Abstract
Purpose
Lung cancer is the leading cause of cancer-related mortality. Intensive care unit (ICU) use among patients with cancer is increasing, but data regarding ICU outcomes for patients with lung cancer are limited.
Patients and Methods
We used the Surveillance, Epidemiology, and End Results (SEER) –Medicare registry (1992 to 2007) to conduct a retrospective cohort study of patients with lung cancer who were admitted to an ICU for reasons other than surgical resection of their tumor. We used logistic and Cox regression to evaluate associations of patient characteristics and hospital mortality and 6-month mortality, respectively. We calculated adjusted associations for mechanical ventilation receipt with hospital and 6-month mortality.
Results
Of the 49,373 patients with lung cancer admitted to an ICU for reasons other than surgical resection, 76% of patients survived the hospitalization, and 35% of patients were alive 6 months after discharge. Receipt of mechanical ventilation was associated with increased hospital mortality (adjusted odds ratio, 6.95; 95% CI, 6.89 to 7.01; P < .001), and only 15% of these patients were alive 6 months after discharge. Of all ICU patients with lung cancer, the percentage of patients who survived 6 months from discharge was 36% for patients diagnosed in 1992 and 32% for patients diagnosed in 2005, whereas it was 16% and 11% for patients who received mechanical ventilation, respectively.
Conclusion
Most patients with lung cancer enrolled in Medicare who are admitted to an ICU die within 6 months of admission. To improve patient-centered care, these results should guide shared decision making between patients with lung cancer and their clinicians before an ICU admission.
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality in the United States (US).1 The percentage of patients with cancer who receive care in an intensive care unit (ICU) at the end of life is increasing.2–4 Approximately 1%5 of the 55,000 patients cared for daily in ICUs6 are admitted for cancer-related organ failure, and 16% of these patients may be diagnosed with lung cancer.7 Mortality estimates for patients with lung cancer admitted to ICUs have varied widely and were based on small cohorts.8–15 Despite recent improvements in the care of critically ill patients,16–19 and the hope that these improvements apply to patients with lung cancer,20 to our knowledge, no studies have examined temporal trends in outcomes among critically ill patients with lung cancer. Finally, patients older than 65 years comprise 56% of all ICU admissions,6 and long-term survival among this population is low, particularly among beneficiaries who receive mechanical ventilation (MV).21
A better understanding of mortality trends and factors associated with mortality may lead to more informed patient-clinician discussions about the prognosis of critical illness among patients with lung cancer. Accordingly, we sought to evaluate the outcomes of patients with lung cancer admitted to an ICU for reasons other than surgical tumor resection.
PATIENTS AND METHODS
Design and Setting
We conducted a retrospective cohort study by using the Surveillance, Epidemiology, and End Results (SEER) –Medicare registry, which is a publicly available database from the National Cancer Institute. The SEER program collects information regarding cancer site, stage, and histology for persons newly diagnosed with cancer who reside in one of the SEER geographic areas. The master enrollment file of Medicare is used to identify which persons in SEER are Medicare beneficiaries and is accurate for the identification of SEER registry cases for people older than 65 years.24 For people who are Medicare eligible, the SEER-Medicare data include claims for covered health care services, including hospital, physician, and hospice bills. We used data from the Patient Entitlement and Diagnosis File and the Medicare Provider Analysis and Review (MedPAR) file. We conducted the study under a data-use agreement with the Centers for Medicare and Medicaid Services (CMS) and received approval from the institutional review board of the Portland Veterans Affairs Medical Center.
Patients
Patients were eligible if they were identified in SEER as receiving a diagnosis of lung cancer between 1992 and 2005 (N = 324,509) and were also enrolled in Medicare during that time. Medicare-use data were available through 2007. We sequentially excluded patients whose lung cancer was diagnosed at autopsy or on a death certificate only (n = 7,650), patients with an in situ stage (n = 195), patients younger than 66 years at the time of diagnosis (n = 56,048), patients initially enrolled in Medicare through the Social Security Disability Income mechanism (n = 25,078), and patients enrolled in health maintenance organizations between 1 year before and 5 years after a lung cancer diagnosis (n = 59,629) because these patients had incomplete claims data and, therefore, were excluded from previous studies that have used Medicare billing records.21,25–30 Patients with more than one primary cancer diagnosis were included, but dates and histologies were based on the lung cancer diagnosis. If patients had more than one diagnosis of primary lung cancer, the date of the first diagnosis was used. Finally, patients admitted to an ICU whose discharge date was after their date of death (n = 153) were excluded from all analyses because this likely indicated data-entry errors.
