Abstract
Purpose
The Cancer and Leukemia Group B (CALGB) C9343 trial found that adjuvant radiation therapy (RT) provided minimal benefits for older women with breast cancer. Although treatment guidelines were changed to indicate that some women could forego RT, the impact of the C9343 results on clinical practice is unclear.
Patients and Methods
We used the Surveillance, Epidemiology, and End Results (SEER) –Medicare data set to assess the use of adjuvant RT in a sample of women ≥ 70 years old diagnosed with stage I breast cancer from 2001 to 2007 who fulfilled the C9343 inclusion criteria. We used log-binomial regression to estimate the relation between publication of C9343 and use of RT in the full sample and across strata of patient and health system characteristics.
Results
Of the 12,925 Medicare beneficiaries in our sample (mean age, 77.7 years), 76.5% received RT. Approximately 79% of women received RT before study publication compared with 75% after (adjusted relative risk of receiving RT postpublication v prepublication: 0.97; 95% CI, 0.95 to 0.98). Although use of RT was lower after the trial within all strata of age and life expectancy, the magnitude of this decrease did not differ significantly by strata. For instance, among patients with life expectancy less than 5 years, RT use decreased by 3.7%, from 44.4% prepublication to 40.7% postpublication. Among patients with life expectancy ≥ 10 years, RT use decreased by 3.0%, from 92.0% to 89.0%.
Conclusion
The C9343 trial had minimal impact on the use of RT among older women in the Medicare population, even among the oldest women and those with shorter life expectancies.
INTRODUCTION
Although cancer is an age-related disease, there is a paucity of data from randomized controlled trials (RCTs) to inform cancer treatment decisions in the older population.1–5 To address this gap, there has been a concerted effort in recent years to generate RCT data on older patients.6 The Cancer and Leukemia Group B (CALGB) C9343 trial is an excellent example of a trial that was specifically designed for older patients with cancer.7 For the majority of women with early-stage invasive breast cancer, the combination of breast-conserving surgery (BCS) and radiation therapy (RT) has become the standard of care.8 However, since many older women with early-stage disease have a low absolute risk of tumor recurrence,9,10 clinicians hypothesized that there may be a subgroup of patients with less aggressive tumor characteristics for whom adjuvant RT could be omitted.11 The C9343 trial addressed this question by randomly assigning women age ≥ 70 years with stage I estrogen receptor–positive disease and tumor size ≤ 2 cm to receive BCS and tamoxifen, with or without RT.
The C9343 trial results were published in September of 2004, showing that RT did not improve 5-year overall or disease-free survival or decrease the rate of mastectomy for recurrence.7 A small but statistically significant improvement in locoregional recurrence was seen for women receiving RT (1% for RT v 4% for no RT). In 2005, the National Comprehensive Cancer Network (NCCN) amended its breast cancer guidelines, stating that adjuvant RT may be omitted in this patient population.12 This option is particularly relevant for older, sicker patients who have limited life expectancy and thus less time at risk for recurrence.7,9,10,13
To determine the extent to which the C9343 results were incorporated into practice, we assessed the use of adjuvant RT in a sample of Medicare beneficiaries who fulfilled the C9343 inclusion criteria. We hypothesized that the impact of the trial findings would vary across subgroups of patient characteristics, with greater reduction in adjuvant RT among patients with limited life expectancy. Because little is known about whether regional health system factors influence the adoption of trial results into clinical practice, we also assessed changes in the use of RT according to health system characteristics.16–20
PATIENTS AND METHODS
Study Design and Data Source
We conducted a retrospective study by using the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) -Medicare database, which is a compilation of clinical and demographic data from 17 tumor registries covering approximately 26% of the US population.21 The program distributes the SEER-Medicare linked database, which contains inpatient, outpatient, and physician Medicare claims for patients ≥ 65 years old. In addition, demographic data and claims are available for a 5% random sample of Medicare beneficiaries, both with and without cancer, who reside within the SEER areas.
Study Sample
We selected female patients with a diagnosis of stage I invasive breast cancer from 2001 through 2007. Inclusion criteria were first or only tumor diagnosis, histology consistent with epithelial origin, age 70 to 94 years, known month of diagnosis, diagnosis not reported from autopsy or death report, received BCS within 9 months of diagnosis, and did not receive a non–breast cancer diagnosis during the time period from initial diagnosis through 12 months postsurgery. Surgery was identified from Medicare claims rather than from SEER so we could determine whether surgery occurred within 9 months of diagnosis. To ensure that patients were likely to have complete Medicare claims, we included only those patients enrolled in fee-for-service Medicare Parts A and B from 24 months before diagnosis through 12 months postsurgery (or death if the patient died within 12 months of surgery). Additional inclusion criteria from the C9343 trial included tumor size ≤ 2 cm and estrogen receptor–positive status, although we also included patients with unknown estrogen receptor status.22 We conducted a sensitivity analysis in which we excluded patients with unknown ER status, but the results did not differ.
