INTRODUCTION
This review describes the basis for US Food and Drug Administration (FDA) approvals of treatments for metastatic breast cancer (MBC). We discuss the end points and regulatory pathways used in these approvals. This review will not address breast cancer drugs approved more than 30 years ago, because they were not approved under contemporary review standards and had broad indications irrespective of disease stage or prior treatment (line of therapy). These drugs include methotrexate, cyclophosphamide, thiotepa, vinblastine, fluorouracil, and doxorubicin. A future article will address the approval of hormonal agents.
The approval of an indication requires substantial evidence of efficacy and an acceptable benefit-to-risk evaluation from adequate and well-controlled investigations.1 Regular approvals of cancer drugs are based on the demonstration of clinical benefit, including improvement in overall survival (OS), disease-related symptoms, or an established surrogate for one of these.
In 1992, new regulations were implemented, allowing for the accelerated approval (AA) of drugs for serious or life-threatening diseases in which the drugs seem to provide benefit over available therapy. AA is usually based on a surrogate end point that is reasonably likely to predict clinical benefit.2 However, AA can also be based on a clinical end point other than survival or irreversible morbidity where there is uncertainty as to the relationship between the end point and ultimate outcome.3 For example, in the adjuvant breast cancer setting, improved disease-free survival may be the basis for AA, but longer follow-up may be needed because the effect of the drug on OS, and longer-term toxicity evaluation may be uncertain.
Sponsors are required to conduct clinical trials to confirm that the drug receiving AA provides clinical benefit. If a company fails to confirm clinical benefit or does not conduct the clinical trial(s) with due diligence, the regulations allow for withdrawal of approval.4
Randomized controlled trials (RCTs) are preferred to single-arm trials (SATs), because safety and efficacy are difficult to interpret in an SAT. Safety is difficult to evaluate in a noncomparative setting where adverse events may be related to either the cancer or drug toxicity. Although objective response rates (ORRs) may be evaluated in SATs, time-dependent end points including OS, progression-free survival (PFS), or time to progression (TTP) should be evaluated in RCTs. Apparent differences in outcome between historical controls and current treatment groups can arise from differences other than drug treatment, including patient selection, improved imaging techniques, or improved supportive care. An RCT minimizes the effect of these differences by providing a direct outcome comparison.
DRUGS APPROVED FOR SECOND- TO THIRD-LINE TREATMENT OF MBC
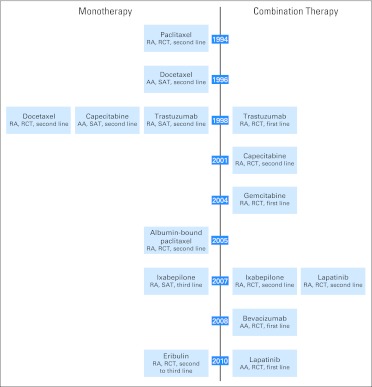
No drugs were approved for MBC between doxorubicin approval in 1974 and paclitaxel approval in 1994. In the last 13 years, the FDA approved eight drugs as monotherapy or in combination regimens: paclitaxel, docetaxel, trastuzumab, capecitabine, protein-bound paclitaxel, lapatinib, ixabepilone, and eribulin (Fig 1). Seven drug were approved under section 505(b)(1) and one drug under section 505(b)(2) of the Food, Drug, and Cosmetic Act.5
Fig 1.
Metastatic breast cancer regulatory approval timeline. AA, accelerated approval; RA, regular approval; RCT, randomized controlled trial; SAT, single-arm trial.
