Abstract
Purpose
To examine population-based improvements in survival and the impact of clinical covariates on outcome among children and adolescents with acute lymphoblastic leukemia (ALL) enrolled onto Children's Oncology Group (COG) clinical trials between 1990 and 2005.
Patients and Methods
In total, 21,626 persons age 0 to 22 years were enrolled onto COG ALL clinical trials from 1990 to 2005, representing 55.8% of ALL cases estimated to occur among US persons younger than age 20 years during this period. This period was divided into three eras (1990-1994, 1995-1999, and 2000-2005) that included similar patient numbers to examine changes in 5- and 10-year survival over time and the relationship of those changes in survival to clinical covariates, with additional analyses of cause of death.
Results
Five-year survival rates increased from 83.7% in 1990-1994 to 90.4% in 2000-2005 (P < .001). Survival improved significantly in all subgroups (except for infants age ≤ 1 year), including males and females; those age 1 to 9 years, 10+ years, or 15+ years; in whites, blacks, and other races; in Hispanics, non-Hispanics, and patients of unknown ethnicity; in those with B-cell or T-cell immunophenotype; and in those with National Cancer Institute (NCI) standard- or high-risk clinical features. Survival rates for infants changed little, but death following relapse/disease progression decreased and death related to toxicity increased.
Conclusion
This study documents ongoing survival improvements for children and adolescents with ALL. Thirty-six percent of deaths occurred among children with NCI standard-risk features emphasizing that efforts to further improve survival must be directed at both high-risk subsets and at those children predicted to have an excellent chance for cure.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy, comprising 25% of cancers occurring before age 15 years and 19% among those younger than age 20 years.1 The 5-year survival rate increased from less than 10% in the 1960s to 77% in 1985 to 1994.1 Survival rate has continued to increase over the past 10 to 15 years.2–11 The National Cancer Institute (NCI) SEER Program reported that 5-year survival for US patients younger than age 15 years with ALL increased from 80.2% to 87.5% between 1990-1994 and 2000-2004.12 Five-year survival rates for adolescents age 15 to 19 years increased from 41.0% in 1980-1984 to 61.1% in 2000-2004.13
The Children's Oncology Group (COG) includes more than 200 member institutions in the United States, Canada, Australia, and New Zealand. Unlike the SEER system, which tracks outcome in five representative states and four metropolitan areas that include approximately 10% of the US population, COG data include patients from all areas of the United States and Canada and provide an opportunity to assess outcome for children with ALL throughout these countries and to examine the prognostic impact of covariates not included in registry data. We report changes in survival among children enrolled onto COG ALL clinical trials between 1990 and 2005 and the extent to which different clinical and biologically defined patient subgroups benefited from treatment improvements.
PATIENTS AND METHODS
Patients
In all, 21,626 eligible children and adolescents younger than age 22 years enrolled onto one of 36 COG ALL clinical trials (Appendix Table A1, online only) between January 1, 1990, and December 31, 2005. Patients were treated on clinical trials that tested treatment intensifications and the need for cranial irradiation, and they used clinical and biologic prognostic variables, including genetic subtype and early treatment response, to risk stratify patients and assign therapies of varying intensity.18 We divided this period into three eras that included similar numbers of patients: 1990-1994 (7,304 patients; median follow-up, 9.13 years), 1995-1999 (7,169 patients; median follow-up, 8.02 years), and 2000-2005 (7,153 patients; median follow-up, 5.35 years). Most patients (92.2%) were treated in the United States, with 5.8% in Canada, and 2% elsewhere. Patients and/or a parent/guardian provided informed consent for clinical trial participation; trials were approved by institutional review boards at COG centers. We analyzed outcome on the basis of clinical features, including age and WBC count at diagnosis, sex, immunophenotype, race, and ethnicity as reported by the patient or parent.
Statistical Analyses
Overall survival estimates were obtained by using the Kaplan-Meier method,19 with SEs calculated by using the method of Peto and Peto.20 Survival time was calculated as the time from study entry to death or date of last contact. Comparisons of survival curves were performed by using the log-rank test.21 Survival curves were truncated at year 15. The cumulative incidence of death due to various causes was determined after adjusting for competing risks.22 Multivariate Cox regression analysis was used to identify prognostic factors affecting overall survival. Survival tree regression was used in both infant and non–infant subsets to identify prognostic factors and explore their association with overall survival.23,24 Data were frozen in September 2009.
Incidence rates for ALL were determined by using published SEER data with additional information on rates obtained directly from the National Institutes of Health and Information Management Services. Total numbers of ALL cases expected to occur in the United States during specific time periods was determined by applying these incidence rates to population statistics derived from US census data.
RESULTS
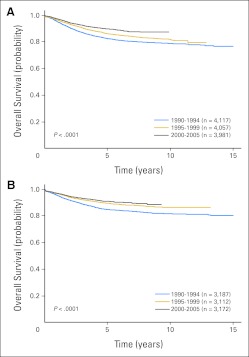
Overall 5- and 10-year survival rates increased significantly over time (Fig 1; P < .001). Five-year survival increased from 83.7% (SE, 0.4%) in 1990-1994 to 87.7% (SE, 0.4%) in 1995-1999 and to 90.4% (SE, 0.5%) in 2000-2005 (Table 1). Similar increases were seen in 10-year survival between 1990-1994 (80.1%; SE, 0.8%) and 1995-1999 (83.9%; SE, 1.3%; P < .001). For the eras from 1990 to 1999, approximately 84% of deaths occurred within 5 years of diagnosis and only 1% occurred more than 10 years following diagnosis (Appendix Tables A2 to A6, online only). Because of these factors and the more limited follow-up for the 2000-2005 era, subsequent analyses focused on 5-year survival.
Fig 1.
Overall survival probability by treatment era for patients enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005.
Table 1.
Five-Year Overall Survival of Patient Subsets by Era
| Patient Group | No. | 5-Year Survival ± SE (%) |
% Reduction2000-2005 v 1990-1994 | P * | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1990-1994 | No. of Patients | 1995-1999 | No. of Patients | 2000-2005 | No. of Patients | ||||
| All patients | 21,626 | 83.7 ± 0.4 | 7,304 | 87.7 ± 0.4 | 7,169 | 90.4 ± 0.5 | 7,153 | 41 | < .001 |
| Age group, years | |||||||||
| < 1 | 461 | 47.9 ± 4.1 | 154 | 48.1 ± 4.3 | 148 | 53.2 ± 5.4 | 159 | 10 | .4520 |
| 1-9.99 | 16,578 | 88.2 ± 0.4 | 5,599 | 91.7 ± 0.4 | 5,523 | 94.1 ± 0.4 | 5,456 | 50 | < .001 |
| ≥ 10 | 4,587 | 70.8 ± 1.2 | 1,551 | 76.9 ± 1.2 | 1,498 | 81.6 ± 1.3 | 1,538 | 37 | < .001 |
| 10-14.99 | 3,072 | 72.8 ± 1.4 | 1,094 | 78.9 ± 1.4 | 1,001 | 84.7 ± 1.5 | 977 | 44 | < .001 |
| ≥ 15 | 1,515 | 66.1 ± 2.3 | 457 | 72.9 ± 2.2 | 497 | 75.9 ± 2.6 | 561 | 29 | .0025 |
| Sex | |||||||||
| Male | 12,155 | 82.7 ± 0.6 | 4,117 | 86.3 ± 0.6 | 4,057 | 89.9 ± 0.6 | 3,981 | 42 | < .001 |
| Female | 9,471 | 84.9 ± 0.7 | 3,187 | 89.5 ± 0.6 | 3,112 | 91.0 ± 0.7 | 3,172 | 40 | < .001 |
| Race | |||||||||
| White | 15,759 | 86.3 ± 0.5 | 5,410 | 88.9 ± 0.5 | 4,890 | 91.1 ± 0.5 | 5,242 | 35 | < .001 |
| Black | 1,474 | 75.3 ± 2.0 | 535 | 80.7 ± 1.9 | 472 | 87.8 ± 2.1 | 425 | 51 | < .001 |
| Other | 4,393 | 77.0 ± 1.2 | 1,359 | 86.3 ± 0.9 | 1,807 | 88.1 ± 1.2 | 1,486 | 48 | < .001 |
| Ethnicity | |||||||||
| Hispanic | 2,589 | 82.0 ± 1.8 | 547 | 86.2 ± 1.4 | 675 | 87.6 ± 1.2 | 1,367 | 31 | .0076 |
| Non-Hispanic | 12,528 | 87.0 ± 0.6 | 3,626 | 88.5 ± 0.6 | 3,377 | 91.4 ± 0.5 | 5,525 | 34 | < .001 |
| Unknown | 6,509 | 80.0 ± 0.7 | 3,131 | 87.1 ± 0.6 | 3,117 | 83.6 ± 3.0 | 261 | 18 | < .001 |
| Immunophenotype | |||||||||
| B cell | 16,880 | 84.9 ± 0.5 | 5,068 | 88.3 ± 0.4 | 5,830 | 91.1 ± 0.5 | 5,982 | 41 | < .001 |
| T cell | 1,831 | 70.7 ± 1.7 | 748 | 80.7 ± 1.7 | 624 | 81.6 ± 2.2 | 459 | 37 | < .001 |
| NCI risk group | |||||||||
| Standard risk | 14,154 | 90.2 ± 0.5 | 4,624 | 92.7 ± 0.4 | 4,674 | 95.0 ± 0.4 | 4,856 | 49 | < .001 |
| High risk | 73.8 + 0.9 | 2,680 | 79.8 ± 0.9 | 2,494 | 82.9 ± 1.1 | 2,286 | 32 | < .001 | |
Abbreviation: NCI, National Cancer Institute.