Construction of Variables
Receipt of RT was ascertained by searching claims for Healthcare Common Procedure Coding System codes (Appendix Table A1, online only). We considered a patient to have received RT if the treatment was initiated within 9 months of BCS and if she had any treatment delivery codes for brachytherapy or at least four treatment delivery codes for external-beam RT.
We assigned time period, either before or after study publication (September 2004), on the basis of whether BCS was performed before January 1, 2004, or on or after September 1, 2004. We excluded patients who received BCS between January and August 2004 because the time period when the majority of these patients would have commenced RT would have overlapped with the publication of C9343. This allowed the two time periods to focus more specifically on patients who were making decisions about RT either before or after the publication of C9343. We created an additional time period variable for use in a sensitivity analysis in which we changed the reference date to March 2005, when the change in the NCCN treatment guidelines was announced.23 The results of this sensitivity analysis did not differ from the main results.
Income was categorized into quintiles based on median household income at the census tract or ZIP code level. To assess comorbidity, we used inpatient, outpatient, and physician claims in the 24 months through 3 months before diagnosis. We searched for International Classification of Disease, ninth revision (ICD-9) diagnosis codes appearing on any inpatient claims or at least two outpatient/physician claims more than 30 days apart.24 The comorbid conditions used were those suggested by Elixhauser et al,25 which we had previously determined to be significantly associated with mortality among a sample of patients with diseases other than cancer (Appendix Table A2, online only).
We estimated life expectancy by using a sample of patients without cancer from the Medicare 5% sample. We calculated mortality rates for each age, sex, and comorbidity stratum. As patients survived into older age categories, we amended the mortality rates to reflect the fact that approximately 20% of patients will move into the next comorbidity category. This value of 20% was selected on the basis of an analysis of our cohort and on clinical judgment. We used life tables to convert the survival probabilities into life expectancies. We validated this approach by using a separate cohort of women and found that, for the life expectancy categories of less than 5, 5 to 10, and ≥ 10 years, 10-year survival was 21.5%, 63.3%, and 80.5%, respectively, indicating that our estimates provided excellent discrimination. For comparison, the 10-year survival of participants in C9343 was approximately 57%.26
We used a sample of female patients from the non-cancer sample to determine county-level mammography screening rates. To distinguish screening from diagnostic procedures, we used a validated algorithm, which we amended to incorporate new procedure codes.27 We calculated the screening rate for each county in which at least 100 patients from our mammography sample resided. The rates were assigned to patients in the breast cancer sample on the basis of county of residence. Hospital bed density was calculated at the county level by using data from the Area Resource File as the total number of hospital beds in 2004 divided by the total population of the county in 2004.
Statistical Analysis
We used χ2 tests to compare the distributions of covariates in the pre- and post-RCT samples. The percentage of patients who received RT was calculated across strata of all covariates separately for the pre- and post-RCT samples, and χ2 tests were used to test whether these values differed significantly.
We used log-binomial regression to assess the association between each independent variable and receipt of RT. Because our outcome was common, this type of analysis provided more accurate estimation of relative risk than logistic regression.28,29 We also repeated the multivariable analysis using life expectancy strata, after removing age and comorbidity. To determine whether the C9343 results might have affected clinical practice differently across subgroups of patient and health system factors, we tested for interactions between each of the covariates and time period. All analyses were performed by using SAS version 9.2 (SAS Institute, Cary, NC). The Yale Human Investigations Committee determined that this study did not qualify as human subjects research.
RESULTS
There were 12,925 Medicare beneficiaries who fulfilled the C9343 inclusion criteria (Table 1). The mean age was 77.7 years (standard deviation, 5.4 years). The overwhelming majority of the patients were white, and nearly half the patients had no comorbidity. Approximately 12% of the sample had a life expectancy of less than 5 years, while 54% and 35% were expected to live 5 to 10 and ≥ 10 years, respectively. The patients in the earlier time period were slightly younger with fewer comorbidities and had a lower median income and longer life expectancy. Overall, 76.5% of the sample received RT. Before the RCT, 78.8% of women received RT, and after the trial 74.5% received RT (P < .001; Table 2). Use of RT tended to be slightly lower post-RCT compared with pre-RCT in all strata of all covariates, although this difference was not always significant. For example, among patients with the lowest income, RT use decreased from 72.3% to 71.2% (P = .61), and for patients with the highest income, RT use decreased from 82.5% to 75.7% (P < .001).