Paclitaxel
Paclitaxel received regular approval (RA) in 1994 for the treatment of MBC progressing after anthracycline therapy. The paclitaxel approval was supported by an RCT in 471 patients with MBC after failure of one or two prior chemotherapy regimens; 67% of these patients had received prior anthracyclines. The treatment arms compared two doses of paclitaxel: 175 mg/m2 and 135 mg/m2 administered as 3-hour intravenous infusions. A significantly longer TTP in the paclitaxel higher-dose arm was the basis for the paclitaxel approval, with a median TTP of 4.2 versus 3.0 months (hazard ratio [HR], 0.75; P = .027).6
Docetaxel
Docetaxel received AA in 1996 for the treatment of progressive MBC after prior chemotherapy. Safety and efficacy were evaluated in six SATs conducted in a total of 309 patients who had had prior therapy for MBC.7 An ORR of 37.9% (95% CI, 31.0 to 44.8) in patients with anthracycline-resistant MBC was the basis for AA. The postmarketing commitment for this AA was to submit results of ongoing RCTs in advanced breast cancer. The TAX304 study, an RCT of 392 patients with a history of prior treatment with an anthracycline-containing regimen,8 was the basis of docetaxel conversion from AA to RA. Patients were randomly assigned to receive either docetaxel 100 mg/m2 intravenously every 3 weeks or to the combination of mitomycin 12 mg/m2 intravenously every 6 weeks plus vinblastine 6 mg/m2 intravenously every 3 weeks. RA was based on a statistically significant survival advantage: median, 11.4 versus 8.7 months (HR, 0.73; 95% CI, 0.53 to 0.93; P = .01). A statistically significant advantage in TTP for patients treated with docetaxel was also observed, with a median of 4.3 versus 2.5 months (HR, 0.75; 95% CI, 0.61 to 0.94; P = .01).
Trastuzumab
Trastuzumab received RA in 1998 as a single agent for the treatment of MBC overexpressing the human epidermal growth factor receptor 2 (HER2) protein in patients who have received one or more chemotherapy regimens for metastatic disease. The trial supporting the approval was a single-agent SAT in 222 patients with HER2-overexpressing MBC. Patients had progressive disease after one (32%) or two (68%) prior chemotherapy regimens for metastatic disease.9 The initial trastuzumab dose was 4 mg/kg intravenously followed by a weekly dose of 2 mg/kg intravenously until disease progression. RA of trastuzumab for second-line MBC was based on an ORR of 14% (2% complete response rate), with a median response duration of 9 months. The trastuzumab approval for first-line treatment of MBC provided supportive evidence for its second-line approval.
Capecitabine
Single-agent capecitabine received AA in 1998 for the treatment of MBC resistant to both paclitaxel and anthracycline-containing regimens. The approval was based on an ORR of 25.6% (95% CI, 13.5 to 41.2) in an SAT in 162 patients with refractory breast cancer.10 The postmarketing commitment was to submit the results of an RCT in a similar patient population.
In 2001, capecitabine was granted RA for use in combination with docetaxel for the treatment of MBC progressing after anthracycline-containing chemotherapy. Results from a single RCT of 511 patients in the indicated population supported the approval.11 Patients were randomly assigned to receive either capecitabine 1,250 mg/m2 twice daily orally for 14 days, in combination with docetaxel 75 mg/m2 intravenously every 3 weeks, or docetaxel monotherapy 100 mg/m2 intravenously every 3 weeks. RA of capecitabine was based on a statistically significant survival improvement (HR, 0.77; 95% CI, 0.63 to 0.95; P = .01). TTP was also significantly prolonged (HR, 0.64; 95% CI, 0.54 to 0.77; P < .001).
Albumin-Bound Paclitaxel
Albumin-bound paclitaxel is a paclitaxel formulation approved under section 505(b)(2) of the Food, Drug, and Cosmetic Act. This regulatory pathway allows the FDA to approve new drug applications that rely on information to which sponsors do not have right of reference.5 Through this pathway, albumin-bound paclitaxel was granted RA for the same MBC indication as paclitaxel, with noninferior ORR as the measure of efficacy. An RCT in 460 patients with progressive MBC after one or two prior anthracycline-containing chemotherapy regimens provided evidence of efficacy and safety.12 Patients were randomly assigned to 3-week cycles of albumin-bound paclitaxel 260 mg/m2 intravenously without corticosteroid premedication or paclitaxel 175 mg/m2 intravenously with corticosteroid premedication. Albumin-bound paclitaxel had a statistically significantly higher ORR compared with paclitaxel (21.5%; 95% CI, 16.2 to 26.7 v 11.1%; 95% CI, 6.9 to 15.1).
Lapatinib
Lapatinib was granted RA for use in combination with capecitabine for the treatment of patients with HER2-overexpressing locally advanced or MBC who have received prior therapy, including anthracyclines, taxanes, and trastuzumab. An RCT of 399 patients overexpressing HER2 3+ or 2+ by immunohistochemistry and confirmed by fluorescent in situ hybridization supported this approval.13,14 Patients were randomly assigned to receive lapatinib 1,250 mg/m2 orally daily plus capecitabine 2,000 mg/m2 orally daily for 14 days or single-agent capecitabine 2,500 mg/m2 orally daily for 14 days.
