Abstract
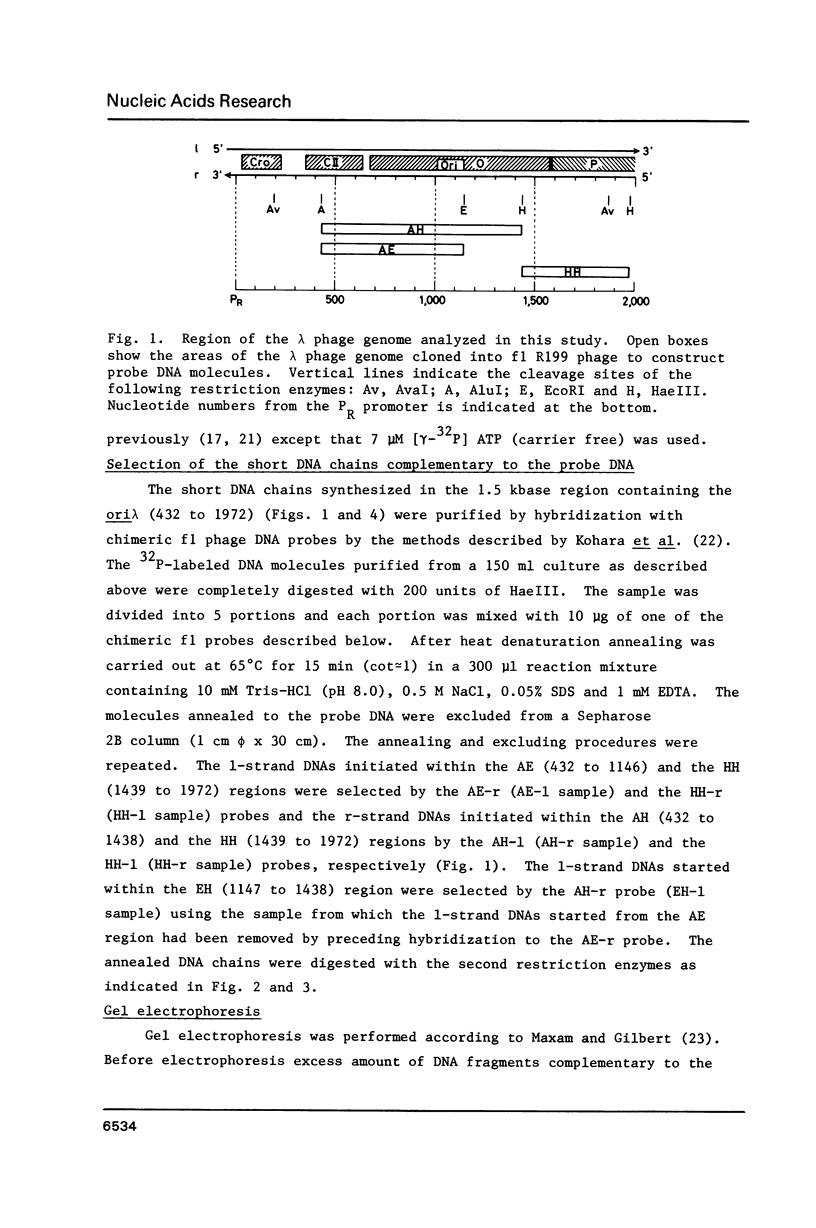
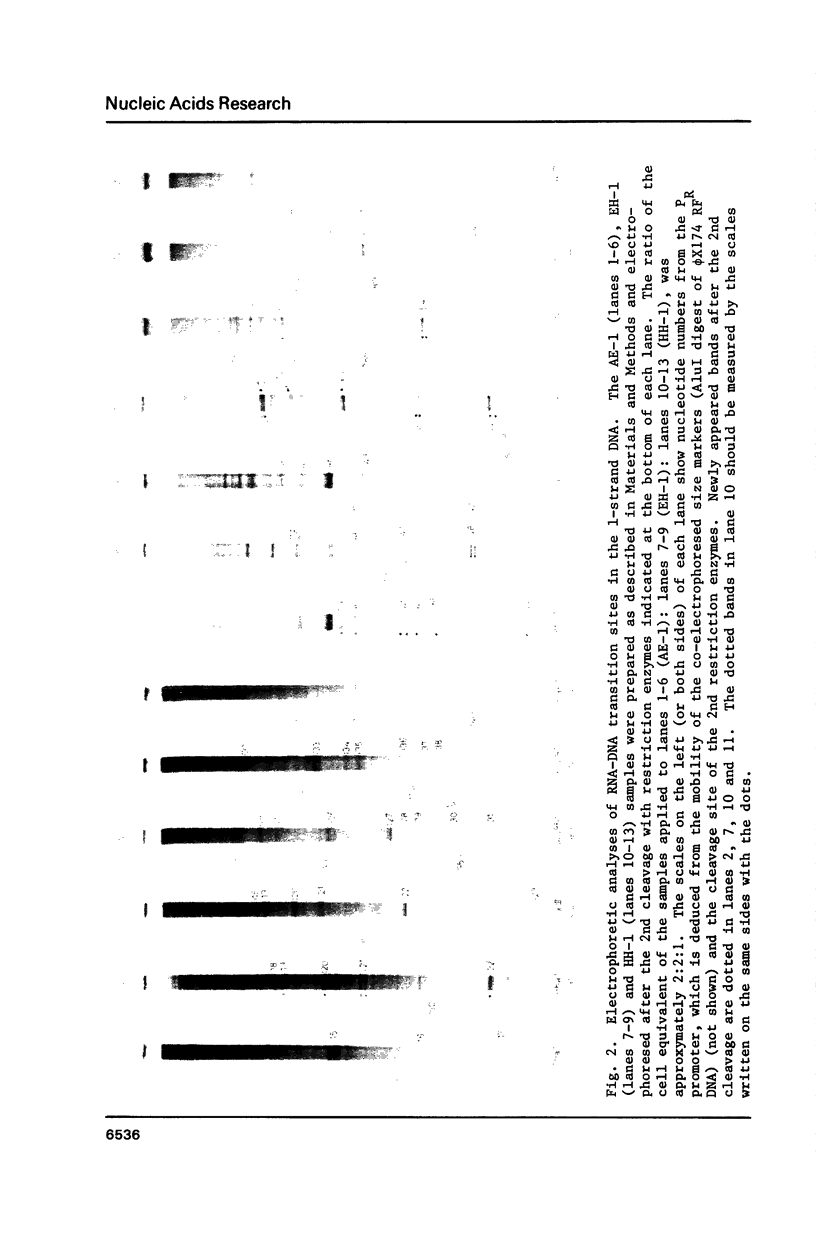
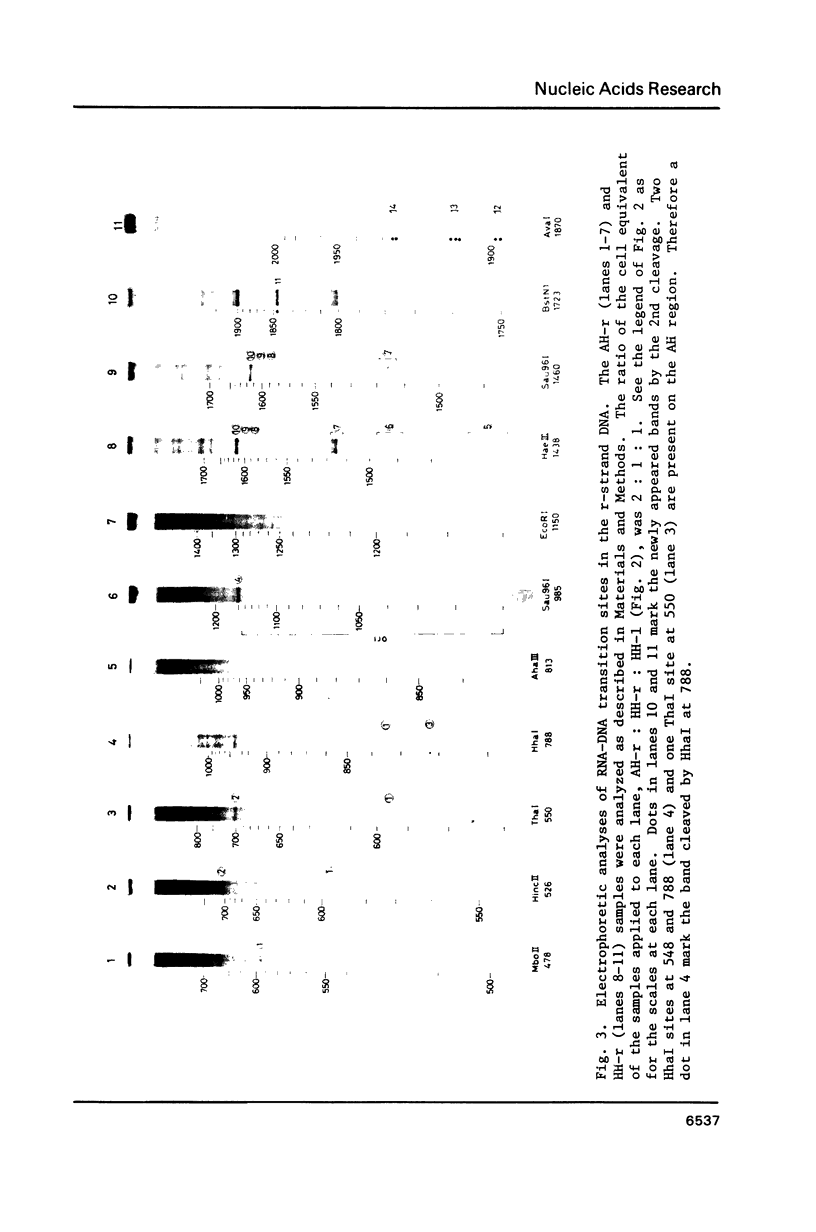
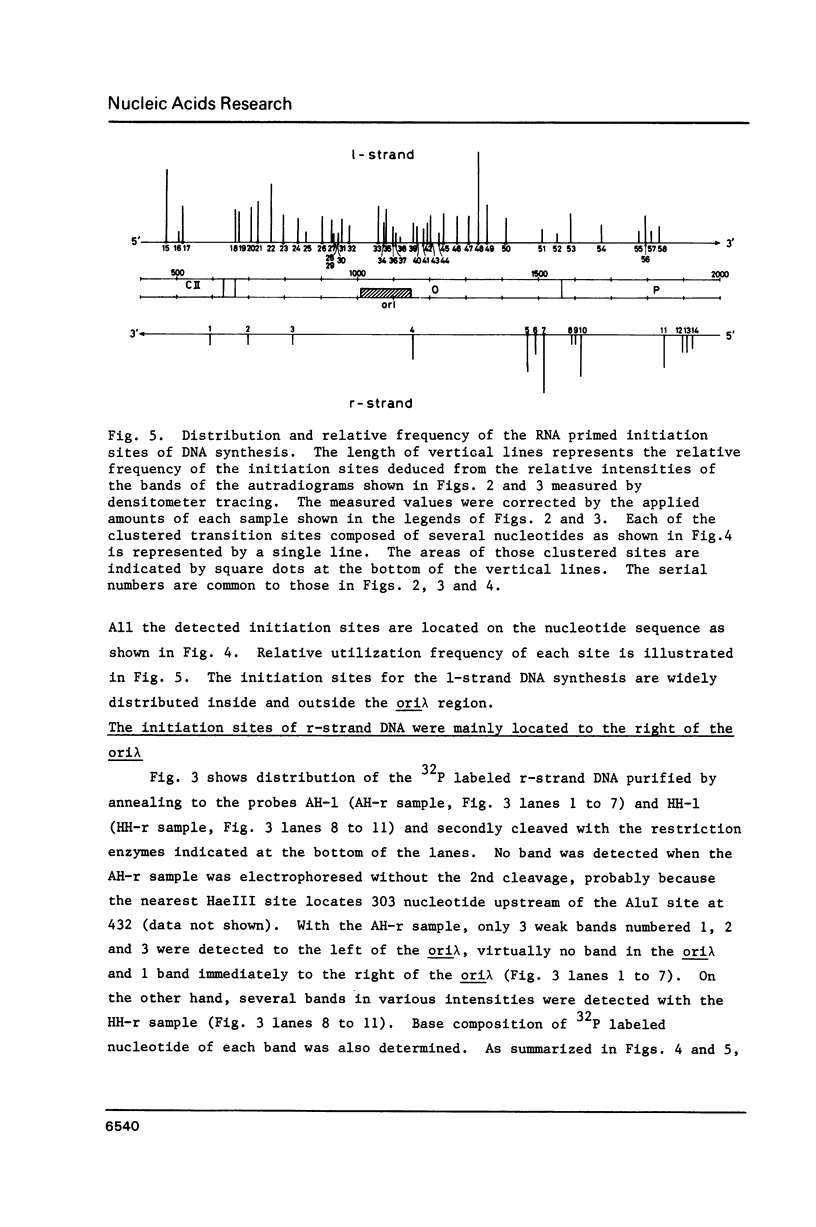
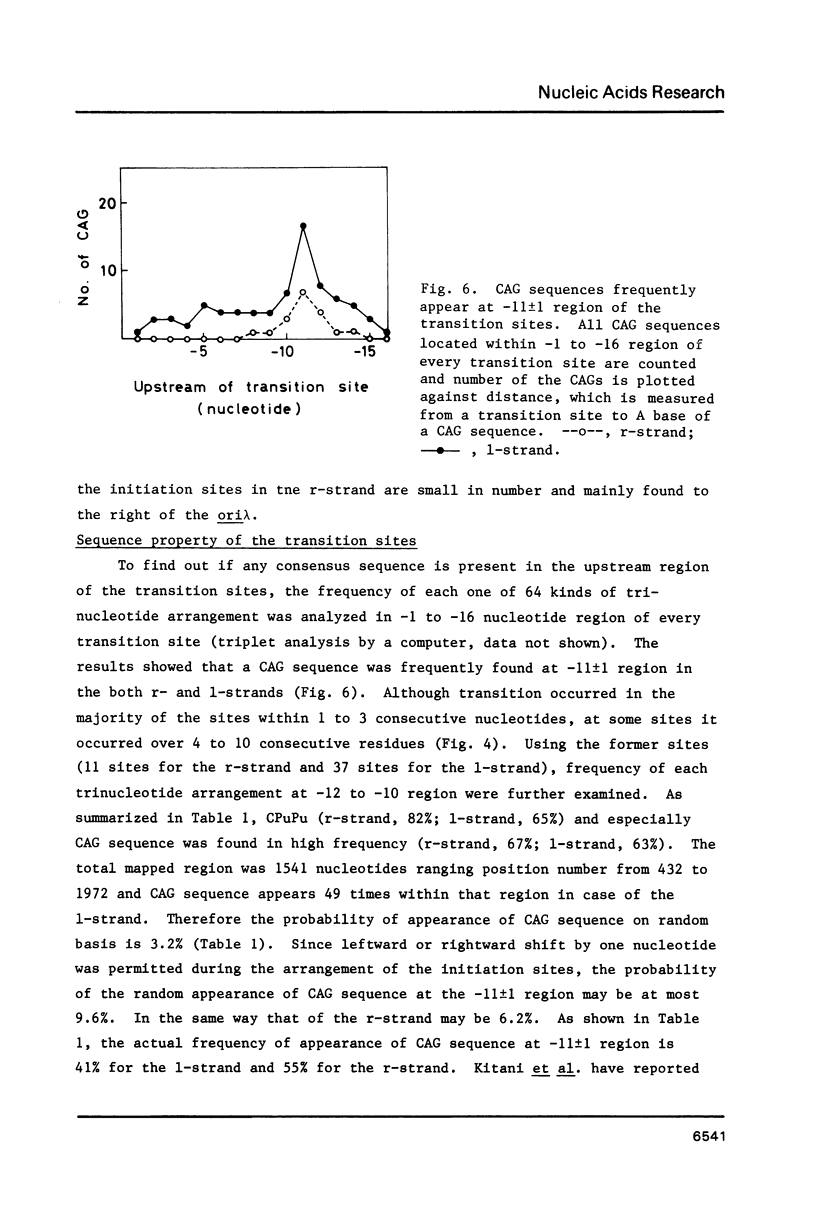
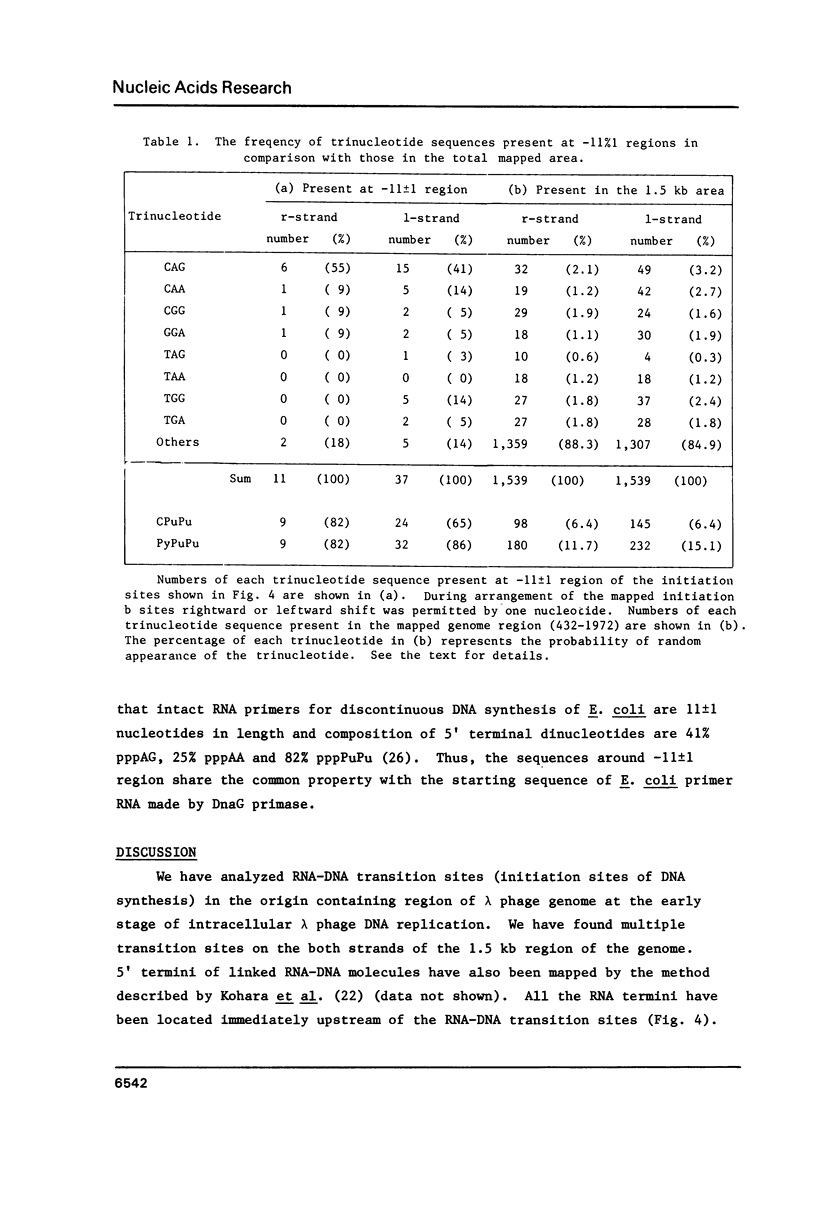
Using DNA molecules synthesized in the early stage of lambda phage infection, deoxynucleotides at the transition sites from primer RNA to DNA synthesis have been mapped in the 1.5 kbase area of the lambda phage genome containing the genetically defined replication origin (ori lambda). Sites in the 1-strand (the polarity of the 1-strand is 5' to 3' from the left to the right direction of the lambda phage genetic map) were distributed both inside and outside of the ori lambda, whereas the sites in the r-strand (the strand in the opposite polarity) were mainly distributed more than three hundred nucleotides apart from the ori lambda to the right. A CPuPu sequence was found at -12 to -10 region of transition sites of the r- and the 1-strands in the frequency of 80% and 70%, respectively, and over 60% of the CPuPu sequences were CAG. Properties of the transition sites are discussed in relation to the primer synthesis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., Vovis G. F., Zinder N. D. Insertion mutant of bacteriophage f1 sensitive to EcoRI. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2699–2702. doi: 10.1073/pnas.76.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope study of the structures of lambdadv DNAs. J Mol Biol. 1974 Jun 15;86(1):69–89. doi: 10.1016/s0022-2836(74)80008-2. [DOI] [PubMed] [Google Scholar]
- Denniston-Thompson K., Moore D. D., Kruger K. E., Furth M. E., Blattner F. R. Physical structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1051–1056. doi: 10.1126/science.929187. [DOI] [PubMed] [Google Scholar]
- Dodson M., Echols H., Wickner S., Alfano C., Mensa-Wilmot K., Gomes B., LeBowitz J., Roberts J. D., McMacken R. Specialized nucleoprotein structures at the origin of replication of bacteriophage lambda: localized unwinding of duplex DNA by a six-protein reaction. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7638–7642. doi: 10.1073/pnas.83.20.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M., Roberts J., McMacken R., Echols H. Specialized nucleoprotein structures at the origin of replication of bacteriophage lambda: complexes with lambda O protein and with lambda O, lambda P, and Escherichia coli DnaB proteins. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4678–4682. doi: 10.1073/pnas.82.14.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama A., Kohara Y., Okazaki T. Initiation sites for discontinuous DNA synthesis of bacteriophage T7. Proc Natl Acad Sci U S A. 1981 Feb;78(2):903–907. doi: 10.1073/pnas.78.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Blattner F. R., McLeester C., Dove W. F. Genetic structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1046–1051. doi: 10.1126/science.929186. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Dove W. F., Meyer B. J. Specificity determinants for bacteriophage lambda DNA replication. III. Activation of replication in lambda ric mutants by transcription outside of ori. J Mol Biol. 1982 Jan 5;154(1):65–83. doi: 10.1016/0022-2836(82)90417-x. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: RNA priming during the initiation of heavy-strand synthesis. J Mol Biol. 1979 Dec 5;135(2):353–368. doi: 10.1016/0022-2836(79)90441-8. [DOI] [PubMed] [Google Scholar]