The P values were computed by comparing the survival curves among all three eras.
Survival improved significantly in all subgroups examined except for infants ≤ 1 year old (Table 1 and Appendix Figs A1 to A6, online only): ages 1 to 9 years, 10+ years, and 15+ years; males and females; self-reported whites, blacks, and other races; self-reported Hispanics, non-Hispanics, and persons of unknown ethnicity; those with B-cell and T-cell ALL; and those with standard-risk (age 1 to 9.99 years; initial WBC < 50,000/μL) or high-risk (age ≥ 10 years and/or initial WBC ≥ 50,000/μL) features by using NCI/Rome25 criteria. The relative reductions in 5-year risk of death between the 1990-1994 and 2000-2005 eras were similar in all non–infant subgroups examined, ranging from 30% to 50% (Table 1). Because the results for infants differed from those of older children, we also analyzed data separately for infants and non–infants (Appendix Tables A7 and A8, online only).
Deaths that occurred after induction failure or relapse were classified as leukemia related, and those that occurred without prior induction failure or relapse were deemed treatment related (Table 2 and Appendix Tables A9 and A10, online only). Among all patients, the 5-year cumulative incidence of death decreased from 16.35% in 1990-1994 to 9.6% in 2000-2005 (P < .001), and the 10-year cumulative incidence of death decreased from 19.86% in 1990-1994 to 16.07% in 1995-1999 (P < .001). This decrease was primarily due to reduction in the 5-year cumulative incidence of death following relapse/disease progression from 12.83% in 1990-1994 to 7.22% in 2000-2005 (P < .001), with similar reductions in 10-year rates between 1990-1994 and 1995-1999. Among infants (Appendix Table A9), the 5-year cumulative incidence of death changed little between 1990-1994 and 2000-2005 (52.1% v 50.3%; P = .45), but the causes of death changed considerably. The 5-year cumulative incidence of death following relapse/disease progression decreased from 43% in 1990-1994 to 27.2% in 2000-2005 (P < .001), and the cumulative incidence of treatment-related death increased from 3.9% in 1990-1994 to 13.9% in 2000-2005 (P < .001).
Table 2.
Cumulative Incidence of Death After Relapse/Disease Progression v Death As a First Event in COG ALL Trials
| Death As a First or Subsequent Event | Cumulative Incidence (%) |
P * | |||
|---|---|---|---|---|---|
| 1990-1994 | 1995-1999 | 2000-2005 | Overall | ||
| 5-year | |||||
| Relapse/disease progression or secondary malignancies as first event | 12.83 | 9.03 | 7.22 | 9.82 | < .001 |
| Treatment-related death prior to relapse/disease progression | 2.16 | 1.92 | 1.57 | 1.89 | .0335 |
| Unknown or unrelated | 1.37 | 1.36 | 0.81 | 1.19 | .0013 |
| Overall | 16.35 | 12.31 | 9.60 | 12.90 | < .001 |
| 10-year | |||||
| Relapse/disease progression or secondary malignancies as first event | 15.80 | 12.45 | — | 12.98 | < .001 |
| Treatment-related death prior to relapse/disease progression | 2.17 | 1.95 | — | 1.91 | .3413 |
| Unknown or unrelated | 1.89 | 1.67 | — | 1.61 | .7149 |
| Overall | 19.86 | 16.07 | 16.50 | < .001 | |
Abbreviations: ALL, acute lymphoblastic leukemia; COG, Children's Oncology Group.
P values in the 5-year category were computed by comparing the corresponding cumulative incidence curves among all three eras; P values in the 10-year category were for comparison between the first two eras (1990-1994 v 1995-1999).
The highest relative risks of death occurred in known high-risk subgroups (Table 3). The relative risk of death was 2.3-fold (1990–1994) to 3.1-fold (2000–2005) higher for patients age 10+ years versus those age 1 to 9.99 years. The differences were even larger when those age 1 to 9.99 years were compared with infants age ≤ 1 year and adolescents age ≥ 15 years. We observed modest but statistically significant sex-based differences in survival, with males having a relative risk of death 1.2- to 1.3-fold higher than that of females. Self-described blacks had an increased relative risk of death compared with whites. Although information about ethnicity was not available for approximately 30% (6,509 of 21,626) of patients, self-described Hispanics (n = 2,589) had a higher relative risk of death than non-Hispanics. Leukemia immunophenotype was prognostic, with patients who had T-cell ALL having a higher relative risk of death than those with B-cell ALL. Patients with NCI high-risk ALL had a 2.4- to 3.6-fold higher relative risk of death than those with standard-risk ALL. However, because most patients have NCI standard-risk clinical features, a significant proportion of deaths occurred among the favorable prognosis subgroups (Table 4). Five-year survival of patients with NCI standard-risk ALL was 90% to 95% between 1990 and 2005 (Table 1 and Appendix Fig A6A), but approximately 36% of total deaths occurred among this subset.
Table 3.
Five-Year RR of Death by Era and Characteristic
| Patient Group | Era |
|||||
|---|---|---|---|---|---|---|
| 1990-1994 |
1995-1999 |
2000-2005 |
||||
| RR | P | RR | P | RR | P | |
| Age, years | ||||||
| 1-9.99 | 1.0 | 1.0 | 1.0 | |||
| ≥ 10 | 2.26 | < .001 | 2.40 | < .001 | 3.10 | < .001 |
| ≥ 15 | 2.61 | < .001 | 2.65 | < .001 | 3.97 | < .001 |
| < 1 | 3.65 | < .001 | 4.92 | < .001 | 7.81 | < .001 |
| Sex | ||||||
| Female | 1.0 | 1.0 | 1.0 | |||
| Male | 1.17 | < .001 | 1.34 | < .001 | 1.16 | .0213 |
| Race* | ||||||
| White | 1.0 | 1.0 | 1.0 | |||
| Black | 1.73 | < .001 | 1.60 | < .001 | 1.37 | .0119 |
| Other | 1.58 | < .001 | 1.16 | .0121 | 1.27 | .0056 |
| Ethnicity* | ||||||
| Non-Hispanic | 1.0 | 1.0 | 1.0 | |||
| Hispanic or Latino | 1.31 | .0024 | 1.16 | .0645 | 1.47 | < .001 |
| Immunophenotype | ||||||
| B cell | 1.0 | 1.0 | 1.0 | |||
| T cell | 1.75 | < .001 | 1.46 | < .001 | 2.04 | < .001 |
| NCI risk group† | ||||||
| Standard risk | 1.0 | 1.0 | 1.0 | |||
| High risk | 2.42 | < .001 | 2.52 | < .001 | 3.59 | < .001 |
NOTE. P values compare RR of the death to the baseline value defined as RR of 1.0 for each characteristic.
