Abstract
The aim of the study was to investigate the efficacy of the use of xylitol-containing tooth-wipes in preventing dental caries in young children. In a double-blinded randomized controlled clinical trial, 44 mothers with active caries and their 6- to 35-month-old children were randomized to xylitol-wipe or placebo-wipe groups. The children’s caries scores were recorded at baseline and 1 year. Salivary levels of mutans streptococci and lactobacilli were enumerated at baseline, 3, 6, and 12 months. Data were analyzed by intent-to-treat modeling with imputation for caries lesions and a linear mixed-effect model for bacterial levels. Significantly fewer children in the xylitol-wipe group had new caries lesions at 1 year compared with those in the placebo-wipe group (P < 0.05). No significant differences between the two groups were observed in levels of mutans streptococci and lactobacilli at all time-points. Daily xylitol-wipe application significantly reduced the caries incidence in young children as compared with wipes without xylitol, suggesting that the use of xylitol wipes may be a useful adjunct for caries control in infants (Clinicaltrials.gov registration number CT01468727).
Keywords: early childhood caries, xylitol, clinical studies/trials, mutans streptococci, lactobacilli, transmission
Introduction
Dental caries is an infectious disease, with mutans streptococci (MS) and lactobacilli (LB) as the two main cariogenic bacteria in humans (Alaluusua and Renkonen, 1983; Ramos-Gomez et al., 2002). Children with early MS colonization have a higher risk of developing caries than those with later colonization or none at all (Alaluusua and Renkonen, 1983; Tenovuo et al., 1990). Therefore, prevention or delay of MS and LB colonization may be advantageous for the prevention of early childhood caries (ECC).
Xylitol, a five-carbon non-cariogenic natural sugar alcohol, has shown potential in caries prevention in children by interfering with MS colonization. Maternal consumption of xylitol gum has been shown to reduce MS colonization and caries significantly in their children up to 10 yrs old (Söderling, 2009). Although maternal transmission is widely accepted as a major route for MS colonization in children (Berkowitz and Jordan, 1975; Davey and Rogers, 1984; Li and Caufield, 1995; Alaluusua et al., 1996), studies have shown that fathers, other caregivers, and playmates are significant alternative sources (Mattos-Graner et al., 2001; Klein et al., 2004; Domejean et al., 2010). In the presence of both maternal and non-maternal sources of MS transmission, it may be more effective to focus on the destination of the bacteria—the children—than on the various sources. Therefore, we hypothesized that direct use of xylitol products in young children is potentially a more effective regimen than maternal use to block MS transmission and to prevent caries in children.
The American Academy of Pediatric Dentistry (2008) has recommended tooth wipes as an important tool for oral hygiene care in infants and toddlers. Xylitol-wipe use has been shown to be safe and well-accepted by both parents and infants (Galganny-Almeida et al., 2007). However, there are no published studies on the effect of xylitol-wipe use on caries prevention and MS acquisition in young children.
The aim of this study was to evaluate the efficacy of daily xylitol-wipe use for 1 yr on caries and levels of MS and LB in young children, in a randomized controlled double-blinded clinical trial.
Materials & Methods
Study Population and Study Design
This was a single-center study. The Committee on Human Research at the University of California, San Francisco (UCSF) approved the study protocol. Sample-size calculation was based on Söderling’s study on the effect of maternal use of xylitol gum on MS transmission to their infants (9.7% of infants with MS at 2 yrs in the xylitol group vs. 48.5% in the fluoride-varnish group) (Söderling et al., 2000). The sample size to detect a difference at alpha (two sided = 0.05, power = 80%) with effect size of 38.8% was estimated as 22 children per group with a 10% attrition rate for the chi-square test. Children aged 6 to 35 mos were selected because a study showed that 83% of children had MS colonization by 36 mos, and that this group of children would benefit the most from tooth-wiping as an additional oral care tool (Caufield et al., 1993).
