SUMMARY
Recurrence of differentiated thyroid cancer can often require further surgical options. Reoperations may carry significant risk of surgical complications; additionally, as the anatomy is subverted, there is the possibility of leaving residual neoplasm. In order to avoid such problems during reoperation for differentiated thyroid cancer recurrence, we have introduced the technique of preoperative ultrasound-guided tattooing localization of the lymphatic structure to be removed with a 4% solution of active charcoal. Using ultrasound guidance, the lesion is identified and 0.5-2 ml of colloidal charcoal is injected near the lesion. The extraction of the needle is accompanied by injection at constant pressure of other charcoal as to leave a trace of colouring along the path of the needle up to the skin. The preoperative injection was well tolerated in all cases. In the last 5 years, we have used this technique in 13 patients with suspected recurrence in the central compartment (all from papillary carcinomas). Postoperative ultrasound and histological examination confirmed the removal of the lesion in all patients; in one case, the lesion was a parathyroid cyst. Complications were observed in two of 13 (15.4%) cases (one transitory hypoparathyroidism, and one transitory vocal cord paresis). Considering our experience, charcoal tattoo localization can be considered a safe, low-cost technique that is extremely useful for facilitating surgical procedures, and reduces the risk of iatrogenic damage.
KEY WORDS: Charcoal tattoo, Differentiated thyroid cancer recurrence, Central compartment of the neck, Surgical complications
RIASSUNTO
Le recidive linfonodali dei carcinomi tiroidei differenziati spesso richiedono ulteriori opzioni chirurgiche. I reinterventi sono caratterizzati da un numero più elevato di complicanze; inoltre, essendo i rapporti anatomici alterati esiste la possibilità di lasciare sul campo operatorio dei residui neoplastici. Al fine di ovviare, almeno parzialmente, a queste problematiche legate ai reinterventi per recidive linfonodali di carcinomi tiroidei differenziati, abbiamo introdotto nella nostra struttura ospedaliera la tecnica della marcatura pre-operatoria, ECOguidata, con una soluzione di carbone vegetale al 4%, della lesione da rimuovere. Sotto guida ecografia, nello spazio adiacente la struttura linfatica, viene iniettata una quantità di circa 0,5-2 ml di soluzione di carbone; l'estrazione dell'ago è accompagnata da una iniezione a pressione costante di altra soluzione in modo da lasciare una traccia di colorante lungo il passaggio dell'ago fino alla cute. L'iniezione pre-operatoria è stata ben tollerata in tutti i casi. Negli ultimi 5 anni abbiamo rioperato 13 pazienti con sospetta recidiva linfonodale nel compartimento centrale (tutte derivanti da carcinomi papillari) utilizzando la tecnica della marcatura con carbone vegetale. L'esame istologico definitivo e gli esami ecografici post-operatori hanno confermato la rimozione delle lesioni sospette in tutti i pazienti; in un caso la lesione è risultata essere una cisti paratiroidea. Abbiamo registrato complicanze chirurgiche in 2 casi (15,4%); in un caso si è trattato di ipoparatiroidismo e nell'altro di paresi della corda vocale, entrambi transitori. Considerando la nostra esperienza possiamo affermare che la marcatura pre-operatoria con carbone vegetale è una tecnica sicura, di costo irrisorio ed estremamente utile al chirurgo durante i reinterventi poiché, agevolandone le manovre, permette una riduzione dei rischi di danni iatrogeni.
Introduction
The incidence of recurrence of differentiated cancer after thyroidectomy has increased following the routine employment of ultrasound (US) and thyroglobulin (Tg) determination. Among patients treated for differentiated thyroid cancer, persistent or recurrent disease occurs in 5-40% of cases 1. Treatment with radioiodine alone can determine complete elimination of a recurrent tumour in many cases. Nevertheless, up to 30% of tumours will not show 131I uptake, and these can be regarded to have a more aggressive behaviour with a poorer prognosis 2.
In these cases, surgical management of the lesions is required. Alternatively, as the impact of early detection and treatment on overall survival is highly debated, a second strategy, namely "wait and see", is sometimes adopted; however, in the majority of cases, this such a policy is refused by patients who prefer to orient themselves towards more radical therapy.
During reoperation, however, the risk of surgical complications is significantly higher 3. The surgeon may encounter problems in identification and preservation of recurrent laryngeal nerves and parathyroid glands due to fibrosis and scar tissue formation in the surgical field, with consequent alteration of normal anatomic relationships.
