Abstract
Described for the first time in 1971, Schimke immuno-osseous dysplasia (SIOD) is an autosomal-recessive multisystem disorder that is caused by bi-allelic mutations of SMARCAL1, which encodes a DNA annealing helicase. To define better the dental anomalies of SIOD, we reviewed the records from SIOD patients with identified bi-allelic SMARCAL1 mutations, and we found that 66.0% had microdontia, hypodontia, or malformed deciduous and permanent molars. Immunohistochemical analyses showed expression of SMARCAL1 in all developing teeth, raising the possibility that the malformations are cell-autonomous consequences of SMARCAL1 deficiency. We also found that stimulation of cultured skin fibroblasts from SIOD patients with the tooth morphogens WNT3A, BMP4, and TGFβ1 identified altered transcriptional responses, raising the hypothesis that the dental malformations arise in part from altered responses to developmental morphogens. To the best of our knowledge, this is the first systematic study of the dental anomalies associated with SIOD.
Keywords: SMARCAL1, tooth morphogenesis, microdontia, hypodontia, molar root hypoplasia, cell signaling
Introduction
Schimke immuno-osseous dysplasia (SIOD, OMIM 242900) is an autosomal-recessive disorder in which the prominent features are spondyloepiphyseal dysplasia, renal dysfunction, T-cell immunodeficiency, and facial dysmorphism (Schimke et al., 1971; Spranger et al., 1991; Boerkoel et al., 2000). The dysmorphic features include a triangular face, broad nasal bridge, bulbous nose tip, small palpebral fissures, dental anomalies, a short neck, hyperpigmented macules, protuberant trunk, and short limbs. Those with severe disease usually die within the first decade, whereas those with milder disease can survive into adulthood.
The only known cause of SIOD is bi-allelic mutations of the SMARCAL1 gene (Boerkoel et al., 2002). This gene encodes a protein from the sucrose non-fermenting 2 (SNF2) family that functions as a DNA annealing helicase (Yusufzai and Kadonaga, 2008). SMARCAL1 participates in the DNA stress response, the processing of stalled DNA replication forks, and gene expression (Bansbach et al., 2009; Ciccia et al., 2009; Postow et al., 2009; Yuan et al., 2009; Yusufzai et al., 2009; Baradaran-Heravi et al., 2012; Bétous et al., 2012). Alterations in gene expression correlate with disease in SIOD patients and model organisms (Baradaran-Heravi et al., 2012).
Prior studies suggest a cell-autonomous mechanism for many features of SIOD. First, mouse SMARCAL1 is expressed in all tissues analogous to those affected in SIOD (Elizondo et al., 2006). Second, SIOD-specific renal failure does not recur in the renal grafts of transplanted SIOD patients (Boerkoel et al., 2000; Clewing et al., 2007a). Third, arterial disease characteristic of SIOD does not affect the renal grafts of SIOD patients (Lücke et al., 2004; Clewing et al., 2007a). Fourth, bone marrow transplant does not prevent renal failure, and renal transplantation does not prevent arterial disease among SIOD patients (Boerkoel et al., 2000; Petty et al., 2000).
Since the formation of the teeth proceeds via a series of precisely orchestrated molecular and morphogenic events (Jernvall and Thesleff, 2000), we hypothesized that the anomalies identified in SIOD patients give insight into the role of SMARCAL1 during development. We therefore profiled the dental anomalies observed in SIOD patients, defined the expression of SMARCAL1 in the developing anlagen, and tested the consequences of SMARCAL1 deficiency on the transcriptional responses to the developmental morphogens wingless-type MMTV integration site family member 3A (WNT3A), bone morphogenetic protein 4 (BMP4), and transforming growth factor β 1 (TGFβ1).
Materials & Methods
Patients
Patients referred to this study gave informed consent to a protocol approved by the Institutional Review Board of Baylor College of Medicine (Houston, TX, USA), the Hospital for Sick Children (Toronto, ON, Canada), or the University of British Columbia (Vancouver, BC, Canada). Autopsy tissues were obtained according to the protocol approved by the University of British Columbia (Vancouver, BC, Canada). The clinical data were obtained from the referring physician. Patients were grouped according to disease severity as previously described (Clewing et al., 2007b).