Abbreviations: NCI, National Cancer Institute; RR, relative risk.
Self-reported race or ethnicity.
Standard, age 1-9.99 years and initial WBC < 50,000/μL; high, age ≥ 10 years and/or initial WBC ≥ 50,000/μL.
Table 4.
Number of Deaths by Era and Presenting Characteristics
| Patient Group | Total No. of Patients | Projected No. of Deaths in 5 Years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1990-1994 |
1995-1999 |
2000-2005 |
1990-2005 |
||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| All patients | 21,626 | 1,194 | 100 | 882 | 100 | 687 | 100 | 2,763 | 100 |
| Age group, years | |||||||||
| ≤ 1 | 461 | 80 | 6.7 | 77 | 8.7 | 74 | 10.8 | 231 | 8.4 |
| 1-9.99 | 16,578 | 662 | 55.5 | 461 | 52.0 | 324 | 46.9 | 1,447 | 52.2 |
| ≥ 10 | 4,587 | 453 | 37.9 | 346 | 39.2 | 283 | 41.3 | 1,082 | 39.1 |
| 10-14.99 | 3,072 | 298 | 24.9 | 211 | 24.0 | 150 | 21.8 | 659 | 23.8 |
| ≥ 15 | 1,515 | 155 | 13.0 | 135 | 15.3 | 135 | 19.7 | 425 | 15.4 |
| Sex | |||||||||
| Male | 12,155 | 714 | 59.8 | 557 | 63.0 | 402 | 58.6 | 1,673 | 60.5 |
| Female | 9,471 | 480 | 40.2 | 326 | 37.1 | 285 | 41.6 | 1,091 | 39.6 |
| Race | |||||||||
| White | 15,759 | 727 | 60.9 | 547 | 61.9 | 493 | 71.8 | 1,767 | 63.9 |
| Black | 1,474 | 129 | 10.8 | 101 | 11.4 | 53 | 7.7 | 283 | 10.2 |
| Other | 4,393 | 341 | 28.6 | 235 | 26.7 | 141 | 20.6 | 717 | 26.0 |
| Ethnicity | |||||||||
| Hispanic | 2,589 | 98 | 8.2 | 93 | 10.5 | 170 | 24.7 | 361 | 13.1 |
| Non-Hispanic | 12,528 | 470 | 39.4 | 388 | 43.9 | 473 | 69.0 | 1,331 | 48.2 |
| Unknown | 6,509 | 626 | 52.4 | 402 | 45.6 | 43 | 6.3 | 1,071 | 38.7 |
| Immunophenotype | |||||||||
| B cell | 16,895 | 768 | 64.3 | 684 | 77.5 | 537 | 78.1 | 1,989 | 72.0 |
| T cell | 1,831 | 121 | 13.7 | 84 | 12.3 | 424 | 15.3 | ||
| NCI risk group | |||||||||
| Standard | 14,002 | 438 | 36.7 | 323 | 36.6 | 233 | 34.0 | 994 | 36.0 |
| High | 7,154 | 677 | 56.7 | 484 | 54.9 | 366 | 53.3 | 1,527 | 55.3 |
Abbreviation: NCI, National Cancer Institute.
For patients older than age 1 year, era, sex, race, immunophenotype, and NCI risk group were all significant prognostic factors in the multivariate Cox regression model (Table 5). To better understand the most important factors predicting risk of death, we performed survival tree regression modeling (Appendix Fig A7A, online only). This analysis showed that NCI risk group was the most significant overall prognostic factor. Among NCI standard-risk patients, era (2000-2005 v 1990-1999) was the most significant prognostic factor. For NCI high-risk patients, age (1 to 14.99 v ≥ 15 years) was the most prognostic factor. Among NCI high-risk patients younger than age 15 years, race was the most significant prognostic factor, with survival for black/other being inferior to that of whites. For adolescents age ≥ 15 years, era was the most significant prognostic factor, with better survival rates in 1995-2005 compared with 1990 to 1994.
Table 5.
Multivariate Cox Regression Analysis for Patients Older Than Age 1 Year
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Sex | |||
| Female v male | 0.83 | 0.77 to 0.90 | < .001 |
| Race | |||
| White v black | 0.84 | 0.73 to 0.97 | .0208 |
| White v other | 0.64 | 0.56 to 0.72 | < .001 |
| Age, years | |||
| 1-9 v 15+ | 0.53 | 0.46 to 0.60 | < .001 |
| 10-14 v 15+ | 0.71 | 0.63 to 0.81 | < .001 |
| NCI risk group | |||
| Standard v high | 0.48 | 0.42 to 0.53 | < .001 |
| Immunophenotype | |||
| B cell v T cell | 0.76 | 0.68 to 0.84 | < .001 |
| Era | |||
| 1995-1999 v 1990-1994 | 0.73 | 0.67 to 0.80 | < .001 |
| 2000-2005 v 1990-1995 | 0.56 | 0.50 to 0.62 | < .001 |
Abbreviation: HR, hazard ratio; NCI, National Cancer Institute.
For infants, age considered as a continuous variable was the most important prognostic factor (Appendix Fig A7B). The best age cutoff among infants was 92 days, with 5-year survival rates of 57.8% and 25.6% for age ≥ 92 days and less than 92 days, respectively (P < .001). Among infants ≥ 92 days old, WBC was the most significant predictor of survival, with higher WBC resulting in poorer outcome.
DISCUSSION
This study, which includes the largest childhood ALL cohort ever reported, documents progressive improvements in survival for children with ALL enrolled onto COG clinical trials between 1990-1994 and 2000-2005. Five-year survival increased from 83.7% to 90.4% during this time. The improved survival was explained primarily by an approximately 44% decrease in the risk of death following relapse/disease progression. Although we examined overall and not event-free survival (EFS) and cannot comment directly on changes in the incidence of relapse, a study of almost 10,000 children treated on COG ALL trials between 1988 and 2002, including 1,961 who relapsed, showed no significant improvements in survival after relapse over time.26 Taken together with results of COG ALL clinical trials showing significant improvements in EFS during this period, we believe that the major reason for improved survival was decreased risk of relapse.14,15,17
Our cohort includes 18,501 (55.8%) of 33,139 US ALL cases in persons age 0 to 14.99 years predicted to occur between 1990 and 2005. Thus, our results are representative of survival following contemporary therapy in the United States and are consistent with previous reports of outcomes for children younger than age 15 years diagnosed with cancer between 1990 and 1994 enrolled onto COG trials.27,28 In contrast, only approximately 25% of adolescents age 15 to 19 years diagnosed with cancer were enrolled onto COG trials between 1990 and 1994.27 Our data are similar, with 33.5% (1,392 of 4,159) of US adolescents age 15 to 19.99 years predicted to develop ALL between 1990 and 2005 enrolling onto COG trials. There are a variety of reasons that children and adolescents with ALL might not be included in COG ALL trials, including participation in trials conducted by other centers,6,8 lack of an open study at the time of diagnosis, having the patient/parent decline participation, or failure to meet eligibility criteria. Most US children with ALL still enroll onto COG trials. In 2009, 1,951 (68%) of 2,869 US children and adolescents age 0 to 19.99 years predicted to develop ALL enrolled onto a COG trial, including 1,758 (69%) of 2,540 of those age 0 to 14.99 years and 168 (51%) of 329 of those age 15 to 19.99 years. The number of older adolescents with ALL enrolling onto COG trials has increased over time, which is an important trend, given the higher survival rates obtained with pediatric versus adult ALL trials for this age group.29–31
Pulte et al12,13 reported survival for US children and adults diagnosed with ALL between 1990 and 2004 by using the SEER 9 Registries database, which includes about 30 million people. Our results show higher survival rates than the SEER-estimated 5-year survival of 87.5% from 2000-2004 for children younger than age 15 years and 61.1% for those age 15 to 19 years. We found 5-year survival rates of 91.4% for US children younger than age 15 years and 74.5% for those age 15 to 19 years in 2000-2005. It is unlikely that the slight difference in time period analyzed (SEER: 2000-2004 v COG: 2000-2005) accounts for these differences. There may be differences based on clinical trial enrollment because the SEER data include all patients reported to tumor registries in the specified areas, although the COG data are based on patients who met eligibility criteria and were offered and accepted clinical trial enrollment. The SEER 9 population is more urban and includes a higher percentage of foreign-born persons than the overall US population; these differences might contribute to observed differences in survival. There was a 13% absolute survival advantage for older adolescents in this COG cohort compared with the SEER estimates, consistent with the significant survival advantages for older adolescents with ALL treated on COG versus adult cooperative group trials.29 These data emphasize that optimal treatment for an older adolescent with ALL is referral to a pediatric center and enrollment onto a pediatric cooperative group trial.