ICU Admission
An ICU admission for reasons other than postoperative surgical lung cancer resection (hereafter described as ICU admission unless otherwise specified) was defined as (1) a MedPAR ICU room charge and/or a procedure code for MV by using the International Classification of Diseases (ninth edition) procedure codes (96.70 to 96.72) in any of the six procedure fields, or a diagnosis related group code that indicated MV (475 or 483) and (2) ICU admissions that occurred greater than 30 days (pre and post) of recording a surgical lung resection procedural code (32.1 to 32.59).31 We only evaluated ICU admissions that occurred up to 5 years after the diagnosis of lung cancer through 2007. Unless specified, we report the first occurrence of an ICU admission for patients with more than one ICU admission.
Covariates
We obtained age, sex, race/ethnicity, histology, and stage at diagnosis from SEER. Income classification was based on the zip code of the subject as supplied by the CMS according to year 2000 census estimates. We calculated a comorbidity index on the basis of inpatient Medicare data from 1 year before the index ICU admission by using the Deyo adaptation of the Charlson index32 with the program supplied by the National Cancer Institute.33 ICU type was obtained from MedPAR. Hospital characteristics (number of beds and teaching status) were based on data supplied by the CMS. The 22 discharge destination codes used by MedPAR were reduced to home, another hospital, skilled nursing facility (SNF), or hospice and were used to estimate functional status at discharge. Procedure codes that included noninvasive ventilation (93.90 and 93.92) were tallied separately from MV. The date of death was obtained from MedPAR with the last date recorded through June 2008. Patients whose date of death was the same day as the date of discharge from the hospital were coded as not surviving the hospital stay.
Analysis
All statistical analyses were performed by using SAS (Version 9.2; SAS Institute, Cary, NC) and SPSS (Version 19.0; SPSS, Chicago, IL). Descriptive statistics included counts with frequencies, means with ranges, and counts per year. Odds ratios were calculated for associations with hospital mortality. We calculated hazard ratios with Cox regression models with the time measured from hospital admission to death or censorship before 6 months after admission. For the adjusted analyses of associations of MV with hospital mortality and 6-month mortality, we a priori adjusted for age (in 5-year increments), sex, race/ethnicity (white, black, or other race), income (in $15,000 increments), comorbidity index (0, 1, 2, or ≥ 3), year of lung cancer diagnosis (continuous), histologic type of cancer (non–small-cell lung cancer, small-cell lung cancer, and unknown), stage (local, regional, distant, and unknown), time from diagnosis to being admitted (divided in quartiles), teaching status (teaching v nonteaching), number of beds (< 250, 251 to 449, and ≥ 450), and type of ICU (medical, cardiac, intermediate, and other). We examined the associations of MV with mortality for effect modification by race/ethnicity, histology, and stage at diagnosis. To assess for effect modification, likelihood ratio tests were conducted to assess interactions between the variables of interest. A variable was classified as an effect modifier if the P value for the interaction was less than 0.05.
RESULTS
Survival Among Patients With Lung Cancer With and Without Mechanical Ventilation
We identified 175,756 patients with lung cancer. From this cohort, 49,373 patients (28%) had at least one ICU admission for reasons other than surgical resection of their lung cancer during the 5-year period after diagnosis and/or through 2007 (Table 1). After the first ICU admission, 37,748 patients (76%) survived to hospital discharge, and 17,099 patients (35%) were still alive 6 months after discharge. Of hospital survivors after an ICU admission, 24,846 patients (66%) were discharged to home, 3,475 patients (9%) were discharged to another hospital, 6,853 patients (18%) were discharged to a SNF, and 1,463 patients (4%) were discharged to hospice. Of the 15,528 patients with an intermediate type of ICU code but without a code for MV, 14,054 patients (91%) survived the hospitalization, and of these patients, 72% of patients were discharged to home, 16% of patients were discharged to an SNF, and 6.1% of patients were discharged to another hospital. Six months after discharge, 41% of all intermediate care ICU patients were still alive.
Table 1.