Immunohistochemistry
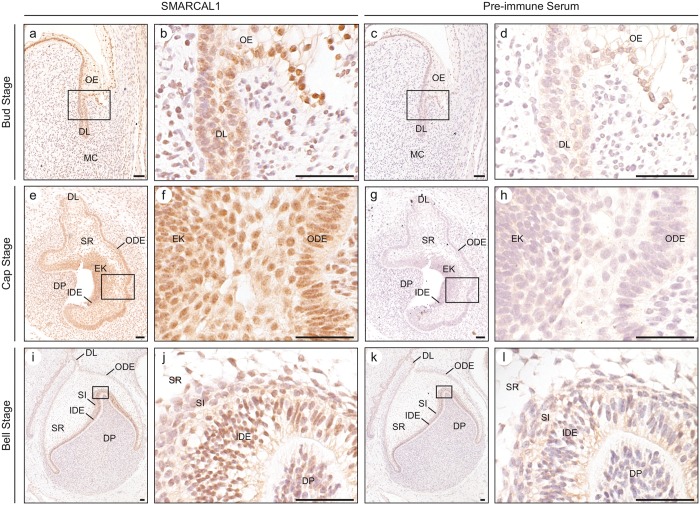
Heat-induced epitope retrieval was conducted with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6). Endogenous peroxidases were inactivated with 3% H2O2 for 30 min. Sections were first blocked with blocking buffer [20% normal goat serum, 10% bovine serum albumin, casein (SP-5020, Vector Laboratories, Burlington, ON, Canada), 0.2% Triton X-100, 1X PBS, pH 7.4], and then incubated with rabbit anti-SMARCAL1 serum (1:200) (Kilic et al., 2005) diluted in blocking buffer, each overnight at 4°C. The sections were washed and then incubated with biotinylated anti-rabbit IgG (1:200, BA-1000, Vector Laboratories). Then sections were washed and incubated with avidin biotinylated horseradish peroxidase complex (PK-6100, Vector Laboratories). Immune complexes were visualized with 3,3′-diaminobenzidine (Dako, Mississauga, ON, Canada), and sections were counterstained with Mayer’s Hematoxylin (Sigma, Oakville, ON, Canada). The staining of adjacent sections with pre-immune serum was used to confirm antiserum specificity (Figs. 2c, 2d, 2g, 2h, 2k, and 2l).
Figure 2.
Analysis of SMARCAL1 protein expression during tooth morphogenesis. (a, b) Photomicrographs of SMARCAL1 immunohistochemical staining of the bud stage of tooth development. SMARCAL1 is expressed in the cells of the oral epithelium, dental lamina, and the mesenchymal cells, which give rise to the dental papilla. (c, d) Photomicrographs of pre-immune staining of the bud stage of tooth development. The cells of the oral epithelium, dental lamina, and mesenchymal cells showed minimal non-specific staining. (e, f) Photomicrographs of SMARCAL1 immunohistochemical staining of the cap stage of tooth development. SMARCAL1 is expressed in the cells of the dental lamina, outer dental epithelium, stellate reticulum, inner dental epithelium, primary enamel knot, and dental papilla. (g, h) Photomicrographs of pre-immune staining of the cap stage of tooth development. The cells of the dental lamina, outer dental epithelium, stellate reticulum, inner dental epithelium, primary enamel knot, and dental papilla did not show non-specific staining. (i, j) Photomicrographs of SMARCAL1 immunohistochemical staining of the bell stage of tooth development. SMARCAL1 is expressed in the cells of the outer dental epithelium, stellate reticulum, stratum intermedium, inner dental epithelium, and dental papilla. (k, l) Photomicrographs of pre-immune staining of the bell stage of tooth development treated with pre-immune rabbit serum. The cells of the dental lamina, outer dental epithelium, stellate reticulum, stratum intermedium, inner dental epithelium, and dental papilla showed minimal non-specific staining. The boxed regions correspond to the higher-magnification images. Abbreviations: DL, dental lamina; DP, dental papilla; EK, primary enamel knot; IDE, inner dental epithelium; MC, mesenchymal cells; ODE, outer dental epithelium; OE, oral epithelium; SI, stratum intermedium; SR, stellate reticulum. Scale bars: 50 µm.
Morphogen Induction of Patient Dermal Fibroblasts
Forty-eight hrs prior to WNT3A, BMP4, or TGFβ1 treatment, 5 × 104 cells were seeded in each well of a 24-well plate. Twenty-four hrs prior to morphogen addition, growth media were replaced with serum-free media. Cells were treated with 100 ng/mL WNT3A, 50 ng/mL BMP4, or 4 ng/mL TGFβ1 (R&D Systems, Minneapolis, MN, USA) for 0, 2, 4, 8, 12, 16, 20, or 24 hrs. For each time-point and treatment, 3 parallel cultures were analyzed for each cell line.
RNA Isolation and Reverse Transcription
RNA was extracted from cells with the the RNeasy 96 Kit (Qiagen, Toronto, ON, Canada), and on-column DNase I digestion (Qiagen) was performed to remove genomic DNA. Reverse transcription was performed with the qScrip™ cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD, USA) according to the manufacturer’s specifications.