In our analyses, survival improvements occurred in every subgroup analyzed with the exception of infants age ≤ 1 year (Table 1 and Appendix Figs A1 to A6). The magnitude of the decrease in risk of death between 1990-1994 and 2000-2005 was generally similar among non–infant subgroups and ranged from approximately 30% to 50%.
There were no survival improvements for infants enrolled onto COG ALL trials between 1990 and 2005 (Appendix Fig A1A). Infants contributed disproportionately to deaths because they accounted for only 2.1% (461 of 21,626) of patients but 8.0% (231 of 2,878) of deaths (P < .001). During this period, the COG pursued several strategies to attempt to increase survival for infants with ALL. Chemotherapy treatment was intensified significantly in the Children's Cancer Group (CCG) 1953 and COG P9407 trials, and the use of stem-cell transplantation in first remission was explored for those with MLL gene rearrangements. Stem-cell transplantation was not beneficial for infants in these COG ALL trials,16 and treatment intensification shifted the causes of death, with a significant decrease in death following relapse/disease progression but a parallel increase in death related to toxicity, with no net improvement in survival (Appendix Table A9). The Interfant99 infant ALL trial (1999–2005) obtained results similar to those of the COG trials, with 4-year EFS of only 48%.32 Although Interfant99 showed a benefit for stem-cell transplantation among a select high-risk subgroup of infants, survival was still poor for these patients.33 Infant ALL is a unique high-risk subset that requires new therapeutic strategies.
Survival for self-reported blacks with ALL improved significantly during the eras examined. The absolute difference in 5-year survival between blacks and whites decreased from 11.0% in 1990-1994 to 3.3% in 2000-2005. There are race-based differences in ALL biology, with blacks having a higher incidence of T-cell ALL and other high-risk features.34 Among the COG patients, 17.7% of blacks versus 9.5% of whites had T-cell ALL (P < .001) and 44.5% of blacks had NCI high-risk features versus 32.9% of whites (P < .001). Thus, black children and adolescents with ALL are predicted to have an inferior survival compared with whites because of the different percentages of ALL subtypes in the two racial groups. However, within these ALL subtypes, although patient numbers are small, our results show decreases in the racial outcome gap between 1990-1994 and 2000-2005. The absolute difference in 5-year survival for blacks versus whites with T-cell ALL decreased from 5.0% in 1990-1994 (94 blacks) to 0.02% in 2000-2005 (57 blacks). Similarly, for children with NCI high-risk B-cell ALL, the gap decreased from 11.1% in 1990-1994 (129 blacks) to 6.6% in 2000-2005 (53 blacks).
Self-described ethnicity is another important risk factor in childhood ALL. Hispanics have inferior outcomes to non-Hispanics.35 Our study confirms this observation, with some differences in the magnitude of differences in the three eras (Tables 1 and 3). Importantly, there was much greater capture of information regarding self-described ethnicity in 2000-2005, with only 3.6% (261 of 7,153) of unknown ethnic group compared with 43.2% (6,284 of 14,473) in 1990-1999. We observed a 1.5-fold higher risk of death for Hispanics versus non-Hispanics in 2000-2005. A variety of reasons might account for the inferior outcome of Hispanics. For example, recent investigations have shown a much higher incidence of certain high-risk leukemia cell genomic alterations in Hispanics enrolled onto COG ALL trials.36
Simple demographic and clinical prognostic factors can identify patient subsets with significantly increased risk of death: infants, adolescents age ≥ 10 or ≥ 15 years, T-cell ALL, and NCI high-risk ALL. The relative risks of death for these subsets ranged from about two- to eight-fold higher than for lower-risk subsets (Table 3). However, 36% of total deaths occurred among patients with NCI standard-risk ALL. Thus, efforts to decrease ALL deaths must focus both on high-risk patient subsets and on the large subset of patients with favorable clinical characteristics. Detailed biologic characterization of lymphoblasts and host germline variability and sophisticated measurements of early treatment response can improve identification of ultra-low-risk patient subsets and identify patients at high risk of treatment failure.18,36–47
We analyzed whether death occurred as a first event or after relapse/induction failure to investigate whether observed survival improvements were due to better front-line antileukemia therapy, better supportive care leading to a decrease in non–leukemia-related death, or both. Five to six times as many deaths occurred following relapse/disease progression compared with toxicity (Table 2). Although the cumulative incidence of deaths related to toxicity is relatively low at approximately 2%, it accounted for a higher percentage of overall deaths as the rate of death from leukemia decreases and was a particular problem among infants. Prevention of treatment-related deaths must be a critical component of efforts to improve childhood ALL survival.
Ten-year survival was 3% to 4% lower than 5-year survival in 1990-1999, when 84% of deaths occurred within 5 years, and only 1% occurred more than 10 years following diagnosis (Appendix Tables A2 to A4). Given this lower 10-year survival rate, the death rates observed in the 2000-2005 era (Appendix Tables A5 and A6), and the shape of the survival curves (Fig 1), we believe that it is extremely unlikely that there will be a significant increase in deaths beyond 5 years for patients diagnosed in 2000-2005, and we anticipate significant improvements in 10-year survival. We anticipate that the 10-year survival rate for children treated on COG ALL trials in 2006-2010 will approach or exceed 90%. The trend from 1990 to 2005 predicts an absolute 2% to 3% increment in survival during each 5-year era. More importantly, randomized COG ALL clinical trials conducted between 1995 and 2005 established superior treatment regimens that then became the baseline therapy for COG trials conducted in 2006 to 2010.4,5,14,15
This report underscores the remarkable improvements in the outcomes for childhood ALL since Farber et al48 first described temporary remissions in 1948. The ongoing discovery of important biologic subsets of ALL36,38–40,46 will further refine risk stratification and facilitate the combination of molecularly targeted therapies with chemotherapy. As proof of principle, addition of imatinib to chemotherapy resulted in a dramatic increase in survival for pediatric Philadelphia chromosome–positive ALL.49 As these changes occur, it will remain essential to closely assess the survival for the majority of US children, adolescents, and young adults with ALL who are treated on COG clinical trials.
Acknowledgment
We thank the thousands of children and parents who participated in these clinical trials and the physicians, nurses, and other medical professionals who participated in their care as well as Sean Altekruse from the National Institutes of Health and Rashid Aminou from Information Management Services for their assistance with SEER data.
Appendix
Table A1.