Patient Demographics and Clinical Characteristics of Cohort Stratified by ICU Admission, Hospital Survival After ICU Admission, and6-Month Survival After ICU Admission
| Demographic or Characteristic | Total ICU Admissions (N = 49,373) |
Hospital Survivors (n = 37,748) |
6-Month Survivors (n = 17,099) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||
| 65-69 | 9,984 | 20.2 | 7,758 | 20.6 | 3,882 | 22.7 |
| 70-74 | 14,384 | 29.1 | 11,068 | 29.3 | 5,320 | 31.1 |
| 75-79 | 13,060 | 26.4 | 9,929 | 26.3 | 4,427 | 25.9 |
| 80-84 | 7,923 | 16.1 | 6,031 | 16.0 | 2,446 | 14.3 |
| 85-89 | 3,159 | 6.4 | 2,307 | 6.1 | 812 | 4.8 |
| > 90 | 863 | 1.8 | 655 | 1.7 | 212 | 1.2 |
| Women | 22,271 | 45.1 | 17,378 | 46.0 | 8,179 | 47.8 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 41,650 | 84.6 | 32,147 | 85.4 | 14,723 | 86.3 |
| Black | 4,216 | 8.6 | 3,050 | 8.1 | 1,234 | 7.2 |
| Other | 3,350 | 6.8 | 2,438 | 6.5 | 1,104 | 6.5 |
| Median annual income, thousands of dollars* | ||||||
| < 15 | 9,263 | 19.8 | 6,882 | 19.2 | 3,306 | 20.4 |
| 15-29.9 | 6,211 | 13.3 | 4,728 | 13.2 | 1,968 | 12.2 |
| 30-44.99 | 12,376 | 26.4 | 9,568 | 26.7 | 4,205 | 26.0 |
| 45-59.99 | 9,719 | 20.7 | 7,511 | 21.0 | 3,423 | 21.1 |
| 60-74.99 | 4,914 | 10.5 | 3,731 | 10.4 | 1,712 | 10.6 |
| ≥ 75 | 4,371 | 9.3 | 3,362 | 9.4 | 1,580 | 9.8 |
| Comorbidity† | ||||||
| 0 | 37,367 | 75.7 | 28,612 | 75.8 | 13,227 | 77.4 |
| 1 | 5,312 | 10.8 | 4,061 | 10.8 | 1,846 | 10.8 |
| 2 | 3,582 | 7.3 | 2,727 | 7.2 | 1,110 | 6.5 |
| ≥ 3 | 3,112 | 6.3 | 2,348 | 6.2 | 916 | 5.4 |
| Stage at diagnosis | ||||||
| Local | 9,782 | 19.8 | 8,224 | 21.8 | 5,498 | 32.2 |
| Regional | 13,293 | 26.9 | 10,374 | 27.5 | 5,478 | 32.0 |
| Distant | 22,538 | 45.7 | 16,360 | 43.3 | 4,889 | 28.6 |
| Unknown | 3,760 | 7.6 | 2,790 | 7.4 | 1,234 | 7.2 |
| Lung cancer histology | ||||||
| NSCLC | 39,645 | 80.3 | 30,775 | 81.5 | 14,214 | 83.1 |
| SCLC | 6,488 | 13.1 | 4,589 | 12.2 | 1,849 | 10.8 |
| Other | 3,240 | 6.6 | 2,384 | 6.3 | 1,036 | 6.1 |
| Teaching status | ||||||
| Teaching hospital | 27,016 | 54.7 | 20,392 | 54.0 | 9,467 | 55.4 |
| Nonteaching hospital | 19,174 | 38.8 | 14,912 | 39.5 | 6,605 | 38.6 |
| Unknown | 3,183 | 6.5 | 2,444 | 6.5 | 1,027 | 6.0 |
| Number of beds | ||||||
| < 250 | 19,888 | 40.3 | 15,299 | 40.5 | 6,671 | 39.0 |
| 251-449 | 18,191 | 36.8 | 13,927 | 36.9 | 6,332 | 37.0 |
| ≥ 450 | 8,193 | 16.6 | 6,145 | 16.3 | 3,097 | 18.1 |
| Unknown | 3,101 | 6.3 | 2,377 | 6.3 | 999 | 5.8 |
| Assisted ventilation | ||||||
| Mechanical ventilation‡ | 10,463 | 21.2 | 4,325 | 11.4 | 1,536 | 9.0 |
| Noninvasive ventilation§ | 587 | 1.2 | 341 | 1.0 | 113 | 0.6 |
| Type of ICU | ||||||
| Medical | 21,859 | 44.3 | 15,528 | 41.1 | 7,025 | 41.1 |
| Surgical | 1,590 | 3.2 | 1,191 | 3.2 | 616 | 3.6 |
| Cardiac | 6,290 | 12.7 | 4,232 | 11.2 | 1,898 | 11.1 |
| Intermediate | 15,932 | 32.3 | 14,259 | 37.8 | 6,494 | 38.0 |
| Other | 2,943 | 6.0 | 2,288 | 6.1 | 990 | 5.8 |
| Unknown | 759 | 1.5 | 250 | 0.7 | 76 | 0.4 |
| Discharge location | ||||||
| Home | NA | 24,846 | 65.8 | 13,418 | 78.5 | |
| Another hospital | NA | 3,475 | 9.2 | 1,122 | 6.6 | |
| Skilled nursing facility | NA | 6,853 | 18.2 | 2,030 | 11.9 | |
| Hospice | NA | 1,463 | 3.9 | 64 | 0.4 | |
| Other | NA | 1,111 | 2.9 | 465 | 2.7 | |
| Length of hospital stay, days | ||||||
| Median | 7 | 7 | 6 | |||
| IQR | 4-12 | 4-12 | 3-10 | |||
| Time from diagnosis, days | ||||||
| Median | 76 | 76 | 120 | |||
| IQR | 19-328 | 19-347 | 23-509 | |||
NOTE. ICU admission was for nonsurgical reasons within 5 years of lung cancer diagnosis. Numbers may not sum secondary to missing data, and percentages are of nonmissing data.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; NA, not applicable; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer.
