Abstract
Purpose
Evidence has suggested a clinically meaningful relationship between self-reported quality of life (QOL) of a patient with cancer at the time of receiving a cancer diagnosis and overall survival (OS). This study evaluated the prognostic value of QOL assessments with regard to OS in a large cohort of patients with lung cancer.
Patients and Methods
A total of 2,442 patients with non–small-cell lung cancer were observed between 1997 and 2007 and completed a single-item measure of overall QOL within the first 6 months of receiving a lung cancer diagnosis; these were dichotomized using an a priori definition of a clinically deficient score (CDS; ≤ 50 v > 50). Kaplan-Meier estimates and Cox models were used to evaluate the prognostic importance of QOL on OS alone and in the presence of covariates. Logistic regression modeling was used to identify which clinical and patient characteristics were related to a clinically meaningful deficit in QOL.
Results
QOL deficits at time of lung cancer diagnosis were significantly associated with OS (hazard ratio [HR], 1.55; P < .001), as were performance status, older age, smoking history, male sex, treatment factors, and stage of disease. The median survival for patients with CDS QOL was 1.6 years versus 5.6 years for patients with non-CDS QOL. After controlling for all these covariates, the indication of a clinically deficient baseline QOL still contributed significantly to the prediction of patient survival (HR, 0.67; P < .001).
Conclusion
Overall QOL measured by a simple single item at the time of lung cancer diagnosis is a significant and independent prognostic factor for survival in patients with lung cancer.
INTRODUCTION
Quality of life (QOL) is a critical aspect of living with lung cancer.1–5 Validated lung cancer–specific QOL measures such as the European Organisation for the Research and Treatment of Cancer and Functional Assessment for Chronic Illness Treatment tools have been applied in clinical studies.6–12 A systematic review exists of QOL assessment in non–small-cell lung cancer (NSCLC).13
There is a strong association between QOL and survival in cancer populations.14–18 Gotay et al19 published a critical systematic review indicating that QOL at time of diagnosis was prognostic for survival. Quinten et al20 carried out a meta-analysis involving more than 10,000 patients with cancer and found that baseline QOL was a prognostic indicator of survival. Efficace et al,9,21 Montazeri et al,22 Mauer et al,23 and our research team24,25 have replicated this finding in various cancer populations.
Information regarding factors associated with long-term cancer survival is expanding.3,26–28 Most work has involved patients with breast cancer, indicating that the QOL of breast cancer survivors typically returns to normal over time. The situation is starkly different for lung cancer, because most patients with lung cancer will not live long after diagnosis. However, Mountain29 reported 2-year survival as 37% to 86% and 5-year survival as 22% to 67% among patients with stage I or II disease; hence, a substantial proportion of patients with early-stage lung cancer will survive with considerable QOL issues.30
Few studies have described the QOL of lung cancer survivors beyond the acute treatment period. A prior study conducted by our group reported on the association between cigarette smoking and QOL up to 3 years after lung cancer diagnosis, with only secondary attention given to other factors possibly associated with QOL.31 Results of our companion study32 indicated that long-term cancer survivors suffered substantial symptom burden that significantly impaired QOL.33
The primary aim of this study was to confirm the prognostic value of QOL at the time of lung cancer diagnosis in predicting survival among patients with lung cancer. A secondary aim was to identify a patient profile associated with poor QOL at the time of diagnosis of lung cancer. The overarching goal of this program of research is to explore which patients with lung cancer have poor QOL and then to design interventions that can be delivered in the cancer care setting to improve QOL, prevent QOL deficits, and perhaps ultimately improve survival.
PATIENTS AND METHODS
Sample
The Mayo Clinic Epidemiology and Genetics of Lung Cancer Research Program has enrolled and prospectively observed patients either diagnosed with and/or treated for lung cancer at the Mayo Clinic (Rochester, MN) since its inception in 1997. Between January 1, 1997, and December 31, 2009, more than 10,000 patients with lung cancer have been enrolled. Procedures for identifying and observing patients with lung cancer enrolled onto this program have been previously described.34 Patients provided informed consent for the study, and it was approved by the relevant ethics committees. Patient follow-up was accomplished by a mailed questionnaire within 6 months after diagnosis and annually thereafter, as described in the parent protocol.34
QOL Assessment
QOL was assessed at all follow-up time points by means of one item from the Lung Cancer Symptom Scale.35,36 The overall QOL item served as the primary end point in the current study. In the primary analysis, overall QOL was considered as a continuous variable, taking integer values from 0 to 100.37–40 A score below 50 was indicative of a need for immediate exploration and intervention for the QOL deficit.41–43 This cutoff has been validated by our research team39,44 and independently by others.45–47
Analysis
The primary aim of the statistical analysis was to explore the prognostic power of a clinically meaningful deficit in QOL (score ≤ 50 on 0 to 100 point scale) in terms of predicting survival. Secondary aims included investigating the impact of concomitant covariates on the prognostic power of the QOL assessment. Ancillary aims included looking at the relationship among various baseline covariates and overall QOL.