The study was stopped early for efficacy based on a planned interim analysis of TTP, the primary end point. The magnitude of the TTP treatment benefit was different between the independent radiology review (IRR) and investigator assessment (INV). This difference could have resulted from unavailability of all radiologic scans to the IRR and choice of different target lesions between the INV and IRR. TTP improvement was statistically significant; median difference in TTP based on IRR was 8.5 weeks (HR, 0.57; 95% CI, 0.43 to 0.77; P < .001) and 5.6 weeks based on INV (HR, 0.72; 95% CI, 0.56 to 0.92; P = .0076).
At the time of approval, the survival data were not mature, with only 32% of the planned mortality events. An updated survival analysis after 2 additional years of follow-up showed no difference in OS (HR, 0.89; 95% CI, 0.71 to 1.10; P = .276).
Ixabepilone
Ixabepilone in combination with capecitabine was granted RA for the treatment of patients with MBC who progress after an anthracycline and taxane. An RCT of 752 patients supported the approval in the indicated population.15 Patients were randomly assigned to receive either the combination of ixabepilone 40 mg/m2 intravenously every 21 days and capecitabine 1,000 mg/m2 twice daily orally for 14 days or capecitabine monotherapy 1,250 mg/m2 twice daily orally for 14 days. The primary end point was PFS as determined by the IRR.
Ixabepilone in combination with capecitabine received RA based on a statistically significant improvement in median PFS of 5.7 versus 4.1 months (HR, 0.69; 95% CI, 0.58 to 0.83; P < .001) with the combination of ixabepilone plus capecitabine compared with capecitabine monotherapy. At the time of the approval, OS data were not mature. The final OS results did not show improvement, with a median OS of 12.9 versus 11.1 months (HR, 0.90; 95% CI, 0.77 to 1.05; P = .19) with ixabepilone plus capecitabine compared with capecitabine monotherapy.
Ixabepilone 40 mg/m2 infused intravenously over 3 hours every 3 weeks was also simultaneously approved as monotherapy for the treatment of MBC in patients who progress after an anthracycline, taxane, and capecitabine. This approval was based on a multicenter SAT in 126 women with MBC whose disease progressed after two or more chemotherapy treatments. The ORR was 12.4% by IRR (95% CI, 6.9% to 19.9%) and 18.3% by INV (95% CI, 11.9% to 26.1%).
Eribulin
In 2010, eribulin was granted RA for the treatment of patients with MBC who have received an anthracycline, taxane, and at least two chemotherapeutic regimens. Safety and efficacy were evaluated in an RCT of 762 patients with MBC who had received at least two chemotherapeutic regimens for the treatment of metastatic disease and experienced disease progression within 6 months of the last chemotherapeutic regimen. Patients were randomly assigned in a ratio of two to one to receive eribulin (n = 508) 1.4 mg/m2 intravenously on days 1 and 8 of a 21-day cycle or the physician's choice of a single-agent therapy selected before randomization (n = 254). The physician's choice agents included vinorelbine (24%), gemcitabine (18%), capecitabine (17%), taxanes (16%), anthracyclines (9%), hormone therapies (4%), and miscellaneous other agents (10%). Patients had received a median of four prior chemotherapy regimens in both arms.
Eribulin approval was based on an improvement in OS, with median OS of 13.1 months for eribulin-treated patients compared with 10.6 months for patients treated in the control arm (HR, 0.81; 95% CI, 0.660 to 0.991; P = .041). The application was also supported by higher ORR in the eribulin treatment arm (11.2%) compared with the control arm (3.9%) and a consistent, but nonsignificant, effect on PFS as determined by independent review (median PFS, 113 v 68 days; HR, 0.87; 95% CI, 0.71 to 1.05; P = .14).
DRUGS APPROVED FOR INITIAL (FIRST-LINE) TREATMENT OF MBC
In the last 10 years, three drugs (trastuzumab, gemcitabine, and bevacizumab) were approved for use in combination with paclitaxel in the first-line treatment of MBC, and lapatinib was approved for use in combination with letrozole for the treatment of postmenopausal women with hormone receptor–positive MBC overexpressing the HER2 receptor.