According to zip code data supplied by the Centers for Medicare and Medicaid Services.
Deyo-Charlson for inpatient codes.
From procedure codes 96.7X and/or diagnosis-related group codes 475 or 483.
From procedure codes 93.90 or 93.92.
Of all ICU patients with lung cancer, 10,463 patients (21%) received MV, 4,325 (41%) of whom survived the hospitalization. Among patients with lung cancer who received MV and survived hospitalization, 46%, 27%, and 5% of patients were discharged to home, SNF, and hospice, respectively. Of 587 patients with a code for noninvasive ventilation, 58% of patients were discharged, and of these patients, 58%, 22%, and 10% of patients were discharged to home, SNF, and hospice, respectively. At 6 months after discharge, 15% of patients who received MV were alive, whereas 19% of patients who received noninvasive ventilation were alive.
Factors Associated With Mortality
Univariate associations of multiple factors with hospital and 6-month mortality are shown in Table 2. Age was relatively weakly associated with hospital mortality. Notably, the number of comorbidities was not associated with hospital mortality, although it was weakly associated with 6-month mortality. The receipt of MV was strongly associated with increased hospital mortality (odds ratio, 8.65; 95% CI, 8.60 to 8.70; P < .001) and 6-month mortality (hazard ratio, 2.49; 95% CI, 2.52 to 2.46; P < .001). Compared with care in a higher-acuity medical ICU, intermediate-level ICU care was associated with a decreased risk of hospital mortality (odds ratio, 0.29; 95% CI, 0.23 to 0.35; P < .001) and 6-month mortality (hazard ratio, 0.74; 95% CI, 0.71 to 0.77; P < .001).
Table 2.
Univariate Associations With Hospital and 6-Month Mortality
| Demographic or Characteristic | Hospital Mortality |
6-Month Mortality |
||
|---|---|---|---|---|
| OR | P | HR | P | |
| Age, years | ||||
| 65-69 | Reference | Reference | ||
| 70-74 | 1.04 | .165 | 1.06 | .001 |
| 75-79 | 1.10 | .003 | 1.15 | < .001 |
| 80-84 | 1.09 | .012 | 1.25 | < .001 |
| 85-89 | 1.29 | < .001 | 1.45 | < .001 |
| ≥ 90 | 1.11 | .223 | 1.50 | < .001 |
| Sex | ||||
| Women | Reference | Reference | ||
| Men | 1.17 | < .001 | 1.11 | < .001 |
| Race/ethnicity | ||||
| Non-Hispanic white | Reference | Reference | ||
| Black | 1.29 | < .001 | 1.16 | < .001 |
| Other | 1.27 | < .001 | 1.06 | .006 |
| Median annual income, thousands of dollars* | ||||
| < 15 | Reference | Reference | ||
| 15-29.9 | 0.91 | .010 | 1.10 | < .001 |
| 30-44.99 | 0.85 | < .001 | 1.05 | .008 |
| 45-59.99 | 0.85 | < .001 | 1.03 | .178 |
| 60-74.99 | 0.92 | .025 | 1.04 | .081 |
| ≥ 75 | 0.87 | .001 | 0.99 | .625 |
| Comorbidity† | ||||
| 0 | Reference | Reference | ||
| 1 | 1.01 | .846 | 1.02 | .413 |
| 2 | 1.03 | .553 | 1.10 | < .001 |
| ≥ 3 | 1.06 | .156 | 1.14 | < .001 |
| Lung cancer histology | ||||
| NSCLC | Reference | Reference | ||
| SCLC | 1.44 | < .001 | 1.23 | < .001 |
| Other | 1.25 | < .001 | 1.16 | < .001 |
| Stage at diagnosis | ||||
| Local | Reference | Reference | ||
| Regional | 1.49 | < .001 | 1.53 | < .001 |