Forty-four mother-child pairs were recruited from the UCSF Pediatric Dental Clinic from January 2007 to January 2008 by flyers, referrals, and active recruitment. Approximately 80% of patients attending this clinic are of low-social-economic status. Eighty-two participants were screened, with 57 eligible and 13 refusals. Inclusion criteria were: (1) mothers with healthy children aged 6 to 35 mos; and (2) mothers who were primary caregivers (>8 hrs per day) and had at least one active caries lesion within a yr. Exclusion criteria included: (1) children who had oral or systemic diseases; and (2) mothers or children who in the previous 3 mos had taken antibiotics or other medication that would affect oral flora. In addition to informed consent, all mothers completed a questionnaire addressing infant oral-care practices, dietary habits, and demographic information. A dental examination was performed at baseline for each mother-child pair and at 1 yr for children only, by two pediatric dental residents (MN and PC). Decayed, missing, and filled tooth surfaces were recorded using the modified criteria of the World Health Organization, which includes non-cavitated caries lesions (D1 or white-spot lesions) without radiographs. Examiner MN performed all baseline examinations, and Examiner PC performed 31 out of 34 examinations at 1 yr. The two dental examiners were trained by one investigator (LZ) to score caries lesions in a standard pediatric dental setting. Cross-calibration on inter-examiner reliability was performed on seven children (15% of the study population). The two examiners showed 100% agreement on caries scoring, with Kappa = 1 (P < 0.01).
Saliva samples were collected from each mother-child pair for MS and LB enumeration. The participants were then randomized to either xylitol-wipe (Spiffies Baby Tooth WipesTM, DR Products Inc., Tucson, AZ, USA) or placebo-wipe groups based on their order of recruitment, using a pre-set computer-generated random number table based on their order of recruitment by ML after a qualification examination. The groups were blinded as Groups A and B. The placebo wipes were custom-synthesized by Dr. Products Inc. for the study and were identical in appearance and composition, except that there was no xylitol in the placebo wipe. Both wipes were grape-flavored, though the xylitol wipe had a sweeter taste than the placebo wipe. Only one investigator (JDBF), who was not involved in any patient contact, dental examinations, and microbiological assays, knew the group assignment. All the other investigators involved in participant contact, microbiological assays, and statistical analysis were blinded.
The wipes were provided to the mothers every 3 mos. Mothers were instructed to use 2 wipes to clean the teeth and gums of the children 3 times daily in addition to daily toothbrushing. The total dosage of xylitol was estimated to be 4.2 g/day for the xylitol-wipe group to achieve the therapeutic dosage as previously determined (Isokangas et al., 1988; Milgrom et al., 2006). A consultation on diet and oral hygiene care for children was given to each guardian according to the guidelines of the American Academy of Pediatric Dentistry. Mothers were called twice monthly to assess compliance with the study protocol and to answer any questions or concerns. Data on compliance and side-effects of wipe use, including allergy, gas, diarrhea, and an open question, were also collected every 3 mos at wipe-pick-up visits. Stimulated saliva samples from mothers at baseline and swab saliva samples from the children at baseline, 3 mos, 6 mos, and 1 yr were collected for MS and LB enumeration. An intend-to-treat model (ITT) was used for data analysis. Therefore, all participants were invited to return at 1 yr for saliva samples and caries examinations. One-year follow-up examinations were completed in January 2009.
Saliva Collection
Paraffin-stimulated saliva was collected from mothers at least 2 hrs after any food or beverage consumption. Oral-swab saliva samples were obtained from infants at least 1 hr after the last feeding as previously described (Khoo et al., 2005; Domejean et al., 2010). Saliva samples from the mothers and infants were transported on ice to the laboratory for bacterial culture within 24 hrs of collection.
Microbiological Assays
The saliva samples were cultured on Mitis-Salivarius-Sucrose-Bacitracin agar (DIFCO, Sparks, MD, USA) for MS and on Rogosa-Tomato-Juice Agar (DIFCO) for LB as previously described (Domejean et al., 2010). The plates were incubated anaerobically (85% N2, 5% CO2, 10% H2) at 37ºC for 72 hrs for subsequent enumeration of MS and LB colonies with the use of a dissecting microscope.
Data Analysis
The primary outcome measures of the study were percentage of children who developed new decayed surfaces at 1 yr and levels of MS and LB over time. Descriptive statistics such as means, standard deviations, and percentages were used to summarize the data by group. Baseline variables were compared between two groups with a t test, Wilcoxon rank-sum test, chi-square test, or Fisher’s exact test as appropriate.
The primary statistical analyses include ITT modeling procedures for new caries lesions at 1 yr and bacteria levels over time. Instead of assuming missing data completely at random in a generalized estimating equation (GEE) model, we imputed missing data for new caries lesions at 1 yr using the following model: P (new caries lesions at 1 yr for children who were caries-free at baseline) = 0, and P (new caries lesions at 1 yr for children who were caries-active at baseline) = 0.7. This imputation model assumes that regardless of treatment groups, 0% of children who were caries-free at baseline and 70% of children who were caries-active at baseline would possibly develop new caries lesions at 1 yr. The imputation model was developed based on our previous study, where 70% of caries-active children developed new caries lesions in 1 yr (Zhan et al., 2006). The Fisher’s exact test was used to compare the proportion of participants who developed new caries lesions, and the Wilcoxon rank-sum test was used to compare the numbers of new decayed surfaces at 1 yr between the two groups. To assess whether the result is sensitive to different assumptions about the nature of the missing data, we conducted a secondary analysis including caries data on only participants who completed the study.
