Abstract
Depth of invasion, a quantifier of vertical growth, is a major cutaneous melanoma staging factor. Stromal penetrance requires pericellular proteolysis regulated by the serine protease and matrix metalloproteinase cascades. The serine protease inhibitor SERPINE1, a poor prognosis biomarker in various cancers, promotes tumor progression likely by titrating the extent and local of plasmin-initiated matrix remodeling. SERPINE1 in human melanoma was assessed using tissue arrays that included primary/ metastatic tumors and normal skin. SERPINE1 was basal layer-restricted in the normal epidermis. SERPINE1 immunoreactivity was evident in 27/28 primary (96%) and 24/26 metastatic tumors (92%); cutaneous metastases (80%) had significantly elevated SERPINE1 levels compared to low signals characteristic of lymph node lesions. Moderate SERPINE1 expression was a general finding in primary melanoma whereas reduced or increased SERPINE1 immunolocalization typified metastatic deposits. The amplitude of SERPINE1 expression may impact melanoma site-specific dissemination, with cutaneous metastases representing a high-SERPINE1 tumor subtype.
Keywords: Dermatopathology, Melanoma, PAI-1, SERPINE1, Metastasis
Background
Conversion of plasminogen to the broad-spectrum protease plasmin via receptor-tethered urokinase-type plasminogen activator (uPA), initiates a serine and matrix metallo-proteinase (MMP) cascade that facilitates cutaneous tumor invasion and metastases (1–3). The predominant physiologic negative regulator of this proteolytic network, the serine protease inhibitor, clade E member 1 (SERPINE1; plasminogen activator inhibitor-1) is itself, paradoxically, linked to aggressive behavior. Elevated tumor SERPINE1 levels signal a poor prognosis and reduced disease-free survival in patients with breast, lung, ovarian and oral carcinomas (1,4–7). This SERPIN maintains an angiogenic `scaffold', stabilizes nascent capillary structure and facilitates tumor stromal penetration through precise control of the proteolytic microenvironment (1,8–10) suggesting an important, perhaps stage-dependent, function in cutaneous tumor dissemination (11–13). SERPINE1 often localizes to incipient epidermal squamous cell carcinoma (SCC) tumor cells and cancer-associated myofibroblasts at the invasive front (2,14–16) likely signaling through engagement of the low-density lipoprotein receptor-related protein 1 (LRP1) to stimulate Jak/Stat1 pathway mobilization and stromal migration (17–19).
TGF-β stimulates both SERPINE1 transcription and melanoma growth/tumor progression. The SKI protein, an inhibitor of the TGF-β-initiated tumor suppression program, is up-regulated in human melanoma and promotes melanoma cell proliferation/migration in vitro as well as melanoma xenograft growth in vivo (20). SKI facilitates TGF-β-dependent c-MYC and SERPINE1 induction in human melanoma and sequesters SMADs in the cytoplasm effectively attenuating p21Waf-1 synthesis. Melanoma resistence to autocrine TGF-β growth inhibition is due to elevated SKI levels and not to defective receptor signaling (20,21). To assess the relevance of this pathway in melanoma in more detail, SERPINE1 expression was evaluated using an array panel of human primary and metastatic melanomas. The data suggest that SERPINE1 immunocytochemistry can be used to stratify patients with respect to metastatic risk and identify cutaneous metastases as a high-expression tumor subtype.
Questions addressed
To evaluate (a) SERPINE1 levels in primary and metastatic melanocytic tumors and (b) define site-associated expression variance.
Experimental design
Human Tumors
Human melanoma and normal skin high-density tissue arrays (#ME207; US Biomax) included 207 tissue samples from 69 biopsied cases (Table S1). Only cases with triplicate representative cores were used in the final data analysis.