Another aspect to be considered during reoperation is oncologic radicality, recurrent nerves and parathyroids may be infact encased in fibrotic tissues making them indistinguishable from pathologic tissue, with the risk of leaving residual neoplasm 4.
In order to circumvent these problems during reoperation in cases of differentiated thyroid cancer recurrence, in our centre during the last few years we have used a technique (adopted from breast diagnostics) that consists in preoperative US-guided tattooing (US-tattoo) localization of the lymphatic structure to be removed.
To our knowledge only two articles have reported the technique of US-tattoo localization by means of charcoal for cervical recurrence after thyroidectomy 2 3 . Herein, we report our experience in terms of facilitating localization and surgical excision of lymphatic metastasis in level VI during reoperation for differentiated thyroid cancer.
Materials and methods
This prospective study was conducted on 13 patients (10 females and 3 males) with an average age of 42 years (range 25-70 years) who were referred to our centre (3 were initially treated in another hospital) with a suspected recurrence of differentiated thyroid cancer in the central compartment. The initial operation and pTNM classifications are shown in Table I . Preoperative evaluation of vocal fold motility and parathyroid function was normal in all patients. Radioiodine treatment was administered to 12; it was not performed in 1 patient affected by microcarcinoma with no metastatic nodes found on prophylactic neck dissection (pT1N0).
Table. I.
Patient and tumour characteristics.
| Case | Sex | Age (yr) | 1st Op | Pat | pTNM | 131I | Rec | FNA and Tg wt | 2nd Op | Node found | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 35 | Tot | Pap | pT2Nx | Yes | 26 | Yes | Lev. III, IV, VI | Yes 4+/5 L. VI 4/15 L. III,IV |
Hypo-parathyroidism |
| 2 | M | 39 | Tot | Pap | pT2Nx | Yes | 23 | Yes | Lev. VI | Yes 3+/5 L. VI |
No |
| 3 | F | 70 | Tot | Pap | pT2Nx | Yes | 28 | Yes | Lev. VI | Yes 2+/8 L. VI |
No |
| 4 | M | 31 | Tot | Pap | pT2Nx | Yes | 48 | Yes | Lev. III, IV, V, VI | Yes 2+/8 L. VI 3/30 L. III-V |
No |
| 5 | F | 26 | Tot | Pap | pT2Nx | Yes | 13 | Yes | Lev. VI | Yes 1+/6 L. VI |
No |
| 6 | F | 25 | Tot + VI | Pap | pT1N0 | No | 3 | Yes (NS) | Single node | Yes (parathyroid cyst) | No |
| 7 | F | 36 | Tot + VI | Pap | pT4N1a | Yes | 18 | Yes | Single node | Yes | No |
| 8 | F | 42 | Tot + VI | Pap | pT1N1a | Yes | 4 | Yes | Single node, fatty tissue |
Yes 1+/1 L. VI |
No |
| 9 | F | 49 | Tot + VI | Pap | pT1N0 | Yes | 22 | Yes | Group of nodes, Lev. III, IV |
Yes 1+/3 L. VI 2/23 L. III,IV |
No |
| 10 | F | 35 | Tot + VI | Pap | pT3N1a | Yes | 14 | Yes | Single node, Lev. II, III, IV |
Yes | No |
| 11 | F | 42 | Tot + VI | Pap | pT3N1a | Yes | 8 | Yes | Single node, Lev. III, IV, V |
Yes (2nd reop.) 2/14 L. III-V |
No |
| 12 | M | 65 | Tot + VI | Pap | pT3N1a | Yes | 15 | Yes (NS) | Single node | Yes | No |
| 13 | F | 46 | Tot + III + IV + VI | Pap | pT4N1b | Yes | 12 | Yes | Single node, fatty tissue |
Yes 1+/3 L. VI |
Nerve paresis |
Op, surgical operation; Pat, pathology; Pap, papillary carcinoma; Tot, total thyroidectomy, Neck dissection: II subdigastric, III supraomohyoid, IV supraclavicular, V posterior triangle, VI central compartment; 131I post operation treatment with radioiodine; Rec, time of recurrence (months); FNA fine needle aspiration biopsy; Tg washout, thyroglobulin determined in the rinse liquid of the syringe after FNA; NS, not significant.