Table 1.
Study Sample Characteristics
| Characteristic | Total Sample (N = 12,925) |
Pre-RCT Dissemination (n = 6,027) |
Post-RCT Dissemination (n = 6,898) |
P* |
|||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age group, years | < .001 | ||||||
| 70-74 | 4,291 | 33.2 | 1,993 | 33.1 | 2,298 | 33.3 | |
| 75-79 | 4,208 | 32.6 | 2,080 | 34.5 | 2,128 | 30.9 | |
| 80-84 | 2,823 | 21.8 | 1,259 | 20.9 | 1,564 | 22.7 | |
| 85-94 | 1,603 | 12.4 | 695 | 11.5 | 908 | 13.2 | |
| Race | .15 | ||||||
| White | 11,979 | 92.7 | 5,611 | 93.1 | 6,368 | 92.3 | |
| Black | 450 | 3.5 | 205 | 3.4 | 245 | 3.6 | |
| Other | 496 | 3.8 | 211 | 3.5 | 285 | 4.1 | |
| No. of comorbidities | < .001 | ||||||
| 0 | 5,828 | 45.1 | 2,857 | 47.4 | 2,971 | 43.1 | |
| 1-2 | 5,235 | 40.5 | 2,376 | 39.4 | 2,859 | 41.5 | |
| ≥ 3 | 1,862 | 14.4 | 794 | 13.2 | 1,068 | 15.5 | |
| Marital status | < .001 | ||||||
| Married | 5,731 | 44.3 | 2,657 | 44.1 | 3,074 | 44.6 | |
| Not married | 6,783 | 52.5 | 3,232 | 53.6 | 3,551 | 51.5 | |
| Unknown | 411 | 3.2 | 138 | 2.3 | 273 | 4.0 | |
| Median income, $ | .03 | ||||||
| < 33,000 | 1,913 | 14.8 | 948 | 15.7 | 965 | 14.0 | |
| 33,000-40,000 | 1,754 | 13.6 | 834 | 13.8 | 920 | 13.3 | |
| 40,001-50,000 | 2,670 | 20.7 | 1,257 | 20.9 | 1,413 | 20.5 | |
| 50,001-63,000 | 2,778 | 21.5 | 1,281 | 21.3 | 1,497 | 21.7 | |
| > 63,000† | 3,810 | 29.5 | 1,707 | 28.3 | 2,103 | 30.5 | |
| Life expectancy, years | < .001 | ||||||
| < 5 | 1,512 | 11.7 | 656 | 10.9 | 856 | 12.4 | |
| 5-10 | 6,960 | 53.9 | 3,198 | 53.1 | 3,762 | 54.5 | |
| ≥ 10 | 4,453 | 34.5 | 2,173 | 36.1 | 2,280 | 33.1 | |
| Tumor size, cm | .49 | ||||||
| < 1 | 5,076 | 39.3 | 2,348 | 39.0 | 2,728 | 39.6 | |
| 1-2 | 7,849 | 60.7 | 3,679 | 61.0 | 4,170 | 60.5 | |
| County-level use of mammography screening, % | .70 | ||||||
| 24.5-42.6 | 7,225 | 55.9 | 3,385 | 56.2 | 3,840 | 55.7 | |
| 42.9-58.6 | 4,992 | 38.6 | 2,306 | 38.3 | 2,686 | 38.9 | |
| Other‡ | 708 | 5.5 | 336 | 5.6 | 372 | 5.4 | |
| County-level hospital bed density (per 100,000 population) | .20 | ||||||
| 0-237 | 5,098 | 39.4 | 2,342 | 38.9 | 2,756 | 40.0 | |
| 238-3,981 | 7,827 | 60.6 | 3,685 | 61.1 | 4,142 | 60.1 | |
Abbreviation: RCT, randomized controlled trial.
P value is for a χ2 test between each covariate and time period.
The income > $63,000 category also includes patients with unknown income because of the small cell sizes in the unknown income category.
Mammography rate was not estimated because there were fewer than 100 female non-cancer patients in the data set residing in that county.
Table 2.
