strial trafficking route from strial capillaries to marginal cells, and secondly by traversing the blood-labyrinth barrier into perilymph and then within (white arrow heads). Alternatively, aminoglycosides pass to the scala tympani through the basilar membrane into extracellular fluids within the organ of Corti by traversing the basolateral membranes (red arrow head). Gentamycin enters the hair cell through mechano-electrical transducer channels; it forms a complex with iron (Fe), a transition metal. These redox active complexes generate ROS/RNS including the superoxide anion radical, hydrogen peroxide, hydroxyl radical and peroxynitrite anion. Reactive species activate JNK (c-Jun N-terminal kinase), which then translocate to the nucleus to activate genes in the cell death pathway by transfer to the mitochondria where they promote the release of cytochrome c (cyt c). In the cytosol, cyt c triggers the activation of a series of caspases followed by apoptosis (programmed cell death) via what is referred to as caspase-dependent cell death. In addition to this caspase-dependent apoptotic pathway, gentamycin may also kill cells via caspase-independent mechanisms.
The second and main point of discussion concerns the mechanism of Q-ter protection against gentamycin ototoxicity. Q-ter is a water-soluble coenzyme Q10 formulation. A therapeutic approach with native CoQ10 is limited by its poor bioavailability in aqueous media and stability problems. In contrast, the Q-ter formulation is more soluble than the native molecule, the chemical moieties of the starting materials are preserved and physicochemical properties such as stability and antioxidant capacity are improved 26 27. In a previous publication 12, we demonstrated the antioxidant efficacy of the water soluble Q-ter in a guinea pig model of acoustic trauma. Thus, considering that the mechanism of cochlear damage by noise and aminoglycosides is reported to be analogous 28, Q-ter protection against gentamycin toxicity, as described in the present paper, is not surprising (see suggested intracellular activated pathway in the inset box of Figure 3). Furthermore, the role of oxidative damage and possible mitochondrial dysfunction in gentamycin ototoxicity can be indirectly hypothesized by the efficacy of our CoQ10 formulation. CoQ10 blocks apoptosis by inhibiting activation of the mitochondrial permeability transition independently of its free radical scavenging activity 9. Another potential protective mechanism of CoQ10 concerns its role as an obligatory cofactor of mitochondrial uncoupling proteins 29 and the activation of these proteins reduces mitochondrial-free radical generation.
There is increasing interest in the utility of CoQ10 to treat neurodegenerative diseases 30. CoQ10 induces mitochondrial uncoupling in the substantia nigra of primates, and this is associated with marked neuroprotection 31. Increased expression of mitochondrial uncoupling proteins protects against brain damage associated with both experimental stroke and epilepsy 32 33. CoQ10 diminishes ischaemia- induced neuronal injury in the hippocampus 34, and protects cultured cerebellar neurons against excitotoxininduced degeneration 35. The potential efficacy of CoQ10 in the treatment of both Parkinson's and Alzheimer's disease has also been described 30.
The data in the present paper may hold additional indication on the novel therapeutic design of Q-ter and its potential use in combating oxidative damage induced by exposure to ototoxic compounds.
Acknowledgements
This research was supported by grants from the Fondi di Ateneo UCSC Rome Italy.
References
- 1.Guthrie OW. Aminoglycoside induced ototoxicity. Toxicology. 2008;249:91–96. doi: 10.1016/j.tox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011;281:28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong SW, Kim LS, Hur D, et al. Gentamycin-induced spiral ganglion cell death: apoptosis mediated by ROS and the JNK signalling pathway. Acta Otolaryngol. 2010;130:670–678. doi: 10.3109/00016480903428200. [DOI] [PubMed] [Google Scholar]
- 4.Ding D, Jiang H, Salvi RJ. Mechanisms of rapid sensory hair-cell death following co-administration of gentamycin and ethacrynic acid. Hear Res. 2010;259:16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopke R, Allen KA, Henderson D. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann N Y Acad Sci. 1999;884:171–191. doi: 10.1111/j.1749-6632.1999.tb08641.x. [DOI] [PubMed] [Google Scholar]
- 6.Priuska EM, Schacht J. Formation of free radicals by gentamycin and iron and evidence for an iron/gentamycin complex. Biochem Pharmacol. 1995;50:1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- 7.Sergi B, Fetoni AR, Ferraresi A, et al. The role of antioxidants in protection from ototoxic drugs. Acta Otolaryngol Suppl. 2004;(552):42–45. [PubMed] [Google Scholar]