Recurrent or suspicious lesions were discovered during follow-up by US and TSH-stimulated Tg determination; the nature of the suspicious lesions was then investigated by cytology (FNA) in association with Tg determination in the washout of fine needle aspiration (FNAB-Tg); in 2 patients, laboratory tests were not useful for diagnosis, but on the basis of US aspects surgical removal was performed. The average time of recurrence was 18 months (range 3-48 months).
In all cases, recurrent lesions were located in the central compartment (level VI); in 5 cases, there was a concomitant localization in other levels of the neck. In these patients, the nodes of lateral compartments were not marked because based on pre-operative examinations selective neck dissection was performed (starting from level II or III to level IV or V). Informed consent was obtained from all patients.
A suspension of active charcoal 40 mg/ml (4%) developed for tattooing breast cancer was used (80 mg active charcoal + 80 mg polysorbate-80 + water for injectable preparations to reach 2 ml).
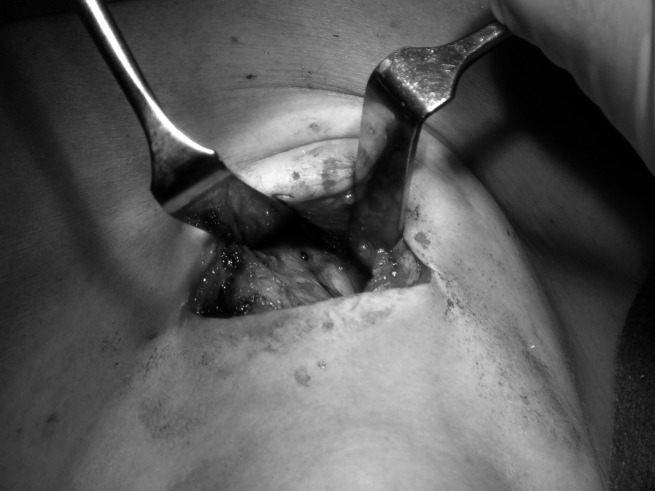
Under US guidance the lesion is identified, and after the needle is carried just near the suspected lesion (we prefer to avoid injecting inside the lesion so as not to compromise histological examination) we proceeded with injection of 0.5-2 ml of charcoal solution. The amount that must be injected depends on the depth of the lesion. In proximity of the suspected neoplasm a store of colouring, which we defined as a charcoal "puddle", takes form (Fig. 1).
Fig. 1.
Paratracheal lymph node marked with charcoal injected next to it.
The extraction of the needle is accompanied by injection at constant pressure of additional charcoal solution in order to leave a trace of colouring along the path of the needle up to the skin, where a tattoo resembling a small nevus will persist for some weeks.
This last expedient provides, during surgical intervention, a valid indication regarding the direction to follow in order to reach the suspected metastatic lesion (Fig. 2).
Fig. 2.
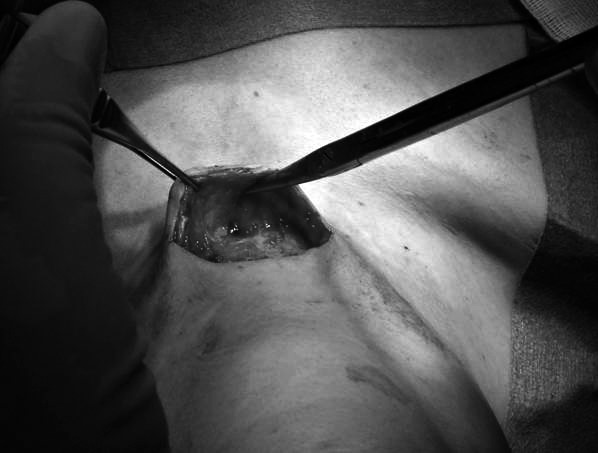
Traces of charcoal are identified since the superficial layers of the dissection providing the direction to follow in order to reach the suspected lesion.
The procedure is executed from a few days to weeks before surgery, as it has been demonstrated that charcoal remains in place for at least 3 months after injection 5 6.
Charcoal injection is performed in an outpatient clinic; after at least 20 min of observation the patient can return home. On the day of the procedure, the patient is advised to rest, avoiding bending the bust and head and raising heavy loads. In case of soreness or pain where the needle was inserted, the use of an analgesic is recommended.
Results
Preoperative injection was well tolerated in all cases; patients complained of the same slight discomfort of a FNA, experienced previously by all patients. No complications related to the procedure of US-tattooing were observed.