Trastuzumab
Trastuzumab in combination with paclitaxel received RA in 1998 for treatment of patients with MBC whose tumors overexpress the HER2 protein and who have not received chemotherapy for metastatic disease. The RCT that supported this indication was conducted in 469 women with MBC who had not received treatment with chemotherapy for metastatic disease.16 Patients with HER2-overexpressing tumors (2+ or 3+ positive by immunohistochemistry) were randomly assigned to receive chemotherapy either alone or in combination with trastuzumab. Patients who had received prior anthracyclines in the adjuvant setting were treated with paclitaxel. For the other patients, chemotherapy consisted of the combination of an anthracycline plus cyclophosphamide. Trastuzumab was administered at an initial dose of 4 mg/kg intravenously followed by a weekly dose of 2 mg/kg. At the time of disease progression, approximately 65% of the patients in the control arm had crossed over to receive trastuzumab.
The basis for RA was an improvement in TTP supported by statistically significant improvements in 1-year survival rates (79% v 68%; P = .03) and ORR (45% v 29%; P < .001). In the overall population, median TTP was 7.2 versus 4.5 months (HR, 0.53; 95% CI, 0.43 to 0.65; P < .001) in the chemotherapy plus trastuzumab arm compared with the chemotherapy alone arm. In the paclitaxel subgroup, median TTP was 6.7 versus 2.5 months with paclitaxel plus trastuzumab compared with paclitaxel alone. In the anthracycline plus cyclophosphamide subgroup, median TTP was 7.6 months with chemotherapy plus trastuzumab versus 5.7 months with chemotherapy alone. OS data were not mature at the time of approval. After approval, median survival was 25.1 months in patients treated with chemotherapy plus trastuzumab compared with 20.3 months in patients treated with chemotherapy alone (HR, 0.80; 95% CI, 0.64 to 1.0; P = .046).
Gemcitabine
In 2004, gemcitabine in combination with paclitaxel was granted RA for the initial treatment of patients with MBC. The RCT that supported this approval enrolled 529 patients with locally advanced or MBC who had received prior adjuvant or neoadjuvant anthracycline chemotherapy.17 Patients were randomly assigned to receive gemcitabine 1,250 mg/m2 on days 1 and 8 with paclitaxel 175 mg/m2 on day 1 or to single-agent paclitaxel 175 mg/m2. Drugs were administered intravenously on a 21-day cycle.
Approval of gemcitabine was based on an improvement in TTP supported by a numeric improvement in OS. Median TTP in patients treated with the gemcitabine plus paclitaxel combination was 5.2 months compared with 2.9 months for paclitaxel alone (HR, 0.65; 95% CI, 0.52 to 0.81; P < .001). In the final OS analysis, median survival was 18.6 months in the gemcitabine plus paclitaxel arm and 15.8 months in the paclitaxel monotherapy arm (HR, 0.86; 95% CI, 0.71 to 1.04).
Bevacizumab
In 2008, bevacizumab in combination with paclitaxel was granted AA for the treatment of patients who have not received chemotherapy for metastatic HER2-negative breast cancer. The E2100 study, an RCT in 722 patients, supported the approval.18 Patients were randomly assigned to receive paclitaxel either alone (90 mg/m2 intravenously weeks 1 to 3 of each 28-day cycle) or in combination with bevacizumab (10 mg/kg intravenously every 2 weeks). The basis for approval was a PFS improvement in an interim analysis. Median PFS in the combination arm was 11.3 months compared with 5.8 months in the paclitaxel alone arm (HR, 0.48; 95% CI, 0.39 to 0.61; P < .001).18,19 A mature analysis of OS (HR, 0.87; 95% CI, 0.72 to 1.04; P = .14) indicated that a detriment in survival was unlikely. One condition of the AA was a requirement to provide verification of the treatment effect on PFS and OS from the ongoing AVADO (Avastin Plus Docetaxel; docetaxel with or without bevacizumab 7.5 mg/kg or 15 mg/kg) and RIBBON1 (Regimens in Bevacizumab for Breast Oncology; taxane/anthracycline with or without bevacizumab) trials.20,21 These trials demonstrated marginal improvements in PFS and no improvements in OS and were discussed publicly at the Oncologic Drugs Advisory Committee (ODAC) in July 2010.22
Lapatinib
In 2010, the FDA granted AA for lapatinib in combination with letrozole for the treatment of postmenopausal women with hormone receptor–positive MBC overexpressing the HER2 receptor and for whom hormonal therapy is indicated. An RCT of 1,286 patients who had not received prior therapy for metastatic disease supported this indication.23 Patients were randomly assigned to receive lapatinib 1,500 mg orally once daily plus letrozole 2.5 mg orally once daily or to placebo plus letrozole 2.5 mg once daily. There were 219 patients (17%) with HER2-positive tumors, 952 patients (74%) with HER2-negative tumors, and 115 patients (9%) without HER2 status.