| Distant | 1.99 | < .001 | 2.51 | < .001 |
| Unknown | 1.84 | < .001 | 1.95 | < .001 |
| Time from diagnosis to admission, days | ||||
| < 19 | Reference | Reference | ||
| 19-75 | 1.27 | < .001 | 1.03 | .053 |
| 76-328 | 1.33 | < .001 | 1.00 | .820 |
| ≥ 329 | 0.96 | .166 | 0.69 | < .001 |
| Teaching status | ||||
| Teaching hospital | Reference | Reference | ||
| Nonteaching hospital | 0.88 | < .001 | 1.02 | .198 |
| Number of beds | ||||
| < 250 | Reference | Reference | ||
| 251-449 | 1.02 | .398 | 0.97 | .006 |
| ≥ 450 | 1.11 | .001 | 0.90 | < .001 |
| Mechanical ventilation | ||||
| No | Reference | Reference | ||
| Yes | 8.65 | < .001 | 2.49 | < .001 |
| Type of ICU | ||||
| Medical | Reference | Reference | ||
| Cardiac | 1.19 | < .001 | 1.04 | .023 |
| Intermediate | 0.29 | < .001 | 0.74 | < .001 |
| Other‡ | 0.82 | .001 | 0.81 | < .001 |
Abbreviations: HR, hazard ratio; ICU, intensive care unit; NSCLC, non–small-cell lung cancer; OR, odds ratio; SCLC, small-cell lung cancer.
According to zip code data supplied by the Centers for Medicare and Medicaid Services.
Deyo-Charlson for inpatient codes.
Surgical, other, and unknown.
Compared with not receiving MV, the adjusted associations for MV receipt were 6.95 (95% CI, 6.89 to 7.01; P < .001) with hospital mortality and 1.21 (95% CI, 1.16 to 1.26; P < .001) for 6-month mortality. The associations of MV receipt with hospital and 6-month mortality were not modified by race/ethnicity and lung cancer histology but were for stage (P for interaction < .001). For hospital mortality, the direction of the association was similar although lower in magnitude for distant stage (local stage, 7.94; 95% CI, 6.85 to 9.21; P < .001 v distant stage, 6.15; 95% CI, 5.65 to 6.70, P < .001).
Trends Over Time Among Patients With Lung Cancer
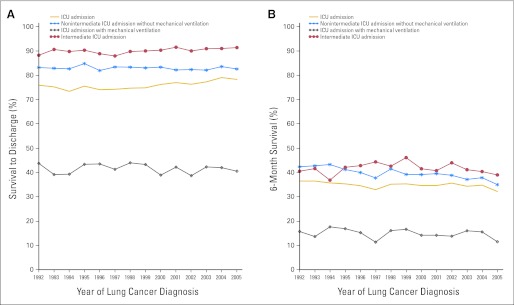
The percentage of patients with lung cancer who survived to hospital discharge after their first ICU admission was 76% for patients diagnosed in 1992 and 78% for patients diagnosed in 2005 (Fig 1A). For patients who received MV, the percentage of patients alive at discharge was 44% for patients diagnosed in 1992 and 40% for patients diagnosed in 2005. Of all ICU patients, the percentage of patients who survived 6 months from discharge was 36% for patients diagnosed in 1992 and 32% for patients diagnosed in 2005, whereas it was 16% and 11%, respectively, for patients who received MV (Fig 1B).
Fig 1.
Survival for patients with lung cancer for the first intensive care unit (ICU) admission for reasons other than postoperative surgical lung cancer resection within 5 years of lung cancer diagnosis by year of diagnosis. (A) Survival to hospital discharge for patients with lung cancer admitted to an ICU. (B) Survival to 6 months after hospital discharge for patients with lung cancer admitted to an ICU. The total cohort was included and categorized by receipt of mechanical ventilation, no receipt of mechanical ventilation and no receipt of care in an intermediate type of ICU, and care in an intermediate type of ICU.