Percentage of Patients Receiving Radiation Therapy Pre- and Post-RCT Dissemination
| Variable | Overall | Pre-RCT Dissemination | Post-RCT Dissemination | P* |
|---|---|---|---|---|
| Overall | 76.5 | 78.8 | 74.5 | < .001 |
| Age group, years | ||||
| 70-74 | 90.1 | 91.3 | 89.1 | .01 |
| 75-79 | 83.5 | 86.0 | 81.0 | < .001 |
| 80-84 | 68.8 | 69.7 | 68.0 | .33 |
| 85-94 | 35.1 | 37.4 | 33.3 | .08 |
| Race | ||||
| White | 76.5 | 78.9 | 74.3 | < .001 |
| Black | 72.2 | 69.3 | 74.7 | .20 |
| Other | 80.7 | 84.4 | 77.9 | .07 |
| No. of comorbidities | ||||
| 0 | 81.5 | 83.0 | 79.9 | .003 |
| 1-2 | 75.5 | 78.3 | 73.1 | < .001 |
| ≥ 3 | 63.7 | 64.9 | 62.8 | .37 |
| Marital status | ||||
| Married | 83.3 | 86.1 | 80.9 | < .001 |
| Not married | 71.1 | 73.1 | 69.3 | < .001 |
| Unknown | 70.6 | 71.7 | 70.0 | .71 |
| Median income, $ | ||||
| < 33,000 | 71.7 | 72.3 | 71.2 | .61 |
| 33,000-40,000 | 76.6 | 78.7 | 74.7 | .05 |
| 40,001-50,000 | 74.7 | 78.1 | 71.7 | < .001 |
| 50,001-63,000 | 78.3 | 79.2 | 77.5 | .27 |
| > 63,000 | 78.7 | 82.5 | 75.7 | < .001 |
| Unknown | 50.0 | 100.0 | 0.0 | .01 |
| Tumor size, cm | ||||
| < 1 | 76.3 | 78.8 | 74.1 | < .001 |
| 1-2 | 76.6 | 78.7 | 74.7 | < .001 |
| County-level use of mammography screening, % | ||||
| 24.5-42.6 | 75.9 | 78.3 | 73.8 | < .001 |
| 42.9-58.6 | 78.0 | 80.3 | 76.1 | < .001 |
| Other† | 70.9 | 72.9 | 69.1 | .26 |
| County-level hospital bed density (per 100,000) | ||||
| 0-237 | 75.3 | 77.4 | 73.4 | < .001 |
| 238-3,981 | 77.3 | 79.6 | 75.2 | < .001 |
Abbreviation: RCT, randomized controlled trial.
P value is for a χ2 test between receipt of radiation therapy and time period within strata of covariates.
Mammography rate was not estimated because there were fewer than 100 female patients without cancer in the data set residing in that county.
One group for whom RT rates did not decrease was black women. Although 69.3% of black women received RT before study publication, 74.7% received RT after (P = .20). In contrast, RT use among white women decreased from 78.9% to 74.3% (P < .001). Although there was a significant racial disparity in use of RT before study publication (P = .001 for black v white), there was no significant difference in use of RT between black and white women after study publication (P = .89). The interaction between time period and black race was of borderline significance (P = .07).
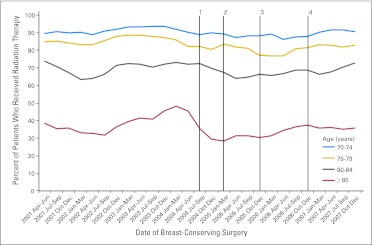
There was substantial variation in receipt of RT across age strata; in the youngest age group, approximately 90% of patients received RT, with the rate decreasing to approximately 35% in the oldest group. Receipt of RT was significantly lower post-RCT among the two youngest age groups (P = .01 for age 70 to 74 years; P < .001 for age 75 to 79 years), although the differences were not significant for the older two age groups. Temporally, there was no drop-off in RT immediately following study publication in the youngest two age categories, although the oldest two categories experienced a slight decrease (Fig 1). However, in all four age categories, the rate of RT use at the end of 2007 was similar to the rate at the beginning of 2001.
Fig 1.
Temporal trend in the use of radiation therapy by age category. 1: September 2004, Cancer and Leukemia Group B (CALGB) C9343 study was published; 2: March 2005, revised National Comprehensive Cancer Network (NCCN) guidelines were announced; 3: November 2005, revised NCCN guidelines were published; 4: December 2006, C9343 update with 8-year follow-up was published.
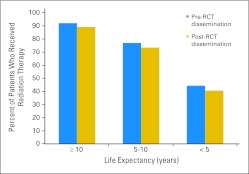
Receipt of RT varied substantially across life expectancy strata. In the pre-RCT sample, 44.4% of women with life expectancy less than 5 years received RT compared with 76.9% and 92.0% in the 5 to 10 and ≥ 10 years categories, respectively (Fig 2). In the post-RCT sample, 40.7% of women with life expectancy less than 5 years received RT compared with 73.4% and 89.0% in the 5 to 10 and ≥ 10 years categories, respectively. Receipt of RT pre- and post-RCT publication differed significantly in the 5 to 10 and ≥ 10 years life expectancy categories but was not statistically different for patients with life expectancy less than 5 years.