For bacteria analysis, a linear mixed-effect model was used to assess differences of logMS and logLB between xylitol-wipe and placebo-wipe groups over time, and changes of logMS and logLB at different visits within each group. The linear mixed-effect model uses all available observations in the data and provides the ITT analysis with assumptions on missing data, and is a widely used standard method for continuous data with repeated measurements over time within the same study population. All data analyses were performed with SAS 9.2 software.
Results
Study Population Demographics at Baseline and Participant Follow-up
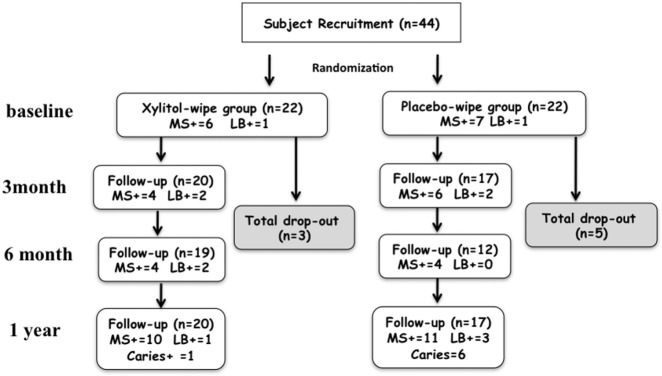
Forty-four mother-child pairs were recruited for the study (n = 22 for each group). All mothers identified themselves as primary caregivers for their children. At 1 yr, 20 individuals from the xylitol-wipe group and 17 from the placebo-wipe group completed the study (Fig. 1). Following the ITT principle, all participants were included in the analyses for comparison on caries lesions, and all available observations were used for comparison of bacterial levels between the two groups (Table 1).
Figure 1.
Flow chart of the follow-up, cariogenic bacterial infection, and caries status of the children in the study: (A) MS+ indicates numbers of children with MS infection; (B) LB+ indicates numbers of children with LB infection; (C) Caries+ indicates numbers of children with new caries lesions.
Table 1.
Participant Demographics at Baseline (mean ± SD)*
| Group | Xylitol n = 22 | Placebo n = 22 |
|---|---|---|
| Mother | ||
| logMS | 5.3 ± 0.9 | 5.4 ± 0.2 |
| logLB | 3.6 ± 2.0 | 3.7 ± 2.1 |
| DMFS** | 22.9 ± 18.3 | 19.1 ± 15.5 |
| Infant | ||
| Age (mos) | 16.7 ± 8.6 | 17.9 ± 8.6 |
| Gender (M/F) | 14:8 | 13:9 |
| Ethnicity | ||
| (White/Hispanic/Asian/other) | 2/15/2/3 | 1/12/7/2 |
| logMS | 1.25 ± 2.28 | 1.10 ± 2.00 |
| logLB | 0.14 ± 0.65 | 0.07 ± 0.34 |
| Total no. of teeth | 10.7 ± 6.0 | 12.9 ± 6.8 |
| No. of tooth surfaces | 45.1 ± 26.7 | 51.5 ± 29.3 |
| dmfs > 0 *** | 2 (9.09%) | 1 (4.55%) |
| Oral Care Questionnaire **** | ||
| Brushed teeth daily | 15 (68%) | 15 (68%) |
| Used fluoride toothpaste | 8 (36%) | 6 (27%) |
| Snacked > 3 times/day | 8 (36%) | 13 (59%) |
| Feed on demand | 9 (41%) | 12 (55%) |
| Sleep with bottle containing liquids other than water | 18 (82%) | 12 (55%) |
No statistically significant differences were found in any baseline variables among mothers and children between the xylitol-wipe and placebo-wipe groups.
DMFS stands for decayed, missing, and filled permanent tooth surfaces, excluding all permanent third molars.
dmfs stands for decayed, missing, and filled primary tooth surfaces.
All questionnaire data are presented as numbers of children (percentage in the group) for diet and oral care habits.