Immunohistochemistry
Deparaffinized/rehydrated sections were immersed in 0.1M citric acid buffer (pH 6.0), treated with 0.3% H2O2 in methanol for 30 min then incubated sequentially in blocking buffer (containing 1.5% normal goat serum), rabbit anti-SERPINE1 serum (1:500) (3) and PBS-washed. Following overlay with biotinylated antibodies (Vector Laboratories), sections were washed, incubated in VectaStain Elite ABC reagent for 30 min, signal developed using diaminobenzidine tetrachloride and hematoxylin QS counterstained. Images were acquired using a NanoZoomer digital pathology system (Hamamatsu Photonics K.K.). Semiquantitative assessment of SERPINE1 immunoreactivity utilized the 'Quickscore' method (22) and following criteria: 0 (negative), 1 (weak), 2 (moderate), 3 (strong) and 4 (intense). The average H-score from three tissue cores was calculated for each case.
Results
SERPINE1 immunoreactivity was primarily restricted to the basal epithelium of the epidermis (Fig. S1). Spinous keratinocytes displayed weak, homogenous staining and the cornified layer was negative. SERPINE1 also localized to capillaries (not shown), as reported previously (3), although the surrounding stroma was negative.
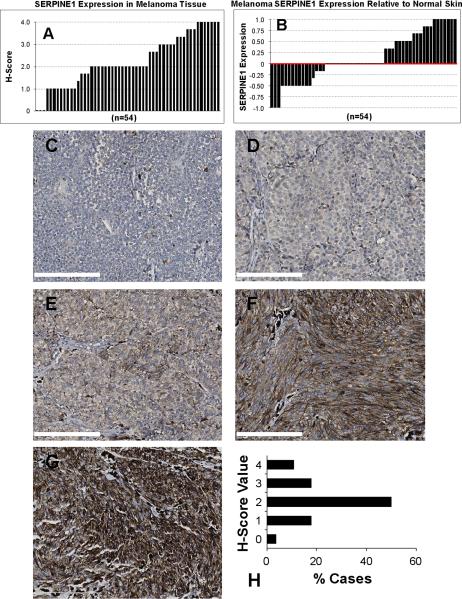
SERPINE1 was detected in 94.4% of the melanomas assayed; only 3 of 54 cases were negative with the majority (68.5%) exhibiting either reduced or increased SERPINE1 expression relative to normal skin (Fig. 1A,B). H-score analysis confirmed that staining intensity was weak in 16/54 tumors and strong in 21/54 with considerable positional variability (Fig. S2). Individual melanoma cells exhibited membranous and cytoplasmic SERPINE1 immunolocalization with additional signal observed in the stroma. Negative staining (H-score = 0) was evident in 1/28 primary tumors (Fig. 1C,H). Compared to normal skin, 5/28 cases had reduced expression (Fig. 1D,H) while the majority (14/28) of primary cutaneous melanomas exhibited moderate signal intensity similar in H-score to normal epidermis (Fig. 1E,H). Significantly elevated SERPINE1 levels were evident in 8/28 primary tumors (Fig 1F–H).
Figure 1. Overview of SERPINE1 expression in human melanoma.
SERPINE1 immunoreactivity is frequently altered in melanoma. Graph (in A) illustrates H-score variability of SERPINE1 staining intensity (range: 0 = negative to 4 = intense) among 54 melanoma samples. Data plotted (in B) depicts SERPINE1 expression in melanoma tumor tissue relative to normal skin. Representative images of negative (C), weak (D), moderate (E), strong (F) and intense (G) SERPINE1 staining of primary malignant melanoma tissue sections. Scale bar = 200 μm for all images. (H) Graphical representation of H-scores derived from SERPINE1 immunoreactivity: (0 = negative, 1 = weak, 2 = moderate, 3 = strong and 4 = intense) displayed as the percentage of cases associated with the indicated immunostaining intensities.