Quantitative PCR
SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories, Mississauga, ON, Canada) was used with the ABI StepOnePlus™ Real-Time PCR System. Expression of the housekeeping gene GAPDH was used as the internal control. The primer sequences used in this study are listed in Appendix Table 1.
Statistics
Graphed quantitative data are presented as mean ± standard deviation of a minimum of 3 independent replicates. The relative quantification of gene expression was calculated by the 2–ΔΔCt method (Livak and Schmittgen, 2001). The ΔCt of the unaffected control cell line was used as the calibrator for relative basal gene expression, and the ΔCt of the cell line of interest at time 0 hrs was used as the calibrator for relative gene expression over time. Standard deviations were calculated from the triplicate samples at each time-point after the 2–ΔΔCt transformations were performed. Data were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey post hoc test for multiple comparisons between cell lines or time-points, with SPSS Statistics (version 20, IBM). A p-value of less than 0.05 was considered statistically significant.
Results
Developmental Tooth Anomalies Are a Common Feature of SIOD
Among SIOD patients with identified bi-allelic SMARCAL1 mutations, 66.0% of patients had dental anomalies. For those patients for whom records were obtained, 46.8% had microdontia and 52.3% had hypodontia (Table and Appendix Table 2). The number of missing teeth ranged from 15 to 0, and the premolars were most frequently absent (Appendix Table 2).
Table.
Summary of Dental Findings in SIOD Patients with Bi-allelic SMARCAL1 Mutations
| Affected Individuals/Total Reported (Percentage) |
||||
|---|---|---|---|---|
| Disease Severity Scorea | Microdontia | Hypodontia | Molar Root Hypoplasia | Other (Frequency) |
| 1 | 0/2 | 0/2 | 0/1 | None |
| (0%) | (0%) | (0%) | ||
| 2 | 0/2 | 1/2 | 0/1 | Retained deciduous molar (1) |
| (0%) | (50%) | (0%) | ||
| 3 | 3/8 | 4/8 | 3/5 | Increased caries (1) |
| (38%) | (50%) | (60%) | Missing permanent premolar (1) | |
| Retained deciduous molar (1) | ||||
| 4 | 7/16 | 7/14 | 6/6 | Abnormal enamel (2) |
| (44%) | (50%) | (100%) | Discoloration (1) | |
| 5 | 5/9 | 6/9 | 5/8 | Increased caries (2) |
| (56%) | (67%) | (63%) | Abnormal enamel (1) | |
| Abnormal dentin (1) | ||||
| Discoloration (1) | ||||
| 6 | 7/9 | 3/7 | 2/2 | Delayed dentition (1) |
| (78%) | (43%) | (100%) | Increased caries (1) | |
| Abnormal dentin (1) | ||||
| Abnormal enamel (1) | ||||
| 7 | 0/1 | 2/2 | 2/2 | Abnormal superior incisors (1) |
| (0%) | (100%) | (100%) | Delayed dentition (1) | |
Patients were grouped according to disease severity as described (Clewing et al., 2007b).
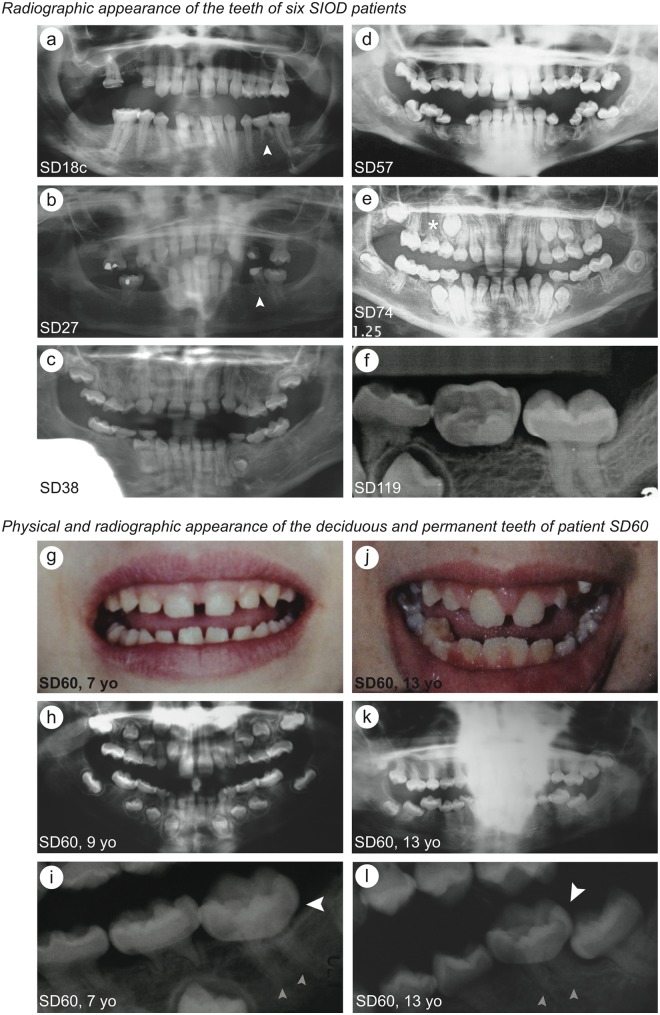
Besides small or absent teeth, 72.0% of SIOD patients had molar root hypoplasia (Appendix Table 2). The disproportion between the molar crown and root ranged from severe in SD38, SD57, SD60, SD74 and SD119 to nearly normal in SD18c (Figs. 1a-1f). The permanent premolars and first molars were commonly malformed, while the incisors and canines were usually normally shaped (Figs. 1a-1l, Appendix Fig. 1).