The AA was based on the results from the subgroup of patients with HER2-overexpressing MBC. The lapatinib plus letrozole arm had a median PFS of 35.4 weeks compared with 13.0 weeks for the placebo plus letrozole arm (HR, 0.71; 95% CI, 0.53 to 0.96; log-rank P = .019). OS data were not mature. As a condition of AA, subsequent RCTs are required to verify and describe the clinical benefit of lapatinib in patients with MBC. Lapatinib in combination with an aromatase inhibitor has not been compared with a trastuzumab-containing chemotherapy regimen for the treatment of MBC.
DISCUSSION
The FDA has used a variety of end points in the approval of nonhormonal agents for the treatment of MBC. Ten products were approved in the last two decades for 14 indications for the treatment of MBC. Of the 10 approvals for second- to third-line treatment of MBC (Table 1), four products (docetaxel, trastuzumab, capecitabine, and ixabepilone) were approved for monotherapy based on durable ORRs in SATs. Docetaxel and capecitabine were granted AA for use as monotherapy based on durable response rates considered reasonably likely to predict for clinical benefit in a population for whom there was no approved drug. Docetaxel and capecitabine AAs were converted to RAs based on OS improvements in subsequent trials. Trastuzumab and ixabepilone RAs for second- and third-line monotherapy indications were supported by simultaneous RAs based on RCTs. The paclitaxel approval in 1994 was based on an RCT comparing two paclitaxel doses. This study design is currently problematic in MBC, because there are many available treatment options. Therefore, as in the eribulin trial, physicians' choice is a reasonable comparator. Physician's choice control arms are acceptable in refractory MBC. Single agents should be considered standard of care, if not FDA approved, for MBC and should be prespecified before randomization. Three of the 10 drug approvals for second- to third-line indications (paclitaxel, lapatinib, and ixabepilone) were based on improvements in TTP or PFS, and three (docetaxel, capecitabine, and eribulin) were based on a 2.5- to 3-month OS improvement.
Table 1.
Second- and Third-Line Metastatic Breast Cancer Drug Approvals
| Year of Approval | Type of Approval | Drugs | No. of Patients | Drug Doses | End Points | HR | 95% CI | Log-Rank P |
|---|---|---|---|---|---|---|---|---|
| 1994 | RA | P | 471 | P: 175 mg/m2 v 135 mg/m2 | TTP: median, 4.2 v 3.0 months | 0.75 | .027 | |
| 1996 | AA | D | 309 | D: 100 mg/m2 | ORR: 37.9% | 31.0 to 44.8 | ||
| 1998 | RA | D | 392 | D: 100 mg/m2 v M: 12 mg/m2 + V: 6 mg/m2 | OS: median 11.4 v 8.7 months | 0.73 | 0.58 to 0.93 | .01 |
| 1998 | RA | T | 222 | T: 4 mg/kg load, 2 mg/kg weekly | ORR: PR, 12%; CR, 2% | 7.73 to 16.3 | ||
| 1998 | AA | C | 162 | C: 2,500 mg/m2 | ORR: 25.6% | 13.5 to 41.2 | ||
| 2001 | RA | C/D | 511 | C: 2,500 mg/m2 + D: 75 mg/m2 v D: 100 mg/m2 | OS: median, 442 v 352 days | 0.77 | 0.63 to 0.95 | .01 |
| 2005 | RA | A-bound P | 460 | A: 260 mg/m2 | ORR: 21.5% | 16.2 to 26.7 | .003 | |
| P: 175 mg/m2 | ORR: 11.1% | 6.9 to 15.1 | ||||||
| 2007 | RA | L/C | 399 | L: 1,250 mg + C: 2,000 mg/m2 v C: 2,500 mg/m2 | TTP: median IND, 27.1 v 18.6 weeks | 0.57 | 0.43 to 0.77 | < .001 |
| TTP: median INV, 23.9 v 18.3 days | 0.72 | 0.56 to 0.92 | .008 | |||||
| OS: median, 75 v 65.8 weeks | 0.89 | 0.71 to 1.10 | .276 | |||||
| 2007 | RA | I | 126 | 40 mg/m2 | ORR: 12.4% DR: median, 6 months | 6.9 to 9.9 | ||
| 2007 | RA | I/C | 752 | I: 40 mg/m2 + C: 2,000 mg/m2 v C: 2,500 mg/m2 | PFS: median, 5.7 v 4.1 months | 0.69 | 0.58 to 0.83 | < .001 |
| OS: median, 12.9 v 11.1 months* | 0.90 | 0.77 to 1.05 | .19 | |||||
| 2010 | RA | E | 762 | E: 1.4 mg/m2 v PC | OS: median, 13.1 v 10.6 months | 0.81 | 0.66 to 0.99 | .041 |
Abbreviations: A, albumin; AA, accelerated approval; C, capecitabine; D, docetaxel; DR, duration of response; E, eribulin; HR, hazard ratio; I, ixabepilone; IND, independent assessment; INV, investigator assessment; L, lapatinib; M, mitomycin; ORR, objective response rate; OS, overall survival; P, paclitaxel; PC, physician's choice; PFS, progression-free survival; RA, regular approval; RR, response rate; T, trastuzumab; TTP, time to progression; V, vinblastine.