During the study period, the percentage of overall ICU patients who survived hospitalization who were discharged to home was 76% for patients diagnosed in 1992 and 64% for patients diagnosed in 2005. Discharges to an SNF or hospice increased from 14% to 20% and 0% to 10%, respectively. For patients diagnosed in 1992 who survived to hospital discharge after receiving MV, 60%, 21%, and 0% of patients were discharged to home, SNF, or hospice, respectively; 45%, 30%, and 16% of patients were discharged to home, SNF, or hospice, respectively for patients diagnosed in 2005.
DISCUSSION
The incidence of patients with cancer who receive ICU care at the end of life is increasing in both the United States2,34 and Canada.4 Among Medicare beneficiaries with stage IIIB or IV lung cancer who died within a year of diagnosis, 18% of patients received care in an ICU in 1993 compared with 25% of patients in 2003.3 In this large observational study, the percentage of patients with lung cancer admitted to an ICU who survived hospitalization and were alive at 6 months did not improve from 1992 to 2005. Of all patients with lung cancer admitted to an ICU, about one quarter of patients died during the hospitalization, one half of patients were discharged home, and the rest of the patients were discharged to hospice, an SNF, or another hospital. Less than 20% of patients who received MV were discharged to home, and only 15% of patients were still alive 6 months after discharge. Finally, MV receipt was strongly associated with increased hospital mortality that was modestly modified by stage.2,34It was surprising that hospital survival among patients with lung cancer admitted to an ICU has not improved. Survival from respiratory failure and sepsis has improved over the last two decades16–19 and a recent review noted improvements in reported survival for critically ill patients with lung cancer when recent to older publications were compared.20 A possible explanation for our finding is that improvements in critical care were balanced by increases in the illness severity of patients. However, this explanation seems unlikely to fully explain the results because mortality was stable over time, even in a more homogeneous group of patients who received mechanical ventilation.
The ability to evaluate individual illness severity by using the SEER-Medicare registry is limited, but patients in our study were probably less severely ill than patients in previous reports; in-hospital mortality was 25% compared with 22% to 85%8,10–15 in previous studies. The lower mortality seen in our study may have been partly due to our decision to include patients who received care in an intermediate-level ICU. Intermediate care units provide care to less acutely ill patients who often do not require life-sustaining services. Patients also received MV less often than in previous studies (21% compared with 40% to 91%, respectively).8,9,11–15 In addition, traditional mortality predictors such as age and comorbidities were weakly or not associated with hospital mortality, which suggested that clinicians may restrict critical care interventions in older and more debilitated patients with lung cancer. Previous studies indicated that clinicians make decisions to limit care on the basis of age and comorbidity,35 and their perception of the risk of death is a strong predictor of decisions to limit life-support interventions.36 By including patients cared for in intermediate ICUs, combined with the factors described, we likely biased our results toward better outcomes. More research into the use of ICU resources may help elucidate how clinicians and patients with lung cancer make decisions regarding the use of critical care.
Mechanically ventilated patients experienced increased mortality compared with nonventilated patients, similar to what other studies have shown among Medicare beneficiaries,21 patients with cancer in general,37 and patients with lung cancer.8,11–15 As an example, 70% of Medicare patients admitted to an ICU who received MV were alive 6 months after discharge.21 In our study, the magnitude and direction of associations between MV receipt and mortality were similar for patients of different race/ethnicities and lung cancer histologies but varied between stages, generally with modestly increased associations for an earlier stage. One explanation for this finding might be that patients with advanced stages experience high mortality regardless of whether they received MV. The result may also indicate that patients with advanced stages of cancer who receive MV have an otherwise lower severity of illness compared with ventilated patients with earlier stages, which again suggests that clinicians and patients limit critical care on the basis of decisions about prognosis.
Our results have important implications for providers who care for patients with lung cancer and highlight that critical care in general, and MV in particular, when delivered to patients with lung cancer, is strongly associated with death and disability. For many patients, receipt of these services may not be consistent with their goals of care. In the SUPPORT trial, 58% of patients with lung cancer in the hospital reported a preference to focus on comfort rather than extending life, and 81% of patients reported not wanting to receive MV indefinitely.38 In addition, there is evidence that clinicians often do not discuss important goals of critical care before their need.11,39 For example, a single center reported that none of the patients with stage IV lung cancer who were admitted to the ICU had a documented discussion of code status before admission. Among the patients admitted to the ICU with a full code status, 77% of patients later changed to their status to do not resuscitate while still in the ICU.11 Furthermore, in an observational study of 603 patients with advanced cancer, the discussion of end-of-life issues was associated with lower rates of ICU and MV use.40 These issues highlight the importance of discussions between patients with lung cancer and their clinicians about post-ICU outcomes.