In the last 5 years, we have reoperated 13 patients for suspected metastatic lesion of differentiated thyroid cancer in level VI using US-tattoo localization.
Immediately before surgery, we performed US examination in the operating theatre to have a better conception of lesion localization (Fig. 3). Preoperative US was especially helpful in defining the anatomical relationships of metastatic lesions, whereas for identification in a surgical field with diffuse scar tissue the trace of charcoal was also highly useful. Suspected lesions were removed successfully in all patients, even if in 1 case we had to perform a second reoperation to localize the metastasis.
Fig. 3.
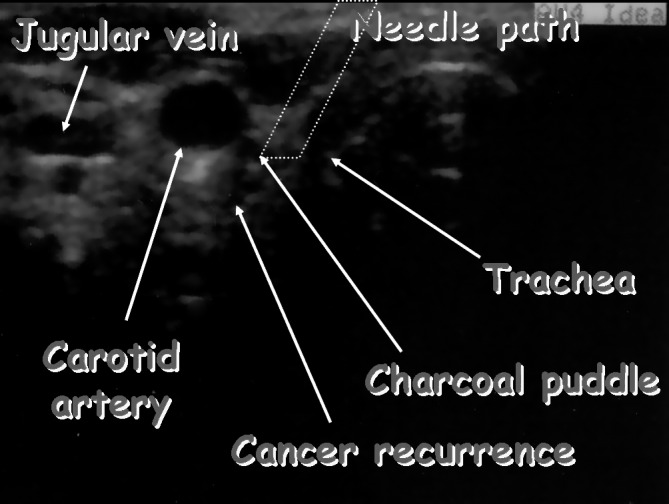
US performed just before surgery showing localization of the lymph node, its anatomical relationships, the "puddle" of charcoal and the path of the injection.
In 5 cases, the first operation was total thyroidectomy, in 7 cases thyroidectomy with dissection of level VI and in 1 case a selective neck dissection was also performed (Table I).
For patients 1-5, reoperation consisted in dissection of level VI (including the marked node) in 3 cases and associated selective neck dissection in 2 cases (Table I).
In the group of patients who firstly underwent central compartment lymphadenectomy, we considered the use of the charcoal tattoo to be important in ensuring inclusion of metastatic nodes in the dissection.
In the other patients who had already undergone a central compartment dissection during the first operation, based on intraoperative findings we removed only the charcoallocalized node, while in others also fatty or connective tissue (suspected of containing other lymphatic nodes) surrounding the targeted lesion.
In this latter group of 8 patients, the removal of metastases in level VI was associated with selective lateral neck dissection in 3 cases (# 9-11).
As previously mentioned, 1 case (# 11) required another reoperation to localize the metastatic lesion. This patient was a young woman with a metastasis at levels VI and V who therefore underwent selective lateral neck dissection (with metastasis in 2 of 14 nodes) and the removal of marked node in level VI. On this occasion, we found unusually widespread diffusion of charcoal in the surgical field; nevertheless, a node was identified and removed that histological examination revealed to be a benign lymphadenopathy. Eight months later and after another unsuccessful treatment with 131I, the patient was operated on again; during the second reoperation we found a similar amount of charcoal in the surgical field.
In this context, we found and removed a lymphatic structure that upon frozen sections was a metastatic node of papillary carcinoma; definitive histological examination confirmed diagnosis.
Following this experience, we have preferred to perform frozen sections during all reoperations.
With the exception of this case we found intraoperatively, following the trace created during the extraction of the needle, the charcoal injected localized close to the suspected lesion.
Histological examination confirmed the removal of a metastatic lesion in all cases with the exception of # 6 where the excised mass was a parathyroid with a benign cyst.
After surgery, we observed no significant complications. One patient (7.6%) had temporary hypoparathyroidism (the pathologist found a parathyroid in the histological specimen), and another (7.6%) had temporary recurrent nerve paralysis.
As mentioned above, we observed 1 (7.6%) false positive case that required a second operation due to spillage of the charcoal. Follow-up with US and Tg determination for at least 6 months confirmed the positive outcome of surgery.
Discussion
Thyroid differentiated carcinoma represents the most common endocrine tumour. Fortunately, its clinical course is favourable considering that for all subjects treated, in spite an increasing number of cases in recent years, the 10-year survival rate is 90%, and the incidence in all deaths for neoplastic diseases is only 0.5% 7.