Updated OS.
Of the four products approved for first-line treatment of MBC (Table 2), two of the RAs (trastuzumab and gemcitabine) had supportive OS data at the time of approval. The other two (bevacizumab and lapatinib) were granted AA based on PFS, with postmarketing requirements to demonstrate clinical benefit in additional RCTs.
Table 2.
First-Line Metastatic Breast Cancer Drug Approvals
| Year of Approval | Type of Approval | Drugs | No. of Patients | Study Arms | End Points | HR | 95% CI | Log-Rank P |
|---|---|---|---|---|---|---|---|---|
| 1998 | RA | T + chemotherapy/P | 469 | T + chemotherapy v chemotherapy | TTP: median, 7.2 v 4.5 months | 0.53 | 0.43 to 0.65 | < .001 |
| T + P v P | TTP: median, 6.7 v 2.5 months | 0.39 | 0.27 to 0.53 | < .001 | ||||
| T + AC v AC | TTP: median, 7.6 v 5.7 months | 0.65 | 0.47 to 0.83 | .002 | ||||
| T + chemotherapy v chemotherapy | OS: median, 25.1 v 20.3 months | 0.80 | 0.64 to 1.00 | .046 | ||||
| T + P v P | OS: median, 22.1 v 18.4 months | 0.79 | 0.56 to 1.11 | .175 | ||||
| T + AC v AC | OS: median, 26.8 v 21.4 months | 0.81 | 0.61 to 1.09 | .162 | ||||
| 2004 | RA | G + P | 529 | G + P v P | TTP: median, 5.2 v 2.9 months | 0.65 | 0.52 to 0.81 | < .001 |
| OS: median, 18.6 v 15.8 months | 0.82 | 0.67 to 1.00 | .048 | |||||
| 2008 | AA | B + P | 722 | B + P v P | PFS: median, 11.3 v 5.8 months | 0.48 | 0.39 to 0.61 | < .001 |
| OS: median, 26.5 v 24.8 months | 0.87 | 0.72 to 1.05 | .14 | |||||
| 2010 | AA | L + letrozole | 1,286 | L + letrozole v letrozole | PFS: median HER2 positive, 8.1 v 3.0 months | 0.71 | 0.53 to 0.96 | .019 |
| PFS: median HER2 negative,13.7 v 13.4 months | 0.90 | 0.77 to 1.05 | .188 |
Abbreviations: AA, accelerated approval; AC, adriamycin plus cyclophasphamide; B, bevacizumab; G, gemcitabine; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; L, lapatinib; OS, overall survival; P, paclitaxel; PFS, progression-free survival; RA, regular approval; T, trastuzumab; TTP, time to progression.
Survival is considered the most unambiguous end point, and when studies can be conducted to adequately assess survival, it is usually the preferred end point of the FDA. However, PFS is acceptable, provided the magnitude in PFS improvement is substantial and associated with a favorable benefit-to-risk analysis. OS is the net effect of both drug toxicity and efficacy and therefore is an important end point in the evaluation of benefit-to-risk profile. Although improving OS is also the ultimate goal in more advanced disease settings such as second- to third-line treatment of MBC, the FDA has accepted an improvement in TTP or PFS as an end point for RA, assuming a delay in disease progression in a highly refractory setting would likely translate into clinical benefit.