Figure 1.
Photographs and dental x-rays showing the dental pathology of patients with identified bi-allelic SMARCAL1 mutations. (a-f) Radiographic appearance of the teeth of six SIOD patients. The small white arrows indicate a retained deciduous molar in SD18c (a) and SD27 (b); the white asterisk indicates a missing permanent premolar in SD74 (e). (g-i) Physical (g) and radiographic (h and i) appearance of the deciduous teeth of patient SD60. (j-l) Physical (j) and radiographic (k and l) appearance of the permanent teeth of patient SD60. Note that the microdontia, thin molar roots, and bulbous molar crowns are evident in both the deciduous and permanent teeth. The large white arrows indicate the bulbous molar crowns in SD60 at 7 yrs of age (i) and 13 yrs of age (l); the small grey arrows indicate the thin molar roots in SD60 at 7 yrs of age (i) and 13 yrs of age (l). Abbreviation: yo, years old.
For the one patient (SD60) for whom we received photographs and radiographs at multiple developmental ages, both the deciduous and permanent dentitions were affected (Figs. 1g-1l). As shown for SD60, most had teeth of normal color and opacity (Figs. 1g-1l).
SMARCAL1 Is Highly Expressed in the Developing Human Tooth
To determine if SMARCAL1 was expressed in the tooth anlagen, we obtained post mortem tissue from 59-day-, 98-day-, and 105-day-gestation fetuses and assessed expression by immunohistochemistry. SMARCAL1 was expressed in all cell types throughout the bud, cap, and bell stages (Figs. 2a, 2b, 2e, 2f, 2i, and 2j; Appendix Table 3). Compared with the oral epithelium, SMARCAL1 expression was moderate to strong in the outer and inner dental epithelia and primary enamel knot, moderate in the stellate reticulum, and weak in the dental papilla and dental lamina (Figs. 2a, 2b, 2e, 2f, 2i, and 2j; Appendix Table 3).
Observing that the premolars and molars were generally more affected than the anterior teeth, we hypothesized that SMARCAL1 was not expressed in the anterior teeth. However immunohistochemical analysis of post mortem tissue from a 98-day-gestation fetus showed that SMARCAL1 was strongly expressed in the incisor, canine, and premolar anlagen as well as in the tooth bud of the permanent premolar (Appendix Fig. 2).