Lymphatic metastasis of differentiated thyroid cancer is commonly considered of negligible importance for prognosis. In numerous studies, in fact, there has been no worsening of the survival rate in the N+ patients regarding those N0 (percentages of survival rates of 10 years up to 95% for the papillary carcinoma, and up to 70% for follicular carcinoma) are reported 8-12.
Moreover, another author reports that, while 56% of patients with papillary carcinoma had a clinically apparent nodal metastasis at the time of initial presentation, only 5% developed subsequent nodal metastasis 13.
Nonetheless, greater attention is being placed on "disease- free survival" considering, in spite of high survival values, the non-negligible incidence of disease recurrence (15-35% even at 20 years), with a meaningful impact on the quality of the life (due to the need for surgical reoperations and/or of elevated doses of radioiodine) 14 15.
Considering this aspect, there is contrasting data in the literature, and various opinions exist regarding the therapeutic approach in N0 cases.
No scoring system (AGES, AMES, DAMES, EORTC) attributes significant importance to lymphatic metastases in terms of prognosis, and therefore no precise indications exist 16-19.
Only the TNM stage system attributes prognostic significance to local recurrences, as classified in stage III patients > 45 years old with a N1 stage neoplasm.
Advocates of elective dissection of the central compartment justify their position by adducing a better locoregional control of disease, the possibility for correct staging, variable radioactive iodine uptake of lymphatic metastases and greater morbidity in surgical recovery; the possibility of damage of recurrent laryngeal nerve can reach 20% (ranging from 0.7 to 4.5% during first operation), and the risk of hypoparathyroidism can reach 30% (8 to 13% in primary procedures) 20 21.
In our experience, notwithstanding the small number of patients, we observed transitory hypoparathyroidism in 1 case (7.6%) and transitory vocal cord paresis another (7.6%).
Critics of systematic dissection of the central compartment assert that iatrogenic injuries are greater, postoperative metabolic therapy is an optimal therapeutic instrument and that only a minimal percentage of micrometastases can evolve into clinically-evident disease, which does not affect the survival rate 10 12 13 22.
In N0 cases, our therapeutic approach, following the indications of many authors, consists of preoperative (by US and when possible FNA) and intraoperative exploration of lymph nodes of the central compartment with dissection in case of clinical suspect 10 22.
In spite of many therapeutic opinions regarding N0 necks in differentiated thyroid cancer, reoperations in the central compartment may be necessary. Also, when a level VI level dissection is performed during first operation, in fact, radicality is very difficult to obtain, especially considering nodes situated on the edge of the mediastinal space.
When reoperative central compartment dissection is required, the morbidity rate is quite high because the surgeon may encounter significant difficulties in the identification and preservation of recurrent laryngeal nerves and parathyroid glands.
To prevent recurrent nerve injuries, it is possible to make use of intraoperative neurological monitoring and/or identification of each nerve low in the tracheo-esophageal groove distant from the thyroid bed with meticulous surgical inferior to superior dissection 4.
Revision surgery of the central compartment places the parathyroid glands at increased risk of devascularization or inadvertent removal. The most important aspect to prevent devascularization is preservation of the inferior thyroid artery. To avoid removal of glands the specimen should be carefully examined for parathyroid tissue; if identified, a biopsy should be performed for histological confirmation by frozen sections with reimplantation in sternocleidomastoid muscle.
In practice, however, fibrosis and multiple pathologic lymph nodes in the dissected specimen can make identification of parathyroid tissue difficult with the risk of inadvertently reimplanting the tumour 4.
In spite of all these procedures, reoperative central compartment dissection remains difficult even when performed by experts. In this context, US-tattoo localization represents an instrument of considerable utility.
Conclusions
Considering our experience, we can affirm that US-tattoo localization of lymphatic metastasis of differentiated thyroid carcinoma is a safe, inexpensive and extremely useful technique in facilitating surgical procedures, particularly difficult in a reoperated neck, and reducing the risk of iatrogenic damage towards parathyroid glands and recurrent laryngeal nerves.