Several approved drug regimens have demonstrated survival benefit. Doxorubicin-based regimens have improved median survival by between 3 to 6 months when compared with regimens that do not contain doxorubicin.24–26 Trastuzumab chemotherapy combinations have increased median survival by 5 months when compared with chemotherapy alone in patients with HER2-positive tumors. Docetaxel monotherapy and the capecitabine plus docetaxel combination have also prolonged OS by 3 months. In the most recent approval, eribulin improved median survival in a heavily pretreated patient population by 2.5 months compared with treatment with physician's choice.
PFS and TTP are appealing end points because they require shorter follow-up and smaller sample sizes to demonstrate efficacy compared with OS. However, the translation of an observed PFS effect into an OS effect is unknown. Furthermore, assessment of progression is complex and includes composite results from radiologic examinations with inherent measurement errors, laboratory tests, and physical examinations. Differences in assessment schedules between treatment arms, missing or incomplete baseline or follow-up assessments, and difficulties in determining progression of unmeasurable disease can introduce systematic bias into the evaluation of progression. Marginal differences in PFS and TTP between treatment arms may result from differences in subjective assessments of progression and may not represent clinically meaningful improvements. Discordance in progression assessments among independent radiology reviewers and investigators typically ranges from 30% to 50% and may introduce uncertainty into the treatment effect size. The magnitude of PFS improvement should be clinically meaningful and large enough to overcome concerns regarding unblinded trials, missing assessments, assessment discordance, and informative censoring. However, what constitutes a meaningful difference is difficult to determine and depends on the benefit-to-risk evaluation. A recent FDA evaluation of 14 trials submitted in support of MBC approvals suggested that PFS and OS have a weak correlation (R2 = 0.067).27 The relationship between PFS and survival in MBC needs to be further explored.
The AA of bevacizumab was the first time PFS was used as the basis for approval of a first-line treatment of MBC. The bevacizumab confirmatory trials—AVADO and RIBBON1—did not demonstrate the same magnitude of PFS benefit observed in the E2100 trial or improvement in OS or patient-reported outcomes. In July 2010, an ODAC meeting was convened to discuss the benefit-to-risk assessment of bevacizumab, considering the results of the confirmatory trials.22 ODAC concluded that the magnitude of PFS from the E2100 trial was not reproduced in the confirmatory studies, and considering the toxicity profile of bevacizumab (hypertension, hemorrhage, GI perforation), the benefit-to-risk evaluation did not favor use of bevacizumab. A hearing was held in June 2011 regarding a proposal to withdraw approval of the MBC indication. On November 18, 2011, the FDA announced its decision to revoke the MBC indication.28
The FDA has approved drugs based on symptom improvement in diseases where symptoms are clearly associated with the underlying cancer (eg, bone pain in metastatic prostate cancer [mitoxantrone] or dysphagia in esophageal cancer [porfimer sodium]). However, this approach is problematic in breast cancer trials enrolling minimally symptomatic patients with ill-defined symptoms that may be related to underlying disease, drug toxicities, or unrelated disease. Although patient-reported outcomes can potentially support drug approval, the instruments that are widely used have not been adequately validated for this purpose.29
Regulatory decisions are generally based on all patients enrolled onto RCTs. Subgroup analyses are exploratory and conducted to confirm consistency of treatment effect in the overall population. Subgroup claims after failure to show benefit in the overall population are considered exploratory. However, benefit demonstration in a preplanned subgroup analysis with prespecified type I error allocation may be acceptable, as noted in the lapatinib plus letrozole approval.
In summary, OS is the preferred end point of the FDA in MBC, because it is both a safety and efficacy measure, and because there are available treatments that improve survival. However, the FDA has acknowledged the potential impact of poststudy therapies on OS. Crossovers and same subsequent therapies in treatment arms may attenuate OS differences.30–32 Crossover therapy, particularly when unplanned, has generally been low, except for the trastuzumab trial with a 65% crossover that did not confound treatment effect on OS. In the gemcitabine trial, crossover was 16%, and approximately 55% of patients in both treatment arms received similar postprogression chemotherapy.17 Similarly, the docetaxel plus capecitabine trial had a 14% crossover rate and 62% postprogression chemotherapy in both treatment arms. Both trials had a 3-month difference in median OS. This amount of crossover did not confound treatment effects on OS in these two examples.