Clinical Trials Included in Present Report
| Trial No. | Trial Name | NCT ID | Accrual Closed | Reference |
|---|---|---|---|---|
| CCG 1881 | Treatment of Newly Diagnosed ALL in Children Aged 2-9 Inclusive With WBC < 10,000/μL | December 15, 1992 | Hutchinson RJ, et al: J Clin Oncol 21:1790-1797, 2003 | |
| CCG 1882 | Treatment of Newly Diagnosed ALL in Children With a Poor Prognosis Excluding Infants and Patients With Lymphoma-Leukemia or FAB L3 Blasts | June 23, 1995 | Nachman JB, et al: N Engl J Med 338:1663-1671, 1998; Nachman J, et al: J Clin Oncol 16:920-930, 1998 | |
| CCG 1883 | Treatment of Newly Diagnosed ALL in Infants Less Than 12 Months of Age | August 25, 1993 | Reaman GH, et al: J Clin Oncol 17:445-455, 1999 | |
| CCG 1891 | Treatment of Newly Diagnosed ALL in Children With an Intermediate Prognosis | July 20, 1990 | Lange BJ, et al: Blood 99:825-833, 2002 | |
| CCG 1901 | Phase III Protocol for the Treatment of Newly Diagnosed Childhood Acute Lymphoblastic Leukemia with Multiple Poor-Risk Factors Exclusive of FAB L3 Leukemia | September 9, 1994 | Heath JA, et al: J Clin Oncol 21:1612-1617, 2003 | |
| CCG 1922 | Phase III Randomized Comparison of Intravenous vs Oral Mercaptopurine During Consolidation and of Prednisone vs Dexamethasone during Induction, Consolidation, and Maintenance in Children with Good-Prognosis and Intermediate-Prognosis ALL Receiving Standard Chemotherapy | August 1, 1995 | Bostrom BC, et al: Blood 101:3809-3817, 2003 | |
| CCG-1952 | Randomized Comparisons of Oral Mercaptopurine vs. Oral Thioguanine and Intrathecal Methotrexate vs. Intrathecal Methotrexate/Cytarabine/Hydrocortisone for Standard Acute Lymphoblastic Leukemia | NCT00002744 | February 1, 2000 | Stork LC, et al: Blood 115:2740-2748, 2010 |
| CCG 1953 | Treatment of Newly Diagnosed ALL in Infants < 1 Year of Age | August 31, 2000 | Hilden JM, et al: Blood 108:441-451, 2006 | |
| CCG-1961 | Treatment of Patients With Acute Lymphoblastic Leukemia With Unfavorable Features: A Phase III Group-Wide Study | NCT00002812 | May 1, 2002 | Seibel NL et al14 |
| CCG 1962 | A Randomized Comparison of PEG and Native E. coli Asparaginases in the Standard Arm of CCG-1952 for Standard Risk ALL | November 10, 1998 | Avramis VI, et al: Blood 99:1986-94, 2002 | |
| CCG-1991 | Escalating Dose Intravenous Methotrexate Without Leucovorin Rescue Versus Oral Methotrexate and Single Versus Double Delayed Intensification for Children With Standard Risk Acute Lymphoblastic Leukemia | NCT00005945 | January 31, 2005 | Matloub Y et al15 |
| POG 8602 | Evaluation of Treatment Regimens in Acute Lymphoid Leukemia of Childhood (ALinC #14) | January 7, 1991 | Harris MB, et al: Leukemia 14:1570-6, 2000 | |
| POG 8698 | Up Front Alternating Chemotherapy vs Up Front Intensive 6-Mercaptopurine/Methotrexate for Acute Lymphocytic Leukemia In Childhood - A Pediatric Oncology Group Pilot Study | January 7, 1991 | Camitta B, et al: J Clin Oncol 12:1383-9, 1994 | |
| POG 8699 | Intensive Intravenous Treatment for Acute Lymphocytic Leukemia in Childhood - A Pediatric Oncology Group Pilot Study | January 7, 1991 | Mahoney DH Jr, et al: Cancer 75:2623-31, 1995 | |
| POG 8704 | T-Cell #3 Protocol - A Pediatric Oncology Group Phase III Study | January 9, 1992 | Amylon MD, et al: Leukemia 13:335-42, 1999 | |
| POG 9005 | Phase III Comparison of Intensification with Intr avenous Mercaptopurine plus Intermediate-Dose Intravenous Methotrexate vs Low-Dose Oral Methotrexate Following Induction with Prednisone/Vincristine/Asparaginase in Children with Low-Risk ALL | September 1, 1994 | Mahoney DH Jr, et al: J Clin Oncol 16:246-54, 1998 | |
| POG 9006 | Up-Front Intensive 6-Mercaptopurine/Methotrexate vs. Up-Front Alternating Chemotherapy for Acute Lymphocytic Leukemia in Childhood - A Randomized Pediatric Oncology Group Phase III Study | November 1, 1994 | Lauer SJ, et al: Leukemia 15:1038-45, 2001 | |
| POG 9086 | Phase I Pilot Study of Therapy for T-cell ALL or NHL | April 24, 1992 | ||
| POG 9107 | Infant Leukemia Protocol - A Pediatric Oncology Groupwide Pilot Study | June 15, 1993 | Maloney KW, et al: Leukemia 14:2276-85, 2000 | |
| POG 9201 | ALinC #16 Treatment for Patients With Lesser Risk Acute Lymphoblastic Leukemia—A Pediatric Oncology Group Phase III Study | November 15, 1999 | Chauvenet AR, et al: Blood 110:1105-11, 2007 | |
| POG 9202 | ALinC #16: Protocol for Patients with Standard Risk Acute Lymphoblastic Leukemia - A Pediatric Oncology Group Limited-Institution Pilot Study | October 1, 1994 | ||
| POG 9203 | ALinC #16: Pilot Study for Patients with High-Risk ALL - A Pediatric Oncology Group Limited-Institution Pilot Study | April 28, 1994 | Salzer WL, et al: J Pediatr Hematol Oncol 29:369-75, 2007 | |
| POG 9295 | T-Cell #4 “A” Pilot (With PEG-l-Asparaginase) - A Pediatric Oncology Group Limited-Institution Pilot Study | December 1, 1993 | ||
| POG 9296 | T-Cell #4 Pilot “B” (With Intravenous Methotrexate/Intravenous 6-Mercaptopurine) - A Pediatric Oncology Group Limited-Institution Pilot Study | June 15, 1993 | ||
| POG 9297 | T-Cell #4 Pilot “C” (With Intermediate Dose Methotrexate/Intravenous 6-Mercaptopurine and High-Dose Cytosine Arabinoside) - A Pediatric Oncology Group Pilot Study | June 15, 1993 | ||
| POG 9398 | Efficacy of recombinant human granulocyte-colony stimulating factor in an Intensive Treatment for T-Cell Leukemia and Advanced-Stage Lymphoblastic Lymphoma of Childhood - A Pediatric Oncology Group-Wide Pilot Study | December 15, 1994 | ||
| POG-9404 | Intensive Treatment for T-Cell Acute Lymphoblastic Leukemia and Advanced Stage Lymphoblastic Non-Hodgkin's Lymphoma: A Pediatric Oncology Group Phase III Study | NCT01230983 | September 10, 2001 | Asselin B, et al: Blood 118:874-883, 2011 |
| POG 9405 | ALinC 16: Protocol for Patients with Newly Diagnosed Standard Risk Acute Lymphoblastic Leukemia (ALL) | December 26, 1995 | ||
| POG 9406 | ALinC 16: Protocol for Patients With Newly Diagnosed High Risk Acute Lymphoblastic Leukemia (ALL) | November 15, 1999 | ||
| POG 9407 | Induction Intensification and Allogeneic Bone Marrow Transplant In Infant ALL: A Children's Oncology Group Pilot Study | NCT00002756 | October 29, 2006 | Dreyer ZE et al16 |
| POG 9605 | ALinC 16: Protocol for Patients With Newly Diagnosed Standard Risk Acute Lymphoblastic Leukemia (ALL) | November 15, 1999 | ||
| POG 9904 | ALinC #17 Treatment for Patients With Low Risk Acute Lymphoblastic Leukemia: A Pediatric Oncology Group Phase III Study | NCT00005585 | April 15, 2005 | Martin PL, et al: Pediatr Blood Cancer 51:58, 2008 |
| POG 9905 | ALinC 17: Protocol for Patients With Newly Diagnosed Standard Risk Acute Lymphoblastic Leukemia (ALL): A Phase III Study | NCT00005596 | April 15, 2005 | Winick N et al17 |
| POG 9906 | ALinC 17: Protocol for Patients With Newly Diagnosed High Risk Acute Lymphoblastic Leukemia (ALL) - Evaluation of the Augmented BFM Regimen: A Phase III Study | NCT00005603 | April 25, 2003 | Bowman WP, et al: Pediatr Blood Cancer 57:569-577, 2011 |
| COG-AALL00P2 | The Use Of Modified BFM +/− Compound 506U78) (NSC# 686673) In an Intensive Chemotherapy Regimen For The Treatment Of T-Cell Leukemia | NCT00016302 | October 4, 2005 | Dunsmore KP, et al: J Clin Oncol 26:539s, 2008 (suppl; abstr 10002) |
| COG-AALL0232 | High Risk B-Precursor Acute Lymphoblastic Leukemia | NCT00075725 | January 21, 2011 | Larsen EC, et al: J Clin Oncol 29:6s, 2011 (suppl; abstr 3) |
Abbreviations: ALinC, acute leukemia in children; ALL, acute lymphoblastic leukemia; BFM, Berlin-Frankfurt-Munster; CCG, Children's Cancer Group; COG, Children's Oncology Group; FAB, French-American-British [leukemia classification system]; ID, identification; NCT, numbered clinical trial; NHL, non-Hodgkin's lymphoma; PEG, polyethylene glycol; POG, Pediatric Oncology Group.