Fig 2.
Radiation therapy rates pre- and post-randomized controlled trial (RCT) dissemination according to life expectancy category.
Several other patient and health system factors were associated with receipt of RT. Increasing age, greater comorbidity, and being unmarried or of unknown marital status decreased the likelihood of receiving RT (Table 3). The association between age and use of RT was strong; compared with patients 70 to 74 years old, patients who were 85 to 94 years old had a risk ratio for receiving RT of 0.41 (95% CI, 0.38 to 0.43). Living in a county in the top two quintiles of mammography screening or in the top three quintiles of hospital bed density increased a patient's likelihood of receiving RT.
Table 3.
Unadjusted and Adjusted RRs for Receipt of Radiation Therapy
| Variable | Unadjusted |
Adjusted* |
||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| Time period | ||||
| Pre-RCT dissemination | 1.00 | — | 1.00 | — |
| Post-RCT dissemination | 0.95 | 0.93 to 0.96 | 0.97 | 0.95 to 0.98 |
| Age group, years | ||||
| 70-74 | 1.00 | — | 1.00 | — |
| 75-79 | 0.93 | 0.91 to 0.94 | 0.94 | 0.93 to 0.96 |
| 80-84 | 0.76 | 0.74 to 0.78 | 0.79 | 0.76 to 0.81 |
| 85-94 | 0.39 | 0.36 to 0.42 | 0.41 | 0.38 to 0.43 |
| Race | ||||
| White | 1.00 | — | 1.00 | — |
| Black | 0.94 | 0.89 to 1.00 | 0.97 | 0.92 to 1.02 |
| Other | 1.05 | 1.01 to 1.10 | 1.01 | 0.96 to 1.05 |
| No. of comorbidities | ||||
| 0 | 1.00 | — | 1.00 | — |
| 1-2 | 0.93 | 0.91 to 0.94 | 0.97 | 0.96 to 0.99 |
| ≥ 3 | 0.78 | 0.75 to 0.81 | 0.89 | 0.86 to 0.92 |
| Marital status | ||||
| Married | 1.00 | — | 1.00 | — |
| Not married | 0.85 | 0.84 to 0.87 | 0.97 | 0.95 to 0.98 |
| Unknown | 0.85 | 0.80 to 0.90 | 0.94 | 0.90 to 0.99 |
| Median income, $ | ||||
| < 33,000 | 1.00 | — | 1.00 | — |
| 33,000-40,000 | 1.07 | 1.03 to 1.11 | 1.03 | 1.00 to 1.06 |
| 40,001-50,000 | 1.04 | 1.01 to 1.08 | 1.01 | 0.98 to 1.04 |
| 50,001-63,000 | 1.09 | 1.05 to 1.13 | 1.03 | 1.00 to 1.06 |
| > 63,000 | 1.10 | 1.06 to 1.13 | 1.03 | 1.00 to 1.06 |
| Unknown | 0.70 | 0.31 to 1.55 | 0.69 | 0.33 to 1.46 |
| Tumor size, cm | ||||
| < 1 | 1.00 | — | ||
| 1-2 | 1.00 | 0.98 to 1.02 | ||
| County-level use of mammography screening, % | ||||
| 24.5-42.6 | 1.00 | — | 1.00 | — |
| 42.9-58.6 | 1.03 | 1.01 to 1.05 | 1.03 | 1.01 to 1.04 |
| Other† | 0.93 | 0.89 to 0.98 | 1.00 | 0.96 to 1.05 |
| County-level hospital bed density (per 100,000) | ||||
| 0-237 | 1.00 | — | 1.00 | — |
| 238-3,981 | 1.03 | 1.01 to 1.05 | 1.03 | 1.01 to 1.04 |
Abbreviations: RCT, randomized controlled trial; RR, risk ratio.
Also adjusted for Surveillance, Epidemiology, and End Results registry.
Mammography rate was not estimated because there were fewer than 100 non-cancer patients residing in that county.
After adjusting for patient and health system factors, the relation between C9343 publication and use of RT was essentially unchanged. Patients who received BCS after study publication were 3% less likely to receive RT than those who received BCS before (risk ratio, 0.97; 95% CI, 0.95 to 0.98). None of the interactions between time period and the covariates were statistically significant. Similarly, when we repeated the adjusted model with age and comorbidity removed and with life expectancy and the life expectancy times time period interaction added in, life expectancy did not significantly moderate the effect of time period on receipt of RT.