In the placebo-wipe group, seven mothers reported rejection of wipe-use by the children, resulting in two drop-outs. Four other children dropped from the study because of moving, loss of interest, or family difficulty. In the xylitol-wipe group, two participants dropped from the study due to moving or loss of interest. Two other participants reported non-compliance due to rejection of wipe-use by the child or family difficulty. No other side-effects related to wipe use were reported by the parents.
All mothers but one in the placebo-wipe group were infected with MS. No statistically significant differences were found in age, caries status, and levels of MS and LB for the mothers and their children between the two groups (P > 0.05). The diet and oral hygiene care data of the children are summarized in Table 1, without significant differences between the groups in any questionnaire item (chi-square or Fisher’s exact test, P > 0.05).
Caries Status at 1 Year
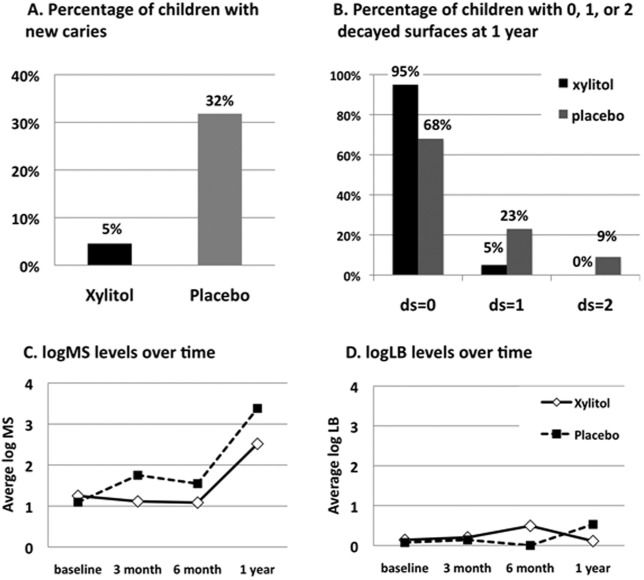
Significantly fewer children in the xylitol-wipe group developed new caries lesions at 1 yr compared with those in the placebo-wipe group (p < 0.05, Fig. 2A). Also, fewer new decayed surfaces were detected in children in the xylitol-wipe group than in the placebo-wipe group (p < 0.05, Fig. 2B).
Figure 2.
Percentage of children with new caries at 1 yr and levels of logMS and logLB in children at different visits.
Similar to the ITT results, in the secondary analysis including only participants who completed the study, fewer children (5% vs. 40%, P = 0.03, Fisher’s exact test) were detected with fewer new caries lesions at 1 yr (mean new ds ± SD as 0.05 ± 0.22 vs. 0.53 ± 0.74, p = 0.01, Wilcoxon rank-sum test) in the xylitol-wipe group than in the placebo-wipe group.
MS and LB Levels
Table 2 depicts logMS and logLB levels in the children at baseline, 3 mos, 6 mos, and 1 yr. MS levels remained stable from baseline to 6 mos in both groups, but doubled or tripled at 1 yr compared with baseline (Fig. 2C), resulting in a significant increase at 1 yr compared with previous visits in both groups (p < 0.01). LB levels remained stable with no significant differences over time within each group (Fig. 2D, P = 0.36). When MS and LB levels were compared between the two groups over time, no significant differences or any significant interaction of treatment regimens with time were detected (linear mixed-effect model, p > 0.1).
Table 2.
Mutans Streptococci and Lactobacilli Levels in the Children at the Follow-up Visits
| logMS |
logLB |
|||
|---|---|---|---|---|
| Xylitol |
Placebo |
Xylitol |
Placebo |
|
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | |
| Baseline | 1.25 ± 2.28 | 1.10 ± 2.00 | 0.14 ± 0.65 | 0.07 ± 0.34 |
| 3 mos | 1.11 ± 2.36 | 1.75 ± 2.52 | 0.20 ± 0.70 | 0.14 ± 0.39 |
| 6 mos | 1.11 ± 2.24 | 1.55 ± 2.22 | 0.49 ± 1.52 | 0 ± 0 |
| 1 yr | 2.52 ± 2.63* | 3.38 ± 2.75* | 0.11 ± 0.48 | 0.53 ± 1.21 |
Statistically significant different from the MS level at baseline, 3 mos, and 6 mos (linear mixed-effect model, p < 0.01).