- Hirose S., Hiraga S., Okazaki T. Initiation site of deoxyribonucleotide polymerization at the replication origin of the Escherichia coli chromosome. Mol Gen Genet. 1983;189(3):422–431. doi: 10.1007/BF00325904. [DOI] [PubMed] [Google Scholar]
- Kitani T., Yoda K., Ogawa T., Okazaki T. Evidence that discontinuous DNA replication in Escherichia coli is primed by approximately 10 to 12 residues of RNA starting with a purine. J Mol Biol. 1985 Jul 5;184(1):45–52. doi: 10.1016/0022-2836(85)90042-7. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Tohdoh N., Jiang X. W., Okazaki T. The distribution and properties of RNA primed initiation sites of DNA synthesis at the replication origin of Escherichia coli chromosome. Nucleic Acids Res. 1985 Oct 11;13(19):6847–6866. doi: 10.1093/nar/13.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986 Apr 5;261(10):4738–4748. [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The bacteriophage lambda O and P protein initiators promote the replication of single-stranded DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3069–3088. doi: 10.1093/nar/12.7.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Zylicz M., Georgopoulos C., McMacken R. Initiation of DNA replication on single-stranded DNA templates catalyzed by purified replication proteins of bacteriophage lambda and Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3988–3992. doi: 10.1073/pnas.82.12.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb M. Mapping missense and nonsense mutation in gene cI of bacteriophage lambda: marker effects. Mol Gen Genet. 1976 Aug 2;146(3):285–290. doi: 10.1007/BF00701252. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Marsh R. C., Worcel A. A DNA fragment containing the origin of replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2720–2724. doi: 10.1073/pnas.74.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Arai K., Okazaki T. Site selection and structure of DNA-linked RNA primers synthesized by the primosome in phage phi X174 DNA replication in vitro. J Biol Chem. 1983 Nov 10;258(21):13353–13358. [PubMed] [Google Scholar]
- Ogawa T., Hirose S., Okazaki T., Okazaki R. Mechanism of DNA chain growth XVI. Analyses of RNA-linked DNA pieces in Escherichia coli with polynucleotide kinase. J Mol Biol. 1977 May 5;112(1):121–140. doi: 10.1016/s0022-2836(77)80160-5. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. Function of RNase H in DNA replication revealed by RNase H defective mutants of Escherichia coli. Mol Gen Genet. 1984;193(2):231–237. doi: 10.1007/BF00330673. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Hirose S., Okazaki T., Ogawa T., Kurosawa Y. Assay of RNA-linked nascent DNA pieces with polynucleotide kinase. Biochem Biophys Res Commun. 1975 Feb 17;62(4):1018–1024. doi: 10.1016/0006-291x(75)90424-6. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Sims J., Capon D., Dressler D. dnaG (primase)-dependent origins of DNA replication. Nucleotide sequences of the negative strand initiation sites of bacteriophages St-1, phi K, and alpha 3. J Biol Chem. 1979 Dec 25;254(24):12615–12628. [PubMed] [Google Scholar]
- Sims J., Dressler D. Site-specific initiation of a DNA fragment: nucleotide sequence of the bacteriophage G4 negative-strand initiation site. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3094–3098. doi: 10.1073/pnas.75.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A. M. DNA replication--bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:201–237. [PubMed] [Google Scholar]
- Sugimoto K., Kohara Y., Okazaki T. Relative roles of T7 RNA polymerase and gene 4 primase for the initiation of T7 phage DNA replication in vivo. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3977–3981. doi: 10.1073/pnas.84.12.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Multiple initiation sites of DNA replication flanking the origin region of lambda dv genome. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7402–7406. doi: 10.1073/pnas.81.23.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purified bacteriophage lambda O protein binds to four repeating sequences at the lambda replication origin. Nucleic Acids Res. 1981 Apr 24;9(8):1789–1799. doi: 10.1093/nar/9.8.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Yoda K., Okazaki T. Primer RNA for DNA synthesis on single-stranded DNA template in a cell free system from Drosophila melanogaster embryos. Nucleic Acids Res. 1983 Jun 11;11(11):3433–3450. doi: 10.1093/nar/11.11.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Gorska I., Taylor K., Georgopoulos C. Bacteriophage lambda replication proteins: formation of a mixed oligomer and binding to the origin of lambda DNA. Mol Gen Genet. 1984;196(3):401–406. doi: 10.1007/BF00436186. [DOI] [PubMed] [Google Scholar]