- 8.Fetoni AR, Ralli M, Sergi B, et al. Protective effects of Nacetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29:70–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Papucci L, Schiavone N, Witort E, et al. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220–28228. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 10.Sergi B, Fetoni AR, Paludetti G, et al. Protective properties of idebenone in noise-induced hearing loss in the guinea pig. Neuroreport. 2006;17:857–861. doi: 10.1097/01.wnr.0000221834.18470.8c. [DOI] [PubMed] [Google Scholar]
- 11.Mordente A, Martorana GE, Minotti G, et al. Antioxidant properties of 2, 3-dimethoxy-5-methyl-6-(10-hydroxydecyl)- 1,4-benzoquinone (idebenone) Chem Res Toxicol. 1998;11:54–63. doi: 10.1021/tx970136j. [DOI] [PubMed] [Google Scholar]
- 12.Fetoni AR, Piacentini R, Fiorita A, et al. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–116. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Lenaz G, Genova ML. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563–573. doi: 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Fetoni AR, Sergi B, Scarano E, et al. Protective effects of alpha-tocopherol against gentamycin-induced Oto-vestibulo toxicity: an experimental study. Acta Otolaryngol. 2003;123:192–197. doi: 10.1080/00016480310001484. [DOI] [PubMed] [Google Scholar]
- 15.Fetoni AR, Sergi B, Ferraresi A, et al. Tocopherol protective effects on gentamycin ototoxicity: an experimental study. Int J Aud. 2004;43:166–171. doi: 10.1080/14992020400050023. [DOI] [PubMed] [Google Scholar]
- 16.Karasawa T, Steyger PS. Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr Biol (Camb) 2011;3:879–886. doi: 10.1039/c1ib00034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fetoni AR, Mancuso C, Eramo SLM, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575–1588. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Fetoni AR, Picciotti PM, Paludetti G, et al. Pathogenesis of presbycusis in animal models: a review. Exp Gerontol. 2011;46:413–425. doi: 10.1016/j.exger.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Warchol ME. Cellular mechanisms of aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2010;18:454–458. doi: 10.1097/MOO.0b013e32833e05ec. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Wang Q, Steyger PS. Acoustic trauma increases cochlear and hair cell uptake of gentamycin. PLoS One. 2011;6:e19130–e19130. doi: 10.1371/journal.pone.0019130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodyear RJ, Gale JE, Ranatunga KM, et al. Aminoglycoside- induced phosphatidylserine externalization in sensory hair cells is s regionally restricted, rapid, and reversible. J Neurosci. 2008;28:9939–9952. doi: 10.1523/JNEUROSCI.1124-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Qi, Kachelmeier A, Steyger PS. Competitive antagonism of fluorescent gentamycin uptake in the cochlea. Hear Res. 2010;268:250–259. doi: 10.1016/j.heares.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai CF, Steyger PS. A systemic gentamycin pathway across the stria vascularis. Hear Res. 2008;235:114–124. doi: 10.1016/j.heares.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada A, Bledsoe S, Jr, Schacht J. An energy-dependent step in aminoglycoside ototoxicity: prevention of gentamycin ototoxicity during reduced. J Res. 1985;19:245–251. doi: 10.1016/0378-5955(85)90144-3. [DOI] [PubMed] [Google Scholar]
- 25.Marcotti W, Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;46:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 27.Corvi Mora P, Canal T, Fortuna F, et al. An innovative technology for improving solubility and antioxidant properties of Coenzyme Q10. Oxygen Society of California Congress"Oxidants And Antioxidants In Biology", Alba. 2005 Sep 7-10; [Google Scholar]
- 28.Abi-Hachem RN, Zine A, Water TR. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Pat CNS Drug Discov. 2010;5:147–163. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]
- 29.Echtay KS, Murphy MP, Smith RA, et al. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 30.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer's and Parkinson's diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 31.Horvath TL, Diano S, Leranth C, et al. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson's disease. Endocrinology. 2003;144:2757–2760. doi: 10.1210/en.2003-0163. [DOI] [PubMed] [Google Scholar]
- 32.Mattiasson I, Lethagen S, Hillarp A. Increased sensitivity to ADP-aggregation in aspirin treated patients with recurrent ischemic stroke? Int Angiol. 2003;22:239–242. [PubMed] [Google Scholar]
- 33.Sullivan PG, Dubé C, Dorenbos K, et al. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrowski RP. Effect of coenzyme Q(10) on biochemical and morphological changes in experimental ischemia in the rat brain. Brain Res Bull. 2000;53:399–407. doi: 10.1016/s0361-9230(00)00406-8. [DOI] [PubMed] [Google Scholar]
- 35.Favit A, Nicoletti F, Scapagnini U, et al. Ubiquinone protects cultured neurons against spontaneous and excitotoxin-induced degeneration. J Cereb Blood Flow Metab. 1992;12:638–645. doi: 10.1038/jcbfm.1992.88. [DOI] [PubMed] [Google Scholar]