SIOD Tooth Anomalies Are Distinct from Other Disorders of DNA Repair
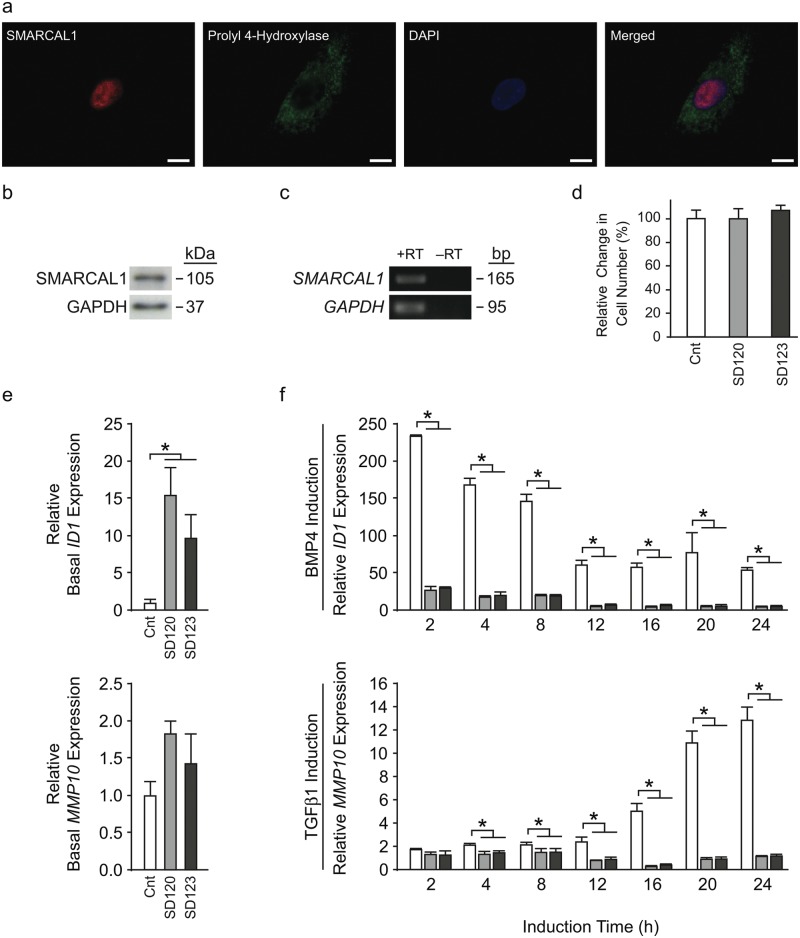
The known function of SMARCAL1 in DNA repair and replication suggests that SMARCAL1 deficiency in the proliferating tooth could lead to cell death or reduced proliferation. To test this hypothesis, we checked the viability and proliferation of dermal fibroblasts cultured from two SIOD patients. Dermal fibroblasts expressed SMARCAL1 mRNA and protein (Figs. 3a-3c), and fibroblasts from SD120 and SD123 exhibited viability and proliferation rates similar to those from an unaffected control (Fig. 3d). Additionally, we profiled the reported dental features of DNA repair and genomic instability disorders. Although microdontia, hypodontia, and short molar roots have been reported for Rothmund-Thomson syndrome, Fanconi anemia, Seckel syndrome, and dyskeratosis congenita (Appendix Table 4), those teeth are distinct from those of SIOD. Together, these observations suggested to us that the dental anomalies observed in SIOD might not arise predominantly from impaired DNA repair or slowing of the cell cycle.
Figure 3.
Expression of SMARCAL1 in cultured human dermal fibroblasts and dysregulated transcriptional responses of SIOD patient dermal fibroblasts upon stimulation with BMP4 or TGFβ1. (a) Photomicrographs showing immunofluorescence of SMARCAL1 (red), fibroblast marker prolyl 4-hydroxylase (green), and DAPI (blue) in cultured human dermal fibroblasts. (b) Photograph of an immunoblot showing expression of SMARCAL1 protein in cultured human dermal fibroblasts. (c) Photograph of an agarose gel of RT-PCR products showing expression of SMARCAL1 mRNA in human dermal fibroblasts (+RT). A ‘no reverse transcription’ negative control (–RT) shows that there is no detectable genomic DNA contamination. (d) The relative cell viability and proliferation rates for SD120 and SD123 patient fibroblast cell lines are graphed relative to unaffected control fibroblasts. (e) The relative basal gene expression levels of fibroblasts from an unaffected control (white bars) and patients SD120 (light grey bars) and SD123 (dark grey bars) were measured by qRT-PCR. Expression of housekeeping gene GAPDH was used as the internal control; expression of each gene was first normalized to GAPDH expression and then graphed relative to the expression of the unaffected control. Error bars represent one standard deviation. * = p < 0.05. (f) The transcriptional responses of fibroblasts from an unaffected control (white bars) and patients SD120 (light grey bars) and SD123 (dark grey bars) were measured by qRT-PCR following induction with the indicated morphogens for 0, 2, 4, 8, 12, 16, 20, or 24 hrs. Expression of housekeeping gene GAPDH was used as the internal control; expression of each gene was first normalized to GAPDH expression and then graphed relative to its expression in the relevant cell line at time = 0 hrs. Error bars represent one standard deviation. Abbreviations: bp, base pairs, Cnt, control; DAPI, 4′6-diamidino-2-phenylindole; h, hours; kDa, kilodalton; RT, reverse transcription. * = p < 0.05. Scale bars = 10 µm.