There were several limitations of this study. First, we used administrative records, and thus, there was certainly misclassification of cancer and exposure information that likely attenuated associations.41 Furthermore, there are multiple reasons why patients with lung cancer require MV; because we did not evaluate ICU admission diagnoses, we could not determine how specific causes of respiratory failure differentially influenced the association between MV mortality. Common causes of respiratory failure in this population include coexisting cardiopulmonary diseases, pneumonia, sepsis, lung cancer and its typical complications, and lung cancer treatments. Studies that can more accurately evaluate specific causes of respiratory failure are warranted to address this concern.
In addition, the SEER-Medicare registry does not include important predictors of ICU mortality, such as severity-of-illness scores, individual laboratory values, or vasopressor use, and communication between patients and clinicians regarding goals of care that have been associated with mortality in previous reports. These limitations are somewhat mitigated by using proxies for illness severity, such as codes for intermediate-level ICU care and MV. Given the large number of patients who received care in an intermediate-level ICU, our overall results were likely biased toward better outcomes. We had data on ICU admissions through 2007, and thus, patients diagnosed with lung cancer after 2002 did not have 5 years of follow-up time, which reduced the total number of patients with lung cancer in the cohort. However, it is unlikely that this affected the conclusions of the study because most patients were admitted within 1 year of diagnosis, the longest time from diagnosis to admittance was not associated with hospital mortality, and the analyses of MV and mortality adjusted for this factor. Finally, the patients in this study were older than 65 years and enrolled in fee-for-service Medicare, and thus, these results should not be considered applicable to other populations, such as younger patients or those enrolled in Medicare HMOs, without additional study.
In the largest study to date on ICU outcomes among patients with lung cancer throughout the US, inpatient and 6-month mortality did not improve for patients diagnosed from 1992 to 2005. Overall, the majority of patients survived to discharge, but only 50% of patients were discharged to home, and approximately one third of patients were still alive 6 months later. Outcomes were poorer for patients who received MV because less than 20% of patients were discharged home, and the vast majority of patients died within 6 months after discharge. The use of these results to guide shared decision making between patients with lung cancer and their clinicians may lead to improvements in patient-centered care.
Acknowledgment
This study was the result of work supported by resources from the Portland Veterans Affairs (VA) Medical Center, the VA Puget Sound Healthcare System, and the Edith Nourse Rogers Memorial VA Hospital.
Footnotes
Supported by a University of Washington Division of Pulmonary and Critical Care Medicine Endowment Grant (C.G.S), a Veterans Affairs (VA) Health Services Research and Development Career Development Award (Grant No. 10-025, C.G.S.), a VA Health Services Research and Development Fellowship (Grant. No. TPM 61-037; L.M.C.), a career development award from the National Cancer Institute (K07 CA 138772; R.S.W.), and the Robert Wood Johnson Foundation Clinical Scholars Program (C.R.C).
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The University of Washington and the Department of Veterans Affairs did not have a role in the conduct of the study, collection, management, analysis, or interpretation of data, or in preparation of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Christopher G. Slatore, Laura M. Cecere, Colin R. Cooke
Collection and assembly of data: Christopher G. Slatore, Jonathan P. Duckart
Data analysis and interpretation: Christopher G. Slatore, Laura M. Cecere, Jennifer L. LeTourneau, Maya E. O'Neil, Jonathan P. Duckart, Renda Soylemez Wiener, Farhood Farjah, Colin R. Cooke
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Horner MJ RL, Krapcho M, Neyman N, et al., editors. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute; 2009. http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 2.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma G, Freeman J, Zhang D, et al. Trends in end-of-life ICU use among older adults with advanced lung cancer. Chest. 2008;133:72–78. doi: 10.1378/chest.07-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587–1591. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groeger JS, Guntupalli KK, Strosberg M, et al. Descriptive analysis of critical care units in the United States: Patient characteristics and intensive care unit utilization. Crit Care Med. 1993;21:279–291. doi: 10.1097/00003246-199302000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Shorr AF, White A, et al. Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 7.Kress JP, Christenson J, Pohlman AS, et al. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160:1957–1961. doi: 10.1164/ajrccm.160.6.9812055. [DOI] [PubMed] [Google Scholar]