Table A2.
Number of Patients Who Died Within 0-4.99, 5-9.99, and ≥ 10 Years of Diagnosis for Patients Enrolled in 1990-1999
| Patient Group | Time to Death (years) |
Total No. of Deaths | |||||
|---|---|---|---|---|---|---|---|
| 0-4.99 |
5-9.99 |
≥ 10 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| All patients | 2,041 | 83.85 | 367 | 15.08 | 26 | 1.07 | 2,434 |
| Age group, years | |||||||
| ≤ 1 | 156 | 98.73 | 2 | 1.27 | 0 | 0 | 158 |
| 1-9.99 | 1,103 | 79.3 | 270 | 19.41 | 18 | 1.29 | 1,391 |
| ≥ 10 | 782 | 88.36 | 95 | 10.73 | 8 | 0.9 | 885 |
| 10-14.99 | 500 | 87.11 | 69 | 12.02 | 5 | 0.87 | 574 |
| ≥ 15 | 282 | 90.68 | 26 | 8.36 | 3 | 0.96 | 311 |
| Sex | |||||||
| Male | 1,248 | 83.26 | 232 | 15.48 | 19 | 1.27 | 1,499 |
| Female | 793 | 84.81 | 135 | 14.44 | 7 | 0.75 | 935 |
| Race | |||||||
| White | 1,257 | 82.48 | 244 | 16.01 | 23 | 1.51 | 1,524 |
| Black | 224 | 86.49 | 34 | 13.13 | 1 | 0.39 | 259 |
| Other | 560 | 86.02 | 89 | 13.67 | 2 | 0.31 | 651 |
| Ethnicity | |||||||
| Hispanic | 184 | 84.79 | 32 | 14.75 | 1 | 0.46 | 217 |
| Non-Hispanic | 841 | 82.78 | 169 | 16.63 | 6 | 0.59 | 1,016 |
| Unknown | 1,016 | 84.6 | 166 | 13.82 | 19 | 1.58 | 1,201 |
| Immunophenotype | |||||||
| B cell | 1,426 | 81.86 | 298 | 17.11 | 18 | 1.03 | 1,742 |
| T cell | 335 | 91.78 | 25 | 6.85 | 5 | 1.37 | 365 |
| Risk group | |||||||
| Standard | 746 | 76.75 | 214 | 22.02 | 12 | 1.23 | 972 |
| High | 1,139 | 87.35 | 151 | 11.58 | 14 | 1.07 | 1,304 |
Table A3.
Number of Patients Age ≤ 1 Year Who Died Within 0-4.99, 5-9.99, and ≥ 10 Years of Diagnosis for Patients Enrolled in 1990-1999
| Patient Group | Time to Death (years) |
Total No. of Deaths | |||||
|---|---|---|---|---|---|---|---|
| 0-4.99 |
5-9.99 |
≥ 10 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Age, years | |||||||
| ≤ 1 | 156 | 98.73 | 2 | 1.27 | 0 | 0 | 158 |
| Sex | |||||||
| Male | 78 | 97.5 | 2 | 2.5 | 0 | 0 | 80 |
| Female | 78 | 100 | 0 | 0 | 0 | 0 | 78 |
| Race | |||||||
| White | 103 | 98.1 | 2 | 1.9 | 0 | 0 | 105 |
| Black | 9 | 100 | 0 | 0 | 0 | 0 | 9 |
| Other | 44 | 100 | 0 | 0 | 0 | 0 | 44 |
| Ethnicity | |||||||
| Hispanic | 18 | 100 | 0 | 0 | 0 | 0 | 18 |
| Non-Hispanic | 73 | 100 | 0 | 0 | 0 | 0 | 73 |
| Unknown | 65 | 97.01 | 2 | 2.99 | 0 | 0 | 67 |
| Immunophenotype | |||||||
| B cell | 123 | 98.4 | 2 | 1.6 | 0 | 0 | 125 |
| T cell | 2 | 100 | 0 | 0 | 0 | 0 | 2 |
Table A4.
Number of Patients Older Than Age 1 Year Who Died Within 0-4.99, 5-9.99, or ≥ 10 Years of Diagnosis for Patients Enrolled in 1990-1999
| Patient Group | Time to Death (years) |
Total | |||||
|---|---|---|---|---|---|---|---|
| 0-4.99 |
5-9.99 |
≥ 10 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| All patients | 1,885 | 82.82 | 365 | 16.04 | 26 | 1.14 | 2,276 |
| Sex | |||||||
| Male | 1,170 | 82.45 | 230 | 16.21 | 19 | 1.34 | 1,419 |
| Female | 715 | 83.43 | 135 | 15.75 | 7 | 0.82 | 857 |
| Race | |||||||
| White | 1,154 | 81.32 | 242 | 17.05 | 23 | 1.62 | 1,419 |
| Black | 215 | 86 | 34 | 13.6 | 1 | 0.4 | 250 |
| Other | 516 | 85.01 | 89 | 14.66 | 2 | 0.33 | 607 |
| Ethnicity | |||||||
| Hispanic | 166 | 83.42 | 32 | 16.08 | 1 | 0.5 | 199 |
| Non-Hispanic | 768 | 81.44 | 169 | 17.92 | 6 | 0.64 | 943 |
| Unknown | 951 | 83.86 | 164 | 14.46 | 19 | 1.68 | 1,134 |
| Immunophenotype | |||||||
| B cell | 1,303 | 80.58 | 296 | 18.31 | 18 | 1.11 | 1,617 |
| T cell | 333 | 91.74 | 25 | 6.89 | 5 | 1.38 | 363 |
Table A5.