WNT3A, BMP4, and TGFβ1 Signaling Is Altered in Cultured SIOD Fibroblasts
Since SMARCAL1 interacts with transcriptionally active chromatin and modulates gene expression (Baradaran-Heravi et al., 2012), and is expressed in major signaling centers coordinating tooth development (Jernvall and Thesleff, 2000), we hypothesized that SMARCAL1 deficiency alters transcriptional responses to morphogens acting on or secreted by these centers. However, we do not have dental cells derived from SIOD patients, and knockdown of SMARCAL1 does not readily recapitulate the features of SIOD (Baradaran-Heravi et al., 2012); therefore, in an initial attempt to address this question, we asked whether SMARCAL1 deficiency cell-autonomously altered transcriptional responses to 3 morphogens involved in tooth formation in mice (Vainio et al., 1993; Unda et al., 2001; Plikus et al., 2005; Hosoya et al., 2008; Liu et al., 2008; Ahn et al., 2010) and for which transcriptional responses have been defined in dermal fibroblasts: WNT3A, BMP4, and TGFβ1 (Appendix Table 5). By qRT-PCR, morphogen treatment induced expression of all target genes analyzed in the unaffected control fibroblasts (Figs. 3e, 3f, Appendix Fig. 3), and SMARCAL1 deficiency altered the basal expression and induction of several targets (Figs. 3e, 3f, Appendix Figs. 3, 4, Appendix Tables 6, 7). Compared with control, BMP4 and TGFβ1 did not induce expression of ID1 and MMP10, respectively (Fig. 3f, Appendix Table 7). Less dramatically, but nonetheless significant, induction of PRDM6 expression by WNT3A was less than observed in the control, and induction of SMAD6 and SMAD7 expression by TGFβ1 was premature compared with that in the control (Appendix Fig. 4, Appendix Table 7).
Discussion
This first comprehensive review of the dental anomalies in SIOD shows that 66.0% of patients with bi-allelic mutations of SMARCAL1 have tooth anomalies and demonstrates that the SMARCAL1 protein is highly expressed in the developing human tooth. Furthermore, as a potential explanation for the cell-autonomous nature of the pathology, this study shows that SMARCAL1 deficiency significantly altered some gene expression responses to the tooth morphogens WNT3A, BMP4, and TGFβ1 in cultured SIOD dermal fibroblasts.
As suggested by da Fonseca (2000), the dental phenotype in SIOD resembles that of dentinogenesis imperfecta (DI) type II, which is characterized by opalescent or translucent teeth with discoloration, increased attrition, short constricted roots, and obliteration of the pulp chambers (Shields et al., 1973). Also, like SIOD, DI type II affects both the deciduous and permanent dentition (Sclare, 1948). However, unlike DI type II, SIOD teeth infrequently have discoloration, enamel hypoplasia, and soft dentin, and the teeth of all SIOD patients reported herein had normal opacity. To the best of our knowledge; therefore, the dental phenotype of SIOD is unique.
The expression of SMARCAL1 in the outer and inner dental epithelia and primary enamel knot suggests that its deficiency could cell-autonomously cause the root and crown malformations of SIOD. Within the developing mouse tooth root, the outer and inner dental epithelia extend apically to give rise to the cervical loop and ultimately to Hertwig’s epithelial root sheath, which contributes to root development by inducing differentiation of mesenchymal cells into odontoblasts and cementoblasts (Zeichner-David et al., 2003). Within the developing crown, primary and secondary enamel knots regulate the size and shape of the crown (Jernvall and Thesleff, 2000).
In addition to its role in DNA repair and replication, SMARCAL1 modulates gene expression (Baradaran-Heravi et al., 2012). Although the mechanism by which it does this is unknown, its annealing helicase activity might modulate the DNA architecture of gene promoters. In bacteria, promoter superhelicity is a major regulator of basal and inductive transcription (Pruss and Drlica, 1989; Hatfield and Benham, 2002). According to this model, deficiency of SMARCAL1 alters the helicity of DNA in regulatory regions and promoters, and this inappropriately impedes or fosters the binding of transcription factors regulating responses to stimuli such as morphogens.
In this model, the tooth malformations observed in SIOD would arise by cell-autonomous alterations in the transcriptional responses to dental morphogens such as WNT3A, TGFβ1, and BMP4. BMP4 is expressed in pre-odontoblasts adjacent to the root sheath epithelium (Yamashiro et al., 2003); TGFβ1 and WNT3A are expressed by the cervical loop (Vaahtokari et al., 1991; Suomalainen and Thesleff, 2010); and BMP4 and TGFβ1 are expressed by the enamel knot (Vaahtokari et al., 1991; Åberg et al., 2004). WNT signaling regulates tooth number, size, and shape (Liu et al., 2008; Ahn et al., 2010). BMP4 mediates inductive epithelial-mesenchymal interactions (Vainio et al., 1993), regulates the formation of enamel knots (Thesleff et al., 2001) as well as Hertwig’s epithelial root sheath (Hosoya et al., 2008), and modulates tooth number, size, and shape (Plikus et al., 2005). TGFβ1 also modulates odontoblast differentiation (Unda et al., 2001). Substantiation of this model, however, requires extensive additional studies.