DISCUSSION
Our results suggest that the C9343 trial resulted in a minimal change in clinical practice among Medicare beneficiaries. Although the difference in use of RT before and after publication of the C9343 study was statistically significant, the use of RT remained high. We found no evidence of differential uptake of the results among patients with limited life expectancy or among the oldest women, indicating that the magnitude of the small decrease in RT was the same within each life expectancy and age category. The use of RT among patients with the shortest life expectancy still exceeded 40% during both time periods.
Our findings have important implications for comparative effectiveness research, which emphasizes the effectiveness of interventions.30 In contrast, most clinical trials emphasize efficacy, which is why older patients with comorbid conditions that may complicate the relationship between treatment and outcome are often excluded.31 C9343 was motivated by a need to generate data on the effectiveness of RT that would be generalizable to older women. Although the US government has invested significant resources in comparative effectiveness research studies,32 our analysis suggests that practice patterns may not necessarily change on the basis of the results of such studies.
There are several reasons why the trial may not have affected practice. First, clinicians may have felt that a median of 5 years of follow-up7 was an insufficient length of time. The meta-analysis of the Early Breast Cancer Trialists' Collaborative Group,33 which was published soon after the revised NCCN guidelines were published, reported that local control at 5 years translated into improved survival at 15 years, so clinicians may have felt the decrease in locoregional recurrence could still result in a survival benefit. However, results from the trial based on a median follow-up of 10.5 years have shown that the reduction in locoregional recurrence continues to be the only significant effect of adjuvant RT.26 Although the absolute risk reduction has increased from 3% to 7%, locoregional recurrence has remained low in both groups (2% v 9% for RT and no RT, respectively).
The continued use of RT may also be due to financial incentives. Higher reimbursement often leads to increased health care resource consumption. Furthermore, physicians are more likely to be influenced by reimbursement when there is uncertainty about a treatment.34 An analysis of the use of androgen deprivation therapy (ADT) for prostate cancer before and after a change in Medicare reimbursement found that, although there was no change in use of ADT for appropriate indications, inappropriate use of ADT decreased significantly once reimbursement was lowered.35 Thus, the results of our analysis may have been different if Medicare had lowered reimbursement for the use of RT in this patient population.
Prior studies indicate that RCT results may affect practice more if they support the adoption of a new treatment or technology rather than the exclusion of an existing one. Yen et al36 assessed the impact of a trial that demonstrated that tamoxifen after BCS and RT for ductal carcinoma in situ decreased the rates of ipsilateral and contralateral breast cancer events at 5 years by 3.3% and 1.4%, respectively, but offered no improvement in survival. Although these results are quite similar to those of the C9343 trial, they were associated with a substantial change in practice, with use of tamoxifen increasing from 24% to 46%. Giordano et al37 found that after the results of CALGB 9344 were presented at a national meeting, the use of taxanes for primary breast cancer increased. Hence, future studies should explore whether positive studies have a differential impact on clinical practice compared with negative studies.
Unexpectedly, our results indicated that the percentage of black patients receiving RT increased by 5% after the dissemination of the trial results. Although the receipt of RT was significantly lower for blacks compared with whites before RCT dissemination but not significantly different after, the interaction between time and black race was only of borderline significance. However, black patients made up only 3.5% of our sample, so it is possible that a significant interaction was obscured by an inadequate number of black patients.
Our study has several limitations. First, although the C9343 results are generalizable only to women who were prescribed tamoxifen following BCS, we did not have the Medicare Part D claims needed to assess tamoxifen use in the entire sample. However, adjuvant tamoxifen was already recommended for all patients with estrogen receptor–positive tumors before our study period,38 and estimates on the use of hormone therapy during this time period range from 70% to more than 80%.39–41 This estimate is supported by the limited Part D claims available to us, which indicated that 74% of patients in our sample diagnosed in 2007 had at least one claim for tamoxifen or an aromatase inhibitor. Second, our comorbidity assessment was based on Medicare claims, which are collected for administrative purposes. However, validation of the life expectancy estimates showed that our approach accurately discriminated between healthier and sicker patients. Finally, we had only 3 years of data after the study was published. It is possible that as additional data from the trial become available, practice patterns will be affected, although the presentation of the 8-year follow-up data in December 2006 does not appear to have had an impact on practice patterns (Fig 1).
Although the C9343 trial was a seminal effort that was widely disseminated and that prompted a change in clinical guidelines, we found that it had minimal impact on clinical practice. Particularly among women with limited life expectancy, the continued use of RT may impose health risks and an added burden to patients while providing no mortality benefit and a relatively small benefit in terms of locoregional recurrence. In addition, unnecessary RT represents a significant cost to Medicare.42 Given the unsustainable level of Medicare spending, data drawn from comparative effectiveness research studies has become increasingly relevant, because it could enable payers to allocate funds in a cost-effective manner without compromising outcomes.43 Although the recent Institute of Medicine report on the National Cancer Institute's Cooperative Group Program issued 12 recommendations for redesigning the program, none of these focused on assessing and optimizing the translation of trial results into practice.44 In settings in which there is a small incremental benefit from treatment, patients and clinicians should be empowered to incorporate evidence into their decision making.