- 8.Boussat S, El'rini T, Dubiez A, et al. Predictive factors of death in primary lung cancer patients on admission to the intensive care unit. Intensive Care Med. 2000;26:1811–1816. doi: 10.1007/s001340000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennens RR, Rosenthal MA, Mitchell P, et al. Outcome of patients admitted to the intensive care unit with newly diagnosed small cell lung cancer. Lung Cancer. 2002;38:291–296. doi: 10.1016/s0169-5002(02)00219-2. [DOI] [PubMed] [Google Scholar]
- 10.Lin YC, Tsai YH, Huang CC, et al. Outcome of lung cancer patients with acute respiratory failure requiring mechanical ventilation. Respir Med. 2004;98:43–51. doi: 10.1016/j.rmed.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Reichner CA, Thompson JA, O'Brien S, et al. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130:719–723. doi: 10.1378/chest.130.3.719. [DOI] [PubMed] [Google Scholar]
- 12.Soares M, Darmon M, Salluh JI, et al. Prognosis of lung cancer patients with life-threatening complications. Chest. 2007;131:840–846. doi: 10.1378/chest.06-2244. [DOI] [PubMed] [Google Scholar]
- 13.Adam AK, Soubani AO. Outcome and prognostic factors of lung cancer patients admitted to the medical intensive care unit. Eur Respir J. 2008;31:47–53. doi: 10.1183/09031936.00031607. [DOI] [PubMed] [Google Scholar]
- 14.Roques S, Parrot A, Lavole A, et al. Six-month prognosis of patients with lung cancer admitted to the intensive care unit. Intensive Care Med. 2009;35:2044–2050. doi: 10.1007/s00134-009-1625-y. [DOI] [PubMed] [Google Scholar]
- 15.Toffart AC, Minet C, Raynard B, et al. Use of intensive care in patients with nonresectable lung cancer. Chest. 2011;139:101–108. doi: 10.1378/chest.09-2863. [DOI] [PubMed] [Google Scholar]
- 16.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 18.Danai PA, Moss M, Mannino DM, et al. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–1440. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 19.Pène F, Percheron S, Lemiale V, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;36:690–696. doi: 10.1097/CCM.0B013E318165314B. [DOI] [PubMed] [Google Scholar]
- 20.Soubani AO, Ruckdeschel JC. The outcome of medical intensive care for lung cancer patients: The case for optimism. J Thorac Oncol. 2011;6:633–638. doi: 10.1097/JTO.0b013e318200f9eb. [DOI] [PubMed] [Google Scholar]
- 21.Wunsch H, Guerra C, Barnato AE, et al. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 22. Reference deleted.
- 23. Reference deleted.
- 24.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 25.SEER-Medicare. Medicare claims files. 2011. http://healthservices.cancer.gov/seermedicare/medicare/claims.html.
- 26.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–1144. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: A population-based study. J Hepatol. 2006;44:158–166. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Schermerhorn ML, O'Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 29.Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361:22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollenbeak CS, Nikkel LE, Schaefer EW, et al. Determinants of Medicare all-cause costs among elderly patients with renal cell carcinoma. J Manag Care Pharm. 2011;17:610–620. doi: 10.18553/jmcp.2011.17.8.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.SEER-Medicare. Calculation of comorbidity weights. 2011 http://healthservices.cancer.gov/seermedicare/program/comorbidity.html#f4. [Google Scholar]
- 34.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 35.Garrouste-Orgeas M, Boumendil A, Pateron D, et al. Selection of intensive care unit admission criteria for patients aged 80 years and over and compliance of emergency and intensive care unit physicians with the selected criteria: An observational, multicenter, prospective study. Crit Care Med. 2009;37:2919–2928. doi: 10.1097/ccm.0b013e3181b019f0. [DOI] [PubMed] [Google Scholar]
- 36.Rocker G, Cook D, Sjokvist P, et al. Clinician predictions of intensive care unit mortality. Crit Care Med. 2004;32:1149–1154. doi: 10.1097/01.ccm.0000126402.51524.52. [DOI] [PubMed] [Google Scholar]
- 37.Soares M, Caruso P, Silva E, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: A prospective multicenter study. Crit Care Med. 2010;38:9–15. doi: 10.1097/CCM.0b013e3181c0349e. [DOI] [PubMed] [Google Scholar]
- 38.Claessens MT, Lynn J, Zhong Z, et al. Dying with lung cancer or chronic obstructive pulmonary disease: Insights from SUPPORT—Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48(suppl 5):S146–S153. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 39.Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25:150–153. doi: 10.1007/s11606-009-1161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver KE, Farley JH. Deciphering surveillance, epidemiology, and end results data analysis: are we seeing the whole picture? Cancer. 2011;117:4112–4115. doi: 10.1002/cncr.26027. [DOI] [PubMed] [Google Scholar]