Discussion
In our study, we focused on the impact of daily xylitol-wipe use on the most important outcome, reduction of new caries lesions. Our study clearly showed a significant reduction of new caries lesions in young children as a result of daily xylitol-wipe use, with 7 times fewer young children from the xylitol-wipe group developing new caries lesions compared with the placebo-wipe group. This result supports the finding of an anti-caries effect of xylitol-syrup use in infants (Milgrom et al., 2009), and indicates that direct xylitol use in infants and young children may play an important role in caries prevention.
Because maternal use of xylitol has successfully reduced MS colonization in children, we hypothesized that direct use of xylitol in young children would be more effective in preventing MS colonization from different sources. Interestingly, we did not find significant reductions of MS and LB in children treated with xylitol wipes, as compared with children in the placebo-wipe group. This result is in agreement with the results of Söderling et al. (2000), who showed no reduction of MS in mothers after xylitol-gum use for 2 yrs, although both MS transmission and caries were significantly reduced in their children. These results suggest that, instead of reducing MS levels, the anti-caries effect of direct and indirect use of xylitol may have been achieved by modifying the virulence of the cariogenic bacteria or the ecology of the oral flora to be less cariogenic or less transmissible.
In our study, MS levels in both groups remained relatively low in the first 6 mos and then increased dramatically at 1 yr. Oral MS colonization can be detected in predentate children and steadily increases after the eruption of primary teeth (Wan et al., 2001, 2003). Li and Caufield reported that MS colonization peaks between 13 and 21 mos of age and steadily increases subsequently (Li and Caufield, 1995). In our study, children were 6 to 35 mos old at baseline and 18 to 47 mos old at 1 yr. These factors may contribute to the dramatic MS increase at 1 yr and support the high risk of caries to children in this age bracket.
Acceptance by the participants is a key to success of all home-use preventive regimens. We found that xylitol wipes were better accepted by the parents and their children, with fewer study drop-outs than for the placebo-wipe group. This suggests that sweetening of wipes may be a factor in acceptance of this oral hygiene practice in infants. The poor acceptance of the non-sweetened placebo wipes could be a confounding factor. To partially address this issue, in the secondary analysis, when only individuals who completed the study were included, we found a consistent anti-caries effect of xylitol wipes over placebo wipes, similar to the results of the ITT analysis. Taken together, these results indicate a real difference in the effectiveness of xylitol wipes over placebo wipes in reducing caries.
There are a few potential limitations of the study. First, the poor acceptance of the placebo wipe limited the sample size, with the potential for unblinding the study to the final examiner and causing bias. Further studies comparing xylitol wipes with placebo wipes with another non-cariogenic sweetener will be useful in further determining the relative effect of non-compliance with the placebo wipes on the overall study. Second, we used active caries experience in mothers as a criterion for high caries risk. As a result, one mother without MS infection was recruited in the study. A combination of pre-screening for MS levels and caries experience in mothers would help to ensure the high-caries-risk status of all mothers. Finally, more extensive cross-examiner calibration may have been useful, though this is less of a concern, since one examiner performed all initial examinations, and the second examiner performed 90% of the final examinations.
Despite these potential weaknesses, the results of this study demonstrated, for the first time, a significant inhibition of new caries formation in young children by daily use of xylitol wipes. The fact that MS and LB increased in both treatment groups to the same level at 1 yr, even though there was a significant reduction in the number of new caries lesions in the xylitol-wipe group, emphasizes the importance of identifying bacterial virulence factors affected by xylitol. Future studies at the genetic level to investigate the effects of xylitol on MS cariogenic properties or oral flora will be crucial in understanding the mechanism behind xylitol’s anti-caries effects.
Footnotes
This research project was supported by the California Society of Pediatric Dentistry Foundation, a Graduate Scientific Research Award from American Academy of Pediatric Dentistry, and NIH/NIDCR grant U54 DE019285. Xylitol and placebo wipes were provided free of charge from DR Products Inc.
We declare that the research project upon which our manuscript is based (1) is original, (2) is not presently under consideration for publication elsewhere, (3) is free of conflict of interest (e.g., not edited by the funding agency or organization), and (4) was conducted according to the highest principles of studies involving human participants.