Acknowledgment
We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare Database. This study used the SEER-Medicare linked database.
Appendix
Table A1.
Health Care Common Procedure Coding System Codes Used to Identify Receipt of Radiation Therapy
| 77402 | 77762 |
| 77403 | 77763 |
| 77404 | 77776 |
| 77406 | 77777 |
| 77407 | 77778 |
| 77408 | 77781 |
| 77409 | 77782 |
| 77411 | 77783 |
| 77412 | 77784 |
| 77413 | 77799 |
| 77414 | 0182T |
| 77416 | 19296 |
| 77418 | 19297 |
| 0073T | 19298 |
| G0174 | C9714 |
| 77761 | C9715 |
Table A2.
Elixhauser Conditions Included in Comorbidity Index
| Congestive heart failure |
| Cardiac arrhythmia |
| Valvular disease |
| Pulmonary circulation disorders |
| Peripheral vascular disorders |
| Paralysis |
| Other neurological disorders |
| Chronic pulmonary disease |
| Diabetes uncomplicated |
| Diabetes complicated |
| Renal failure |
| Liver disease |
| AIDS/HIV |
| Rheumatoid arthritis/collagen |
| Coagulopathy |
| Weight loss |
| Fluid and electrolyte disorders |
| Blood loss anemia |
| Deficiency anemia |
| Alcohol abuse |
| Drug abuse |
| Psychoses |
| Depression |
Footnotes
See accompanying editorial on page 1577; listen to the podcast by Dr Buchholz at www.jco.org/podcasts
Supported by Grant No. 1R01C4149045-01 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Cary P. Gross, Medtronic Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Pamela R. Soulos, James B. Yu, Kenneth B. Roberts, Cary P. Gross
Collection and assembly of data: Pamela R. Soulos, Jessica B. Long
Data analysis and interpretation: Pamela R. Soulos, Ann C. Raldow, Jeph Herrin, Jessica B. Long, Cary P. Gross
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Gross CP, Wong N, Dubin JA, et al. Enrollment of older persons in cancer trials after the Medicare reimbursement policy change. Arch Intern Med. 2005;165:1514–1520. doi: 10.1001/archinte.165.13.1514. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Gross CP, Herrin J, Wong N, et al. Enrolling older persons in cancer trials: The effect of sociodemographic, protocol, and recruitment center characteristics. J Clin Oncol. 2005;23:4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 5.Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer. 1994;74:2208–2214. doi: 10.1002/1097-0142(19941001)74:7+<2208::aid-cncr2820741737>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Cohen HJ, Muss HB. The Cancer and Leukemia Group B Cancer in the Elderly Committee: Addressing a major cancer need. Clin Cancer Res. 2006;12:3606s–3611s. doi: 10.1158/1078-0432.CCR-06-9007. [DOI] [PubMed] [Google Scholar]
- 7.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 9.Howard-McNatt M, Hughes KS, Schnaper LA, et al. Breast cancer treatment in older women. Surg Oncol Clin N Am. 2005;14:85–102. doi: 10.1016/j.soc.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Gajdos C, Tartter PI, Bleiweiss IJ, et al. The consequence of undertreating breast cancer in the elderly. J Am Coll Surg. 2001;192:698–707. doi: 10.1016/s1072-7515(01)00832-8. [DOI] [PubMed] [Google Scholar]
- 11.Whelan T. A trial of two questions. J Clin Oncol. 2002;20:4135–4138. doi: 10.1200/JCO.2002.20.20.4135. [DOI] [PubMed] [Google Scholar]
- 12.Carlson RW, McCormick B. Update: NCCN breast cancer Clinical Practice Guidelines. J Natl Compr Canc Netw. 2005;3(suppl 1):S7–S11. [PubMed] [Google Scholar]
- 13.Velanovich V, Gabel M, Walker EM, et al. Causes for the undertreatment of elderly breast cancer patients: Tailoring treatments to individual patients. J Am Coll Surg. 2002;194:8–13. doi: 10.1016/s1072-7515(01)01132-2. [DOI] [PubMed] [Google Scholar]
- 14. Reference deleted.
- 15. Reference deleted.