PFS is an acceptable end point for approval if it is measured properly and of sufficient magnitude to provide a favorable benefit-to-risk analysis. However, survival should also be measured to ensure that a new therapy does not lead to survival decrement. Interim analyses of PFS are discouraged, because they may result in a trial being stopped before accrual is complete, provide an overestimate of the treatment effect, or be underpowered to detect a survival difference. Early discussion with the FDA on the appropriate setting in which to use PFS is encouraged during trial design.
Footnotes
Presented orally at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Patricia Cortazar
Data analysis and interpretation: Patricia Cortazar, Rajeshwari Sridhara
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1. 21 Code of Federal Regulations, Section 314.126.
- 2. 21 Code of Federal Regulations, Sections 314.510 and 601.41.
- 3. 21 Code of Federal Regulations, Sections 314.510 and 601.41.
- 4. 21 Code of Federal Regulations, Sections 314.530 and 601.43.
- 5.US Food and Drug Administration. Guidance for Industry: Applications Covered by Section 505(b)(2) http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079345.pdf.
- 6.Nabholtz JM, Bontenbal GM, Spielmann M, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1858–1867. doi: 10.1200/JCO.1996.14.6.1858. [DOI] [PubMed] [Google Scholar]
- 7.Van Oosterom AT. Docetaxel (Taxotere): An effective agent in the management of second-line breast cancer. Semin Oncol. 1995;22(suppl 13):22–28. [PubMed] [Google Scholar]
- 8.Nabholtz JM, Senn HJ, Bezwoda WR, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. J Clin Oncol. 1999;17:1413–1424. doi: 10.1200/JCO.1999.17.5.1413. [DOI] [PubMed] [Google Scholar]
- 9.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 10.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 11.O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: Phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 13.Cameron D, Casey M, Press M, et al. A Phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progresses on trastuzumab: Update efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 14.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 15.Thomas E, Gomez H, Li R, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 17.Albain K, Nag S, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26:3950–3957. doi: 10.1200/JCO.2007.11.9362. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 19.Gray R, Bhattacharya S, Bowden C, et al. Independent review of E2100: A phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miles D, Chan A, Romieu G, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. J Clin Oncol. 2008;26(suppl):43s. abstr LBA1011. [Google Scholar]
- 21.Robert N, Dieras V, Glapsy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2–negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. Advisory Committees: 2010 Meeting Materials, Oncologic Drugs Advisory Committee. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/ucm195226.htm.
- 23.Johnson S, Pippen J, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 24.A'Hern R, Smith I, Ebbs S. Chemotherapy and survival in advanced breast cancer: The inclusion of docorubicin in Cooper type regimens. Br J Cancer. 1993;67:801–805. doi: 10.1038/bjc.1993.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paridaens R, Biganzoli L, Bruning P, et al. Paclitaxel versus doxorubicin as first-line single agent chemotheraspy for metastatic breast cancer: A European Organisation for Research and Treatment of Cancer randomized study with cross-over. J Clin Oncol. 2000;18:724–733. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- 26.Fossati R, Confalonieri C, Torri V, et al. Cytotoxic and hormonal treatment of metastatic breast cancer: A systematic review of published randomized trials involving 31,510 women. J Clin Oncol. 1998;16:3439–3460. doi: 10.1200/JCO.1998.16.10.3439. [DOI] [PubMed] [Google Scholar]
- 27.Cortazar P, Zhang J, Sridhara R, et al. Relationship between OS and PFS in metastatic breast cancer (MBC): Review of FDA submission data. J Clin Oncol. 2011;29(suppl):88s. abstr 1035. [Google Scholar]
- 28.US Food and Drug Administration. Commissioner Statement: FDA commissioner removes breast cancer indication from Avastin label. http://www.fda.gov/NewsEvents/Newsroom/UCM279485.
- 29.US Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures—Use in Medical Product Development to Support Labeling Claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed]
- 30.Broglio K, Berry D. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: A review of recent randomized controlled clinical trials. J Clin Oncol. 2010;28:1958–1962. doi: 10.1200/JCO.2009.25.5414. [DOI] [PubMed] [Google Scholar]
- 32.Korn E, Freidlin B, Abrams J. Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J Clin Oncol. 2011;29:2439–2442. doi: 10.1200/JCO.2011.34.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]