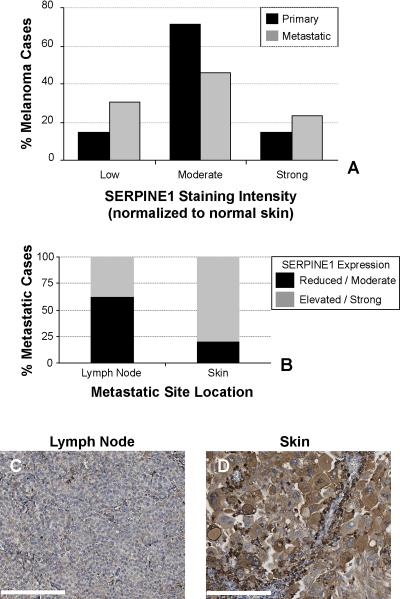
The percentage of unchanged SERPINE1 immunoreactive cases (H-score mean ± SD = 2.24±1.13) declined from 71.4% in primary malignancies to 46.2% in metastatic melanomas (Fig. 2A). Such extreme variations in SERPINE1 staining intensity suggested a need to assess metastatic deposits. Compared to primary tumors, distal metastases demonstrating reduced SERPINE1 staining (H-score = 0 – 1.11) increased 16.5%. Markedly elevated SERPINE1 expression H-scores (3.37 – 4) were also more frequently associated with metastases (14.3% primary vs. 23.1% metastatic tumors) (Figs. 2B,S2) although there was an obvious metastatic site-related variation in SERPINE1 levels. Indeed, 62% of lymph node metastasis exhibited reduced SERPINE1 immunoreactivity (H-score = 0 – 1.11) (Fig. 2B) while a majority (80%) of cutaneous metastasis partitioned to the high-expressing group (H-score = 3.37 – 4) (Fig. 2B,C).
Figure 2. Increased SERPINE1 expression correlates with melanoma metastasis.
A switch in SERPINE1 immunoreactivity is associated with the progression from primary to metastatic melanoma. Graphed (in A) is the percentage of cases associated with reduced (H-score = 0 – 1.11), unchanged (H-score = 1.12 – 3.36) and elevated (H-score = 3.37 – 4) immunostaining. Graph (in B) depicts the anatomical site distribution of metastatic melanoma tissue sections. The plot reflects the percentage of cases with weak + moderate vs strong + intense SERPINE1 staining. Representative images of SERPINE1 localization in melanoma in a negative lymph node (C) and a positive cutaneous (D) metastases. Scale bar = 200 μm for all images.
Conclusions
The main findings are: SERPINE1 (a) is frequently expressed in both primary and metastatic melanomas and (b) elevated expression characterizes cutaneous vs. lymph node metastases. Focal proteolysis at the cell surface is controlled primarily by regulation of plasmin generation with subsequent mobilization of a complex tissue remodeling cascade (2). During tumor progression, increased MMP-10 expression and its activation by catalytic-levels of plasmin, create a proteolytic axis that accelerates collagen degradation through MMP-1“superactivation” while enhancing MMP-7, -8,-9, and -13-dependent matrix proteolysis (2,3). Recent transcript profiling has, in fact, identified increased MMP1 and SERPINB3/B4 in primary melanoma (23). SERPINE1 upregulation in melanoma cells or stromal elements within the tumor microenvironment, may shift this proteolytic balance to optimize creation of a migratory “scaffold.” SERPINE1, moreover, is itself a substrate for extracellular proteases (2,24,25) and the “cleaved” molecule is unable to bind its target plasminogen activators (uPA/tPA) to limit plasmin-based proteolysis. The active, latent, plasmin- or MMP-processed forms of SERPINE1 still can interact with LRP1 to enhance cell migration into physiological scaffolds (17) and stimulate LRP1-dependent motility via engagement of Jak/Stat1 signaling (17–19,24,26). While active SERPINE1 is internalized in a complex with uPA/uPAR/LRP1, the latent and cleaved species, with preserved motile function, remain matrix-embedded likely serving as a reservoir to maintain cell movement (24). Since increased melanoma SERPINE1 expression correlates with metastases, promotes migratory/invasive behavior and has prognostic implications (24,27–30), it may be a candidate for focused therapies.