Number of Patients Who Died Within 0-4.99 or 5-9.99 Years of Diagnosis for Patients Enrolled In 2000-2005
| Patient Group | Time to Death (years) |
Total No. of Deaths | |||
|---|---|---|---|---|---|
| 0-4.99 |
5-9.99 |
||||
| No. of Patients | % | No. of Patients | % | ||
| All patients | 618 | 91.56 | 57 | 8.44 | 675 |
| Age group, years | |||||
| ≤ 1 | 73 | 100 | 0 | 0 | 73 |
| 1-9.99 | 285 | 88.79 | 36 | 11.21 | 321 |
| ≥ 10 | 260 | 92.53 | 21 | 7.47 | 281 |
| 10-14.99 | 140 | 93.33 | 10 | 6.67 | 150 |
| ≥ 15 | 120 | 91.6 | 11 | 8.4 | 131 |
| Sex | |||||
| Male | 363 | 90.75 | 37 | 9.25 | 400 |
| Female | 255 | 92.73 | 20 | 7.27 | 275 |
| Race | |||||
| White | 445 | 90.82 | 45 | 9.18 | 490 |
| Black | 48 | 92.31 | 4 | 7.69 | 52 |
| Other | 125 | 93.98 | 8 | 6.02 | 133 |
| Ethnicity | |||||
| Hispanic | 154 | 91.12 | 15 | 8.88 | 169 |
| Non-Hispanic | 425 | 91.4 | 40 | 8.6 | 465 |
| Unknown | 39 | 95.12 | 2 | 4.88 | 41 |
| Immunophenotype | |||||
| B cell | 482 | 90.6 | 50 | 9.4 | 532 |
| T cell | 81 | 97.59 | 2 | 2.41 | 83 |
| Risk group | |||||
| Standard | 203 | 88.65 | 26 | 11.35 | 229 |
| High | 341 | 91.67 | 31 | 8.33 | 372 |
Table A6.
Number of Patients Who Died Within 0-4.99 or 5-9.99 Years of Diagnosis for Patients Older Than Age 1 Year Enrolled in 2000-2005*
| Patient Group | Time to Death (years) |
Total No. of Deaths | |||
|---|---|---|---|---|---|
| 0-4.99 |
5-9.99 |
||||
| No. of Patients | % | No. of Patients | % | ||
| All patients | 545 | 90.53 | 57 | 9.47 | 602 |
| Sex | |||||
| Male | 318 | 89.58 | 37 | 10.42 | 355 |
| Female | 227 | 91.9 | 20 | 8.1 | 247 |
| Race | |||||
| White | 390 | 89.66 | 45 | 10.34 | 435 |
| Black | 45 | 91.84 | 4 | 8.16 | 49 |
| Other | 110 | 93.22 | 8 | 6.78 | 118 |
| Ethnicity | |||||
| Hispanic | 142 | 90.45 | 15 | 9.55 | 157 |
| Non-Hispanic | 373 | 90.31 | 40 | 9.69 | 413 |
| Unknown | 30 | 93.75 | 2 | 6.25 | 32 |
| Immunophenotype | |||||
| B cell | 416 | 89.27 | 50 | 10.73 | 466 |
| T cell | 81 | 97.59 | 2 | 2.41 | 83 |
All the deaths that occurred in infants age ≤ 1 year who were enrolled in 2000-2005 occurred within 5 years of initial diagnosis.
Table A7.
Five-Year Overall Survival of Infants (age ≤ 1 year) by Era
| Patient Group | No. | 5-Year Survival ± SE (%) |
% Reduction2000-2005 v 1990-1994 | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1990-1994 |
1995-1999 |
2000-2005 |
|||||||
| % | No. of Patients | % | No. of Patients | % | No. of Patients | ||||
| Age, years | |||||||||
| ≤ 1 | 461 | 47.9 ± 4.1 | 154 | 48.1 ± 4.3 | 148 | 53.2 ± 5.4 | 159 | 10 | .4520 |
| Sex | |||||||||
| Male | 233 | 46.2 ± 5.6 | 82 | 46.9 ± 6.5 | 65 | 45.8 ± 7.0 | 86 | −1 | .9299 |
| Female | 228 | 49.9 ± 6.1 | 72 | 49.0 ± 5.7 | 83 | 61.6 ± 8.0 | 73 | 23 | .2754 |
| Race | |||||||||
| White | 322 | 46.4 ± 5.1 | 101 | 50.1 ± 5.3 | 99 | 54.3 ± 6.0 | 122 | 15 | .5909 |
| Black | 25 | — | 12 | — | 6 | — | 7 | — | — |
| Other | 114 | 48.5 ± 8.2 | 41 | 45.7 ± 7.7 | 43 | 48.3 ± 12.3 | 30 | 0 | .8509 |
| Ethnicity | |||||||||
| Hispanic | 63 | 64.3 ± 13.6 | 14 | 38.5 ± 10.7 | 22 | 54.0 ± 13.0 | 27 | −29 | .4606 |
| Non-Hispanic | 274 | 52.9 ± 5.5 | 83 | 54.8 ± 6.0 | 76 | 53.8 ± 6.5 | 115 | 2 | .9982 |
| Unknown | 124 | 36.7 ± 6.7 | 57 | 52.0 ± 7.2 | 50 | 47.1 ± 14.0 | 17 | 16 | .8070 |
| Immunophenotype | |||||||||
| B cell | 382 | 45.9 ± 4.8 | 115 | 48.7 ± 4.8 | 120 | 54.3 ± 5.6 | 147 | 16 | .2434 |
| T cell | 6 | — | 2 | — | 2 | — | 2 | — | — |
Table A8.
Five-Year Overall Survival of Non-Infants (older than age 1 year) by Era
| Patient Group | Total No. of Patients | 5-Year Survival ± SE (%) |
% Reduction 2000-2005 v 1990-1994 | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1990-1994 |
1995-1999 |
2000-2005 |
|||||||
| % | No. of Patients | % | No. of Patients | % | No. of Patients | ||||
| All patients | 21,165 | 84.4 ± 0.4 | 7,150 | 88.5 ± 0.4 | 7,021 | 91.3 ± 0.4 | 6,994 | 44 | < .001 |
| Sex | |||||||||
| Male | 11,922 | 83.4 ± 0.6 | 4,035 | 86.9 ± 0.6 | 3,992 | 90.9 ± 0.6 | 3,895 | 45 | < .001 |
| Female | 9,243 | 85.8 ± 0.6 | 3,115 | 90.6 ± 0.6 | 3,029 | 91.8 ± 0.7 | 3,099 | 42 | < .001 |
| Race | |||||||||
| White | 15,437 | 87.0 ± 0.5 | 5,196 | 89.7 ± 0.5 | 4,289 | 92.0 ± 0.5 | 5,412 | 38 | < .001 |
| Black | 1,449 | 75.7 ± 2.0 | 509 | 81.3 ± 1.8 | 517 | 88.4 ± 2.1 | 423 | 52 | < .001 |
| Other | 4,279 | 77.8 ± 1.2 | 1,445 | 87.3 ± 0.9 | 1,675 | 89.2 ± 1.2 | 1,159 | 51 | < .001 |
| Ethnicity | |||||||||
| Hispanic | 2,526 | 82.5 ± 1.8 | 533 | 87.8 ± 1.4 | 653 | 88.3 ± 1.1 | 1,340 | 33 | .0036 |
| Non-Hispanic | 12,254 | 87.9 ± 0.6 | 3,543 | 89.3 ± 0.6 | 3,301 | 92.3 ± 0.5 | 5,410 | 36 | < .001 |
| Unknown | 6,385 | 80.8 ± 0.7 | 3,074 | 87.9 ± 0.6 | 3,067 | 86.3 ± 2.9 | 244 | 28 | < .001 |
| Immunophenotype | |||||||||
| B cell | 16,518 | 85.8 ± 0.5 | 4,957 | 89.1 ± 0.4 | 5,716 | 92.0 ± 0.5 | 5,845 | 44 | < .001 |
| T cell | 1,825 | 70.8 ± 1.7 | 746 | 80.8 ± 1.7 | 622 | 81.5 ± 2.2 | 457 | 37 | < .001 |
Table A9.