- 16.Smith GL, Xu Y, Shih YC, et al. Breast-conserving surgery in older patients with invasive breast cancer: Current patterns of treatment across the United States. J Am Coll Surg. 2009;209:425–433.e2. doi: 10.1016/j.jamcollsurg.2009.06.363. [DOI] [PubMed] [Google Scholar]
- 17.Chagpar AB, Studts JL, Scoggins CR, et al. Factors associated with surgical options for breast carcinoma. Cancer. 2006;106:1462–1466. doi: 10.1002/cncr.21728. [DOI] [PubMed] [Google Scholar]
- 18.Smith GL, Shih YC, Xu Y, et al. Racial disparities in the use of radiotherapy after breast-conserving surgery: A national Medicare study. Cancer. 2010;116:734–741. doi: 10.1002/cncr.24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nattinger AB, Gottlieb MS, Veum J, et al. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–1107. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 20.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097–1101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 21.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 22.Smith BD, Gross CP, Smith GL, et al. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 23.Carlson R, McCormick B. Update: Breast cancer guidelines. Presented at the 10th Annual Conference of the National Comprehensive Cancer Network; March 16-20, 2005; Hollywood, FL. [Google Scholar]
- 24.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Hughes KS, Schnaper LA, Cirrincione C, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer. J Clin Oncol. 2010;28:69s. doi: 10.1200/JCO.2012.45.2615. (suppl; abstr 507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith-Bindman R, Quale C, Chu PW, et al. Can Medicare billing claims data be used to assess mammography utilization among women ages 65 and older? Med Care. 2006;44:463–470. doi: 10.1097/01.mlr.0000207436.07513.79. [DOI] [PubMed] [Google Scholar]
- 28.McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 29.Robbins AS, Chao SY, Fonseca VP. What's the relative risk? A method to directly estimate risk ratios in cohort studies of common outcomes. Ann Epidemiol. 2002;12:452–454. doi: 10.1016/s1047-2797(01)00278-2. [DOI] [PubMed] [Google Scholar]
- 30.Washington, DC: The National Academies Press; 2009. Institute of Medicine: Initial National Priorities for Comparative Effectiveness Research. [Google Scholar]
- 31.Sox HC. Defining comparative effectiveness research: The importance of getting it right. Med Care. 2010;48:S7–S8. doi: 10.1097/MLR.0b013e3181da3709. [DOI] [PubMed] [Google Scholar]
- 32.Sox HC. Comparative effectiveness research: A progress report. Ann Intern Med. 2010;153:469–472. doi: 10.7326/0003-4819-153-7-201010050-00269. [DOI] [PubMed] [Google Scholar]
- 33.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Andersen R, Brook R, et al. The effects of payment method on clinical decision-making: Physician responses to clinical scenarios. Med Care. 2004;42:297–302. doi: 10.1097/01.mlr.0000114918.50088.1c. [DOI] [PubMed] [Google Scholar]
- 35.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363:1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 36.Yen TW, Kuerer HM, Ottesen RA, et al. Impact of randomized clinical trial results in the national comprehensive cancer network on the use of tamoxifen after breast surgery for ductal carcinoma in situ. J Clin Oncol. 2007;25:3251–3258. doi: 10.1200/JCO.2006.10.2699. [DOI] [PubMed] [Google Scholar]
- 37.Giordano SH, Duan Z, Kuo YF, et al. Impact of a scientific presentation on community treatment patterns for primary breast cancer. J Natl Cancer Inst. 2006;98:382–388. doi: 10.1093/jnci/djj090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institutes of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement: Adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst Monogr. 2001;30:5–15. [PubMed] [Google Scholar]
- 39.Harlan LC, Clegg LX, Abrams J, et al. Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987-2000. J Clin Oncol. 2006;24:872–877. doi: 10.1200/JCO.2005.03.5840. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y, Dong W, Feig BW, et al. Patterns of treatment for early stage breast cancers at the M. D. Anderson Cancer Center from 1997 to 2004. Cancer. 2009;115:2041–2051. doi: 10.1002/cncr.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen TW, Czypinski LK, Sparapani RA, et al. Socioeconomic factors associated with adjuvant hormone therapy use in older breast cancer survivors. Cancer. 2011;117:398–405. doi: 10.1002/cncr.25412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howlander N, Noone AM, Krapcho M, et al. Bethesda, MD: 2011. SEER Cancer Statistics Review, 1975-2008. [Google Scholar]
- 43.Neumann PJ, Tunis SR. Medicare and medical technology: The growing demand for relevant outcomes. N Engl J Med. 2010;362:377–379. doi: 10.1056/NEJMp0912062. [DOI] [PubMed] [Google Scholar]
- 44.Washington, DC: The National Academies Press; 2010. Institute of Medicine: A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. [PubMed] [Google Scholar]