Supplementary Material
Acknowledgements
Supported by NIH grant GM57242 to PJH. RMK and PJH designed the research study, analyzed data and wrote the paper; DB, SPH and CEH performed the experiments; RMK conducted the immunohistochemical analyses; SPH and PJH developed the antibody reagents. All specimen spots on the array slides were confirmed by a pathologist. Each tissue collected was consented to by both the hospital and individual; a legal consent form was obtained and the rights to hold research uses for any purpose of further commercialized uses were waived.
Footnotes
Conflict of interests The authors declare no conflicting interests.
References
- 1.Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins-Port CE, Ye Q, Mazurkiewicz JE, Higgins PJ. TGF-β1+EGF-initiated invasive potential in transformed human keratinocytes is coupled to a plasmin/MMP-10/MMP-1-dependent collagen remodeling axis: role for PAI-1. Cancer Res. 2009;69:4081–4091. doi: 10.1158/0008-5472.CAN-09-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freytag J, Wilkins-Port CE, Higgins CE, et al. PAI-1 regulates the invasive phenotype in human cutaneous squamous cell carcinoma. J Oncol. 2009;2009:1–12. doi: 10.1155/2009/963209. article no. 963209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hundsdorfer B, Zeilhofer HF, Bock KP, Dettmar P, Schmitt M, Horch HH. The prognostic importance of urinase type plasminogen activators (uPA) and plasminogen activator inhibitors (PAI-1) in the primary resection of oral squamous cell carcinoma. Mund Kiefer Gesichtschir. 2004;8:173–179. doi: 10.1007/s10006-003-0520-x. [DOI] [PubMed] [Google Scholar]
- 5.Annecke K, Schmitt M, Euler U, et al. uPA and PAI-1 in breast cancer: review of their clinical utility and current validation in the prospective NNBC-3 trial. Adv Clin Chem. 2008;45:31–45. doi: 10.1016/s0065-2423(07)00002-9. [DOI] [PubMed] [Google Scholar]
- 6.Harbeck N, Schmitt M, Paepke S, Allgayer H, Kates RE. Tumor-associated proteolytic factors uPA and PAI-1: critical appraisal of their clinical relevance in breast cancer and their integration into decision-support algorithms. Crit Rev Clin Lab Sci. 2007;44:179–201. doi: 10.1080/10408360601040970. [DOI] [PubMed] [Google Scholar]
- 7.Vairaktaris E, Yapijakis C, Serefoglou Z, et al. Plasminogen activator inhibitor-1 polymorphism is associated with increased risk for oral cancer. Oral Oncol. 2006;42:888–992. doi: 10.1016/j.oraloncology.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Bajou K, Masson V, Gerard RD, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- 10.Higgins SP, Samarakoon R, Higgins CE, Freytag J, Wilkins-Port CE, Higgins PJ. TGF-β1-induced expression of the anti-apoptotic PAI-1 protein requires EGFR signaling. Cell Commun Insights. 2009;2:1–11. doi: 10.4137/cci.s2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajou K, Noël A, Gerard RD, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 12.Maillard C, Jost M, Rømer MU, et al. Host plasminogen activator inhibitor-1 promotes human skin carcinoma progression in a stage-dependent manner. Neoplasia. 2005;7:57–66. doi: 10.1593/neo.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacharach E, Itin A, Keshet E. Apposition-dependent induction of plasminogen activator inhibitor type 1 expression: a mechanism for balancing pericellular proteolysis during angiogenesis. Blood. 1998;92:939–945. [PubMed] [Google Scholar]
- 14.Illemann M, Hansen U, Nielsen HJ, et al. Leading-edge myofibroblasts in human colon cancer express plasminogen activator inhibitor-1. Am J Clin Pathol. 2004;122:256–265. doi: 10.1309/F32X-WQ20-T568-H8VP. [DOI] [PubMed] [Google Scholar]