A finding not explained by this model is why SMARCAL1 deficiency affects molars more severely than anterior teeth. One possible speculation is that the more complex development of molars, which require the induction of secondary and tertiary enamel knots (Jernvall and Thesleff, 2000; Luukko et al., 2003), renders the molar more susceptible to the consequences of SMARCAL1 deficiency on transcriptional responses to tooth morphogens. Alternatively, SMARCAL1 deficiency may not affect the expression of required genes in the developing anterior teeth as much as it does in the developing molar root. Future studies will test these speculations and define the differential dependence of developing molars on SMARCAL1 function.
Furthermore, the failure of BMP4 and TGFβ1 to appropriately induce expression of some target genes in SIOD patient–derived dermal fibroblasts is consistent with other observations of SIOD. First, supplemental growth hormone fails to improve growth rate and stature for 93% of SIOD patients (C.F.B., unpublished observations; Boerkoel et al., 2000). Second, 40-50% of patients have a decreased response to thyroid-stimulating hormone and require levothyroxine supplementation (Boerkoel et al., 2000). Third, the bone marrow failure and anemia associated with SIOD frequently do not respond to treatment with stem cell factor and erythropoietin, respectively (Boerkoel et al., 2000).
In summary, our findings show that dental anomalies are common among SIOD patients and that SMARCAL1 is highly expressed in the developing tooth. Furthermore, the finding that SMARCAL1 deficiency alters transcriptional responses to morphogens in cultured fibroblasts suggests a mechanism for the dental pathology of SIOD. These observations also provide a model for understanding how SMARCAL1 deficiency could give rise to other malformations characteristic of SIOD.
Supplementary Material
Acknowledgments
We are grateful to the patients and family members who have contributed to this study. We thank Dr. Donald Shuen for critical review of this manuscript.
Footnotes
This work was supported by the March of Dimes (6-FY02-136 to C.F.B.), the Gillson Longenbaugh Foundation (C.F.B.), the Dana Foundation (C.F.B.) and the New Development Award, Microscopy, and Administrative Cores of the Mental Retardation and Developmental Disabilities Research Center at Baylor College of Medicine (C.F.B.), the Burroughs Wellcome Foundation (Grant 1003400 to C.F.B.), the National Institute of Diabetes, Digestive, and Kidney Diseases, National Institutes of Health (R03 DK062174 and R21 DK065725 to C.F.B.), New Investigator Award from the SickKids Foundation – Canadian Institutes of Health Research Institute of Human Development, Child and Youth Health (C.F.B.), the Association Autour D’Emeric et D’Anthony (C.F.B.), and The Little Giants Foundation (C.F.B.).
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Åberg T, Wang XP, Kim JH, Yamashiro T, Bei M, Rice R, et al. (2004). Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol 270:76-93 [DOI] [PubMed] [Google Scholar]
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. (2010). Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development 137:3221-3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansbach CE, Betous R, Lovejoy CA, Glick GG, Cortez D. (2009). The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev 23:2405-2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran-Heravi A, Cho KS, Tolhuis B, Sanyal M, Morozova O, Morimoto M, et al. (2012). Penetrance of bi-allelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression. Hum Mol Genet [Epub ahead of print 3/13/2012] (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bétous R, Mason AC, Rambo RP, Bansbach CE, Badu-Nkansah A, Sirbu BM, et al. (2012). SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev 26:151-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerkoel CF, O’Neill S, Andre JL, Benke PJ, Bogdanovic R, Bulla M, et al. (2000). Manifestations and treatment of Schimke immuno-osseous dysplasia: 14 new cases and a review of the literature. Eur J Pediatr 159:1-7 [DOI] [PubMed] [Google Scholar]
- Boerkoel CF, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, et al. (2002). Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat Genet 30:215-220 [DOI] [PubMed] [Google Scholar]
- Ciccia A, Bredemeyer AL, Sowa ME, Terret ME, Jallepalli PV, Harper JW, et al. (2009). The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev 23:2415-2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewing JM, Antalfy BC, Lucke T, Najafian B, Marwedel KM, Hori A, et al. (2007a). Schimke immuno-osseous dysplasia: a clinicopathological correlation. J Med Genet 44:122-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewing JM, Fryssira H, Goodman D, Smithson SF, Sloan EA, Lou S, et al. (2007b). Schimke immunoosseous dysplasia: suggestions of genetic diversity. Hum Mutat 28:273-283 [DOI] [PubMed] [Google Scholar]