Cumulative Incidence of Death After Relapse/Disease Progression v Death As a First Event for Infants Age ≤ 1 Year by Cause in COG ALL Trials
| Death As a First or Subsequent Event | Cumulative Incidence (%) |
P | |||
|---|---|---|---|---|---|
| 1990-1994 | 1995-1999 | 2000-2005 | Overall | ||
| 5-year | |||||
| Relapse/disease progression or secondary malignancies as first event | 43.03 | 15.89 | 27.21 | 28.92 | < .001 |
| Treatment-related death prior to relapse/disease progression | 3.90 | 27.17 | 13.87 | 14.79 | < .001 |
| Unknown or unrelated | 5.21 | 8.88 | 5.72 | 6.56 | .2774 |
| Overall | 52.14 | 51.94 | 46.80 | 50.27 | .4503 |
| 10-year | |||||
| Relapse/disease progression or secondary malignancies as first event | 43.73 | 15.89 | — | 29.20 | < .001 |
| Treatment-related death prior to relapse/disease progression | 3.90 | 27.17 | — | 14.79 | < .001 |
| Unknown or unrelated | 5.21 | 9.86 | — | 6.95 | .1454 |
| Overall | 52.83 | 52.92 | 50.93 | .6231 | |
Abbreviations: ALL, acute lymphoblastic leukemia; COG, Children's Oncology Group.
Table A10.
Cumulative Incidence of Death After Relapse/Disease Progression v Death As a First Event for Patients Older Than Age 1 Year by Cause in COG ALL Trials
| Death As a First or Subsequent Event | Cumulative Incidence (%) |
P | |||
|---|---|---|---|---|---|
| 1990-1994 | 1995-1999 | 2000-2005 | Overall | ||
| 5-year | |||||
| Relapse/disease progression or secondary malignancies as first event | 12.18 | 8.89 | 6.74 | 9.40 | < .001 |
| Treatment-related death prior to relapse/disease progression | 2.12 | 1.39 | 1.28 | 1.60 | < .001 |
| Unknown or unrelated | 1.29 | 1.20 | 0.70 | 1.08 | < .001 |
| Overall | 15.58 | 11.48 | 8.72 | 12.07 | < .001 |
| 10-year | |||||
| Relapse/disease progression or secondary malignancies as first event | 15.20 | 12.38 | — | 12.62 | < .001 |
| Treatment-related death prior to relapse/disease progression | 2.13 | 1.42 | — | 1.62 | .0012 |
| Unknown or unrelated | 1.82 | 1.50 | — | 1.49 | .4130 |
| Overall | 19.15 | 15.31 | 15.74 | < .001 | |
Abbreviations: ALL, acute lymphoblastic leukemia; COG, Children's Oncology Group.
Fig A1.
Overall survival probability for patients with acute lymphoblastic leukemia of different ages enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005; (A) infants age ≤ 1 year, and persons age (B) 1 to 9.99 years, (C) 10 years or older, and (D) 15 years or older.
Fig A2.
Overall survival probability for (A) males and (B) females enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005.
Fig A3.
Overall survival probability for (A) whites, (B) blacks, and (C) persons of other ethnicities enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005.
Fig A4.
Overall survival probability for persons who reported themselves as (A) Hispanic, (B) non-Hispanic, or (C) unknown ethnicity enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005.
Fig A5.
Overall survival probability by leukemia immunophenotype for patients with (A) B-cell and (B) T-cell acute lymphoblastic leukemia enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005.
Fig A6.
Overall survival probability by National Cancer Institute risk group for patients with (A) standard-risk (age 1-9.99 years and initial WBC count < 50,000/μL) and (B) high-risk (age 10 years or older and/or initial WBC count > 50,000/μL) acute lymphoblastic leukemia enrolled onto Children's Oncology Group trials in 1990-1994, 1995-1999, and 2000-2005.
Fig A7.
Survival tree regression analysis was performed by using complete data for (A) patients older than age 1 year (yr); and (B) infants age ≤ 1 year. NCI, National Cancer Institute; OS, overall survival.
Footnotes
Listen to the podcast by Dr Silverman at www.jco.org/podcasts
Supported by Chair's Grant No. U10 CA98543, Statistics and Data Center Grant No. U10 CA98413, Community Clinical Oncology Program Grant No. U10 CA95861, and Human Specimen Banking Grant No. U24 CA114766 to the Children's Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Presented as an abstract at the 40th Congress of the International Society of Pediatric Oncology, Berlin, Germany,October 2-6, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Paul S. Gaynon, EUSA Pharma, sanofi-aventis, Teva Pharmaceutical Industries, Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Paul S. Gaynon, Enzon Pharmaceuticals, Sigma Tau Pharmaceuticals, Genzyme Research Funding: Paul S. Gaynon, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Stephen P. Hunger, Meenakshi Devidas, Bruce M. Camitta, Naomi J. Winick, Gregory H. Reaman, William L. Carroll
Provision of study materials or patients: Naomi J. Winick
Collection and assembly of data: Meenakshi Devidas, Gregory H. Reaman, William L. Carroll
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ries LAG, Smith MA, Gurney JG, et al., editors. NIH Pub. No. 99-4649. Bethesda, MD: 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. [Google Scholar]
- 2.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: Results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004) Lancet Oncol. 2009;10:957–966. doi: 10.1016/S1470-2045(09)70228-1. [DOI] [PubMed] [Google Scholar]
- 4.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: A report from the Children's Oncology Group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the Children's Cancer Group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children's Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 8.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conter V, Aricò M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 10.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, et al. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–319. doi: 10.1038/leu.2009.258. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell C, Richards S, Harrison CJ, et al. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980-2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. 2008;100:1301–1309. doi: 10.1093/jnci/djn276. [DOI] [PubMed] [Google Scholar]
- 13.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 14.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2011;118:243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreyer ZE, Dinndorf PA, Camitta B, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: A report from the Children's Oncology Group. J Clin Oncol. 2011;29:214–222. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winick N, Martin PL, Devidas M, et al. Delayed intensification (DI) enhances event-free survival (EFS) of children with B-precursor acute lymphoblastic leukemia (ALL) who received intensification therapy with six courses of intravenous methotrexate (MTX): POG 9904/9905–A Childrens Oncology Group Study (COG) ASH Annual Meeting Abstracts. 2007;110:583. [Google Scholar]
- 18.Schultz KR, Pullen DJ, Sather HN, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: A combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG) Blood. 2007;109:926–935. doi: 10.1182/blood-2006-01-024729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 23.Breiman L, Friedman JH, Olshen RA, et al. Wadsworth, Belmont: 1984. Classification and Regression Trees. [Google Scholar]
- 24.LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics. 1992;48:411–425. [PubMed] [Google Scholar]
- 25.Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleyer WA, Tejeda H, Murphy SB, et al. National cancer clinical trials: Children have equal access; adolescents do not. J Adolesc Health. 1997;21:366–373. doi: 10.1016/S1054-139X(97)00110-9. [DOI] [PubMed] [Google Scholar]
- 28.Bleyer WA, Tejeda HA, Murphy SB, et al. Equal participation of minority patients in U.S. national pediatric cancer clinical trials. J Pediatr Hematol Oncol. 1997;19:423–427. doi: 10.1097/00043426-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nachman JB, La MK, Hunger SP, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: A report from the Children's Oncology Group. J Clin Oncol. 2009;27:5189–5194. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 32.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): An observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 33.Mann G, Attarbaschi A, Schrappe M, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: Results from the Interfant-99 Study. Blood. 2010;116:2644–2650. doi: 10.1182/blood-2010-03-273532. [DOI] [PubMed] [Google Scholar]
- 34.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: A Pediatric Oncology Group Study. J Clin Oncol. 2000;18:813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 35.Kadan-Lottick NS, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 36.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children's Oncology Group study. Blood. 2008;111:5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullighan CG, Collins-Underwood JR, Phillips LA, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treviño LR, Yang W, French D, et al. Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet. 2009;41:1001–1005. doi: 10.1038/ng.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang JJ, Cheng C, Yang W, et al. Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. JAMA. 2009;301:393–403. doi: 10.1001/jama.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang H, Chen IM, Wilson CS, et al. Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood. 2010;115:1394–1405. doi: 10.1182/blood-2009-05-218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: Correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010;116:4874–4884. doi: 10.1182/blood-2009-08-239681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 46.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: A genome-wide classification study. Lancet Oncol. 2009;10:125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 48.Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787–793. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- 49.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A Children's Oncology Group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]