- 15.Offersen BV, Nielsen BS, Høyer-Hansen G, et al. The myofibroblast is the predominant plasminogen activator inhibitor-1-expressing cell type in human breast carcinomas. Am J Pathol. 2003;163:1887–1899. doi: 10.1016/S0002-9440(10)63547-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maquerlot F, Galiacy S, Malo M, et al. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. Am J Pathol. 2006;169:1624–1632. doi: 10.2353/ajpath.2006.051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg N, Goyal N, Strawn TL, et al. Plasminogen activator inhibitor-1 and vitronectin expression level and stoichiometry regulate vascular smooth muscle cell migration through physiological collagen matrices. J Thromb Haemost. 2010;8:1847–1854. doi: 10.1111/j.1538-7836.2010.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 19.Kamikubo Y, Neels JG, Degryse B. Vitronectin inhibits plasminogen activator inhibitor-1-induced signalling and chemotaxis by blocking plasminogen activator inhibitor-1 binding to the low-density lipoprotein receptor-related protein. Int J Biochem Cell Biol. 2009;41:578–585. doi: 10.1016/j.biocel.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Lin Q, Box N, et al. SKI knockdown inhibits human melanoma tumor growth in vivo. Pigment Cell Melanoma Res. 2009;22:761–772. doi: 10.1111/j.1755-148X.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, Chen D, Timchenko NA, Medrano EE. SKI promotes Smad3 linker phosphorylations associated with the tumor-promoting trait of TGFβ. Cell Cycle. 2010;9:1684–1689. doi: 10.4161/cc.9.9.11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immune-histochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauerer A, Roesch A, Hafner C, Stempfl T, Wild P, Meyer S, Landthaler M, Vogt T. Identification of new genes associated with melanoma. Exp Dermatol. 2011;20:502–507. doi: 10.1111/j.1600-0625.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 24.Czekay R-P, Wilkins-Port CE, Higgins SP, et al. PAI-1: an integrator of cell signaling and migration. Intl J Cell Biol. 2011;2011:1–9. doi: 10.1155/2011/562481. article 562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audenaert AM, Knockaert I, Collen D, Declerck PJ. Conversion of plasminogen activator inhibitor-1 from inhibitor to substrate by point mutations in the reactive site loop. J Biol Chem. 1994;269:19559–19564. [PubMed] [Google Scholar]
- 26.Hou SX, Zheng Z, Chen X, Perrimon N. The JAK/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell. 2002;3:765–778. doi: 10.1016/s1534-5807(02)00376-3. [DOI] [PubMed] [Google Scholar]
- 27.Stabuc B, Markovic J, Bartenjev I, Vrhovec I, Medved U, Kocijancic B. Urokinase-type plasminogen activator and plasminogen activator inhibitor type 1 and type 2 in stage I malignant melanoma. Oncol Rep. 2003;10:635–639. [PubMed] [Google Scholar]
- 28.Quax PH, van Muijen GN, Weening-Verhoeff EJ, et al. Metastatic behavior of human melanoma cell lines in nude mice correlates with urokinase-type plasminogen activator, its type-1 inhibitor, and urokinase-mediated matrix degradation. J Cell Biol. 1991;115:191–199. doi: 10.1083/jcb.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks TD, Slomp J, Quax PH, et al. Antibodies to PAI-1 alter the invasive and migratory properties of human tumour cells in vitro. Clin Exp Metastasis. 2000;18:445–453. doi: 10.1023/a:1011882421528. [DOI] [PubMed] [Google Scholar]
- 30.van Muijen GNP, Danen EHJ, de Vries TJ, Quax PHA, Verhejen JH, Ruiter DJ. Properties of metastasizing and nonmetastasizing human melanoma cells. Recent Results Cancer Res. 1995;139:105–122. doi: 10.1007/978-3-642-78771-3_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.