References
- Alaluusua S, Renkonen O. (1983). Streptococcus mutans establishment and dental caries experience in children from 2 to 4 years old. Scand J Dent Res 91:453-457 [DOI] [PubMed] [Google Scholar]
- Alaluusua S, Mättö J, Grönroos L, Innilä S, Torkko H, Asikainen S, et al. (1996). Oral colonization by more than one clonal type of mutans streptococcus in children with nursing-bottle dental caries. Arch Oral Biol 41:167-173 [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatric Dentistry Clinical Affairs Committee-Infant Oral Health Subcommittee; American Academy of Pediatric Dentistry Council on Clinical Affairs (2008). Guideline on infant oral health care. Pediatr Dent 30(7 Suppl):90S-93S [PubMed] [Google Scholar]
- Berkowitz RJ, Jordan HV. (1975). Similarity of bacteriocins of Streptococcus mutans from mother and infant. Arch Oral Biol 20:725-730 [DOI] [PubMed] [Google Scholar]
- Caufield PW, Cutter GR, Dasanayake AP. (1993). Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res 72:37-45 [DOI] [PubMed] [Google Scholar]
- Davey AL, Rogers AH. (1984). Multiple types of the bacterium Streptococcus mutans in the human mouth and their intra-family transmission. Arch Oral Biol 29:453-460 [DOI] [PubMed] [Google Scholar]
- Domejean S, Zhan L, DenBesten PK, Stamper J, Boyce WT, Featherstone JD. (2010). Horizontal transmission of mutans streptococci in children. J Dent Res 89:51-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galganny-Almeida A, Queiroz MC, Leite AJ. (2007). The effectiveness of a novel infant tooth wipe in high caries-risk babies 8 to15 months old. Pediatr Dent 29:337-342 [PubMed] [Google Scholar]
- Isokangas P, Alanen P, Tiekso J, Mäkinen KK. (1988). Xylitol chewing gum in caries prevention: a field study in children. J Am Dent Assoc 117:315-320 [DOI] [PubMed] [Google Scholar]
- Khoo G, Zhan L, Hoover C, Featherstone JD. (2005). Cariogenic virulence characteristics of mutans streptococci isolated from caries-active and caries-free adults. J CA Dent Assoc 33:973-980 [PubMed] [Google Scholar]
- Klein MI, Florio FM, Pereira AC, Hofling JF, Gonçalves RB. (2004). Longitudinal study of transmission, diversity, and stability of Streptococcus mutans and Streptococcus sobrinus genotypes in Brazilian nursery children. J Clin Microbiol 42:4620-4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Caufield PW. (1995). The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J Dent Res 74:681-685 [DOI] [PubMed] [Google Scholar]
- Mattos-Graner RO, Li Y, Caufield PW, Duncan M, Smith DJ. (2001). Genotypic diversity of mutans streptococci in Brazilian nursery children suggests horizontal transmission. J Clin Microbiol 39:2313-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. (2006). Mutans streptococci dose response to xylitol chewing gum. J Dent Res 85:177-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom P, Ly KA, Tut OK, Mancl L, Roberts MC, Briand K, et al. (2009). Xylitol pediatric topical oral syrup to prevent dental caries: a double-blind randomized clinical trial of efficacy. Arch Pediatr Adolesc Med 163:601-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez FJ, Weintraub JA, Gansky SA, Hoover CI, Featherstone JD. (2002). Bacterial, behavioral and environmental factors associated with early childhood caries. J Clin Pediatr Dent 26:165-173 [DOI] [PubMed] [Google Scholar]
- Söderling EM. (2009). Xylitol, mutans streptococci, and dental plaque. Adv Dent Res 21:74-78 [DOI] [PubMed] [Google Scholar]
- Söderling E, Isokangas P, Pienihakkinen K, Tenovuo J. (2000). Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants. J Dent Res 79:882-887 [DOI] [PubMed] [Google Scholar]
- Tenovuo J, Lehtonen O, Aaltonen A. (1990). Caries development in children in relation to the presence of mutans streptococci in dental plaque and serum antibodies against whole cells and protein antigen I/II of Streptococcus mutans . Caries Res 24:59-64 [DOI] [PubMed] [Google Scholar]
- Wan AK, Seow W, Purdie D, Bird P, Walsh L, Tudehope D. (2001). Association of Streptococci mutans colonization and oral developmental nodules in predentate infants. J Dent Res 80:1945-1948 [DOI] [PubMed] [Google Scholar]
- Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. (2003). A longitudinal study of Streptococcus mutans colonization in infants after tooth eruption. J Dent Res 82:504-508 [DOI] [PubMed] [Google Scholar]
- Zhan L, Featherstone JD, Gansky SA, Hoover CI, Fujino T, Berkowitz RJ, et al. (2006). Antibacterial treatment needed for severe early childhood caries. J Public Health Dent 66:174-179 [DOI] [PubMed] [Google Scholar]