- da Fonseca MA. (2000). Dental findings in the Schimke immuno-osseous dysplasia. Am J Med Genet 93:158-160 [DOI] [PubMed] [Google Scholar]
- Elizondo LI, Huang C, Northrop JL, Deguchi K, Clewing JM, Armstrong DL, et al. (2006). Schimke immuno-osseous dysplasia: a cell autonomous disorder? Am J Med Genet A 140:340-348 [DOI] [PubMed] [Google Scholar]
- Hatfield GW, Benham CJ. (2002). DNA topology-mediated control of global gene expression in Escherichia coli . Annu Rev Genet 36:175-203 [DOI] [PubMed] [Google Scholar]
- Hosoya A, Kim JY, Cho SW, Jung HS. (2008). BMP4 signaling regulates formation of Hertwig’s epithelial root sheath during tooth root development. Cell Tissue Res 333:503-509 [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. (2000). Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19-29 [DOI] [PubMed] [Google Scholar]
- Kilic SS, Donmez O, Sloan EA, Elizondo LI, Huang C, Andre JL, et al. (2005). Association of migraine-like headaches with Schimke immuno-osseous dysplasia. Am J Med Genet A 135:206-210 [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, et al. (2008). Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 313:210-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402-408 [DOI] [PubMed] [Google Scholar]
- Lücke T, Marwedel KM, Kanzelmeyer NK, Hori A, Offner G, Kreipe HH, et al. (2004). Generalized atherosclerosis sparing the transplanted kidney in Schimke disease. Pediatr Nephrol 19:672-675 [DOI] [PubMed] [Google Scholar]
- Luukko K, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Kettunen P. (2003). Identification of a novel putative signaling center, the tertiary enamel knot in the postnatal mouse molar tooth. Mech Dev 120:270-276 [DOI] [PubMed] [Google Scholar]
- Petty EM, Yanik GA, Hutchinson RJ, Alter BP, Schmalstieg FC, Levine JE, et al. (2000). Successful bone marrow transplantation in a patient with Schimke immuno-osseous dysplasia. J Pediatr 137:882-886 [DOI] [PubMed] [Google Scholar]
- Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JG, et al. (2005). Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol Dev 7:440-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow L, Woo EM, Chait BT, Funabiki H. (2009). Identification of SMARCAL1 as a component of the DNA damage response. J Biol Chem 284:35951-35961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss GJ, Drlica K. (1989). DNA supercoiling and prokaryotic transcription. Cell 56:521-523 [DOI] [PubMed] [Google Scholar]
- Schimke RN, Horton WA, King CR. (1971). Chondroitin-6-sulphaturia, defective cellular immunity, and nephrotic syndrome. Lancet 2:1088-1089 [DOI] [PubMed] [Google Scholar]
- Sclare R. (1948). Hereditary opalescent dentine. Br Dent J 84:164-166 [PubMed] [Google Scholar]
- Shields ED, Bixler D, el-Kafrawy AM. (1973). A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol 18:543-553 [DOI] [PubMed] [Google Scholar]
- Spranger J, Hinkel GK, Stoss H, Thoenes W, Wargowski D, Zepp F. (1991). Schimke immuno-osseous dysplasia: a newly recognized multisystem disease. J Pediatr 119(1 Pt 1):64-72 [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Thesleff I. (2010). Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn 239:364-372 [DOI] [PubMed] [Google Scholar]
- Thesleff I, Keranen S, Jernvall J. (2001). Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res 15:14-18 [DOI] [PubMed] [Google Scholar]
- Unda FJ, Martin A, Hernandez C, Perez-Nanclares G, Hilario E, Arechaga J. (2001). FGFs-1 and -2, and TGF beta 1 as inductive signals modulating in vitro odontoblast differentiation. Adv Dent Res 15:34-37 [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Vainio S, Thesleff I. (1991). Associations between transforming growth factor beta 1 RNA expression and epithelial-mesenchymal interactions during tooth morphogenesis. Development 113:985-994 [DOI] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. (1993). Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75:45-58 [PubMed] [Google Scholar]
- Yamashiro T, Tummers M, Thesleff I. (2003). Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res 82:172-176 [DOI] [PubMed] [Google Scholar]
- Yuan J, Ghosal G, Chen J. (2009). The annealing helicase HARP protects stalled replication forks. Genes Dev 23:2394-2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kadonaga JT. (2008). HARP is an ATP-driven annealing helicase. Science 322:748-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T, Kong X, Yokomori K, Kadonaga JT. (2009). The annealing helicase HARP is recruited to DNA repair sites via an interaction with RPA. Genes Dev 23:2400-2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichner-David M, Oishi K, Su Z, Zakartchenko V, Chen LS, Arzate H, et al. (2003). Role of Hertwig’s epithelial root sheath cells in tooth root development. Dev Dyn 228:651-663 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.