References
- 1.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 2.Hartl DM, Chami L, Al Ghuzlan A, et al. Charcoal suspension tattoo localization for differentiated thyroid cancer recurrence. Ann Surg Oncol. 2009;16:2602–2608. doi: 10.1245/s10434-009-0572-8. [DOI] [PubMed] [Google Scholar]
- 3.Kang TW, Shin JH, Han BK, et al. Preoperative ultrasound- guided tattooing localization of recurrences after thyroidectomy: safety and effectiveness. Ann Surg Oncol. 2009;16:1655–1659. doi: 10.1245/s10434-009-0431-7. [DOI] [PubMed] [Google Scholar]
- 4.Kim MK, Mandel SH, Baloch Z, et al. Morbidity following central compartment reoperation for recurrent or persistent thyroid cancer. Arch Otolaryngol Head Neck Surg. 2004;130:1214–1216. doi: 10.1001/archotol.130.10.1214. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu MC, Bonhomme-Faivre L, Rouzier R, et al. Tattooing breast cancers treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2007;14:2233–2238. doi: 10.1245/s10434-006-9276-5. [DOI] [PubMed] [Google Scholar]
- 6.Bonhomme-Faivre L, Depraetere P, Savelli MP, et al. Charcoal suspension for tumor labelling modifies macrophage activity in mice. Life Sci. 2000;66:817–827. doi: 10.1016/s0024-3205(99)00654-2. [DOI] [PubMed] [Google Scholar]
- 7.Biffoni M, Scipioni P, Macrina N, et al. Surgical treatment of differentiated thyroid cancer recurrence. L'Endocrinologo. 2009;10:143–148. [Google Scholar]
- 8.Moley JF, Wells SA. Compartment-mediated dissection for papillary thyroid cancer. Langenbecks Arch Surg. 1999;384:9–15. doi: 10.1007/s004230050167. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren CI, Hall P, Dickman PW, et al. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106:524–531. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- 10.Shaha A. Treatment of thyroid cancer based on risk groups. J Surg Oncol. 2006;94:683–691. doi: 10.1002/jso.20697. [DOI] [PubMed] [Google Scholar]
- 11.Kupferman ME, Patterson M, Mandel SJ, et al. Patterns of lateral neck metastasis in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:857–860. doi: 10.1001/archotol.130.7.857. [DOI] [PubMed] [Google Scholar]
- 12.Hassanain M, Wexler M. Conservative management of welldifferentiated thyroid cancer. Can J Surg. 2010;53:109–118. [PMC free article] [PubMed] [Google Scholar]
- 13.Shaha AR, Shah JP, Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg. 1996;172:692–694. doi: 10.1016/s0002-9610(96)00310-8. [DOI] [PubMed] [Google Scholar]
- 14.Hay ID, Bergstralh EJ, Grant CS, et al. Impact of primary surgery on outcome in 300 patients with pathologic tumornode- metastasis stage III papillary thyroid carcinoma treated at one institution from 1940 through 1989. Surgery. 1999;126:1173–1181. doi: 10.1067/msy.2099.101435. [DOI] [PubMed] [Google Scholar]
- 15.Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18:559–567. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 16.Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990;19:545–576. [PubMed] [Google Scholar]
- 17.Cady B, Rossi R. Surgery of the thyroid and parathyroid glands. Philadelphia: W.B. Saunders Co.; 1991. [Google Scholar]
- 18.Pasieka JL, Zedenius J, Auer G, et al. Addition of nuclear DNA content to the AMES risk-group classification for papillary thyroid cancer. Surgery. 1992;112:1154–1159. [PubMed] [Google Scholar]
- 19.Betka J, Mrzena L, Astl J, et al. Surgical treatment strategy for thyroid gland carcinoma nodal metastases. Eur Arch Otorhinolaryngol. 1997;254:S169–S174. doi: 10.1007/BF02439753. [DOI] [PubMed] [Google Scholar]
- 20.Scheumann GF, Seeliger H, Musholt TJ, et al. Completion thyroidectomy in 131 patients with differentiated thyroid carcinoma. Eur J Surg. 1996;162:677–684. [PubMed] [Google Scholar]
- 21.Noguchi S, Murakami N, Kawamoto H. Classification of papillary cancer of the thyroid based on prognosis. World J Surg. 1994;18:552–557. doi: 10.1007/BF00353763. [DOI] [PubMed] [Google Scholar]
- 22.Davidson HC, Park BJ, Johnson JT. Papillary thyroid cancer: controversies in the management of neck metastasis. Laryngoscope. 2008;118:2161–2165. doi: 10.1097/MLG.0b013e31818550f6. [DOI] [PubMed] [Google Scholar]