Covariates considered in this study included a collection of baseline characteristics with purported association with QOL, as suggested by previous authors.16,18,31 These variables can be broadly grouped into demographic (age, sex, race, comorbidities), social (employment status, marital status, years of education), smoking history (pack years, never, former, recent quitter, still smoking) disease-related (histology, stage, grade), and treatment-related (chemotherapy, radiation, surgery) characteristics. In previous studies, we examined the variability of time since diagnosis within the 6-month eligibility period for this study and found no impact on the findings reported herein.32,34 Given the prevalence of cigarette smoking in patients with lung cancer, smoking classification was assessed in several ways. First was pack years, defined as the number of packs of cigarettes smoked over time. For example, a participant who smoked one pack of cigarettes per day for 20 years would have a 20-year pack history. Participants were also classified according to smoking status at the time they completed the QOL item: never smoker (< 100 lifetime cigarettes), former smoker (quit > 12 months), recent quitter (quit > 30 days but < 12 months), or current smoker (any tobacco usage in the past 30 days).
Collinearity among covariates was examined in previous studies34 via the methods of Belsey et al,48 including variance inflation factors and index numbers so that overlap among the independent variables did not affect the resultant findings. In two previous studies, we carried out extensive investigations for redundancy among the various covariates and only included in this study those that were identified as significant independent prognostic indicators on survival.49,50
The primary aim was accomplished using Kaplan-Meier survival estimates and associated multivariate Cox proportional hazards models to assess the prognostic power of QOL for survival in the presence of the aforementioned covariates. The secondary aim of identifying which patient and disease characteristics were associated with a report of a clinically meaningful deficit in QOL was explored using univariate Fisher's exact and t-tests followed by a stepwise logistic regression modeling process.
Power Considerations
The large sample (2,442 patients with lung cancer) available through the Mayo Clinic Epidemiology and Genetics of Lung Cancer Research Program provides considerable precision for all analytic procedures. Any percentage reported on the total sample is accurate to within 2% of the population percentage with 95% confidence. Any average QOL score is accurate to within 4% times the standard deviation, or less than one point on a 100-point scale.51 A Fisher's exact test has 80% power to detect a difference of 6% between the incidence rates of men and women reporting a deficit in QOL. Finally, a Kaplan-Meier–based survival analysis log-rank test has 80% power to detect a median difference of 1 month in the median survival between those who report a clinically meaningful deficit in QOL versus those who do not. This power calculation assumes a 12-month median survival in the group not reporting a deficit in QOL. Because power was plentiful for this study, it was more important to examine the effect sizes observed instead of the P values.
RESULTS
Sample Characteristics
A total of 2,442 patients with lung cancer who completed the overall QOL item at least once within 6 months of receiving their lung cancer diagnosis were included in the analysis. Demographic variables are presented for the entire sample and classified by QOL score in Table 1 . Over the 11-year study period, 120 (5%) of the 2,442 patients with lung cancer survived, and 2,320 (95%) died. A majority of patients were men, white, and married; had good performance status and early disease stage; were never or former smokers who had quit smoking more than 20 years ago; and had undergone surgical treatment.
Table 1.
Patient Demographics and Clinical Characteristics by First QOL Assessment (N = 2,442)
| Characteristic | QOL > 50 (n = 1,932) |
QOL ≤ 50 (n = 510) |
Total (N = 2,442) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age, years | .005 | ||||||
| < 50 | 198 | 10 | 31 | 6 | 229 | 9 | |
| 50 to < 65 | 707 | 37 | 180 | 35 | 887 | 36 | |
| 65 to 80 | 914 | 47 | 276 | 54 | 1,190 | 49 | |
| > 80 | 113 | 6 | 23 | 5 | 136 | 6 | |
| Smoker category | < .001 | ||||||
| Never | 360 | 19 | 61 | 12 | 421 | 17 | |
| Former | 1,035 | 54 | 266 | 52 | 1,301 | 53 | |
| Recent quitter/abstinent | 316 | 16 | 104 | 20 | 420 | 17 | |
| Current/persistent | 221 | 11 | 79 | 16 | 300 | 13 | |
| Treatment | < .001 | ||||||
| Surgery | 1,431 | 74 | 277 | 55 | 1,708 | 70 | |
| Radiation or chemotherapy only | 199 | 10 | 89 | 17 | 288 | 12 | |
| Radiation plus chemotherapy | 225 | 12 | 108 | 21 | 333 | 14 | |
| Other | 77 | 4 | 36 | 7 | 113 | 4 | |
| Sex | < .001 | ||||||
| Female | 975 | 51 | 175 | 34 | 1,150 | 47 | |
| Male | 957 | 49 | 335 | 66 | 1,292 | 53 | |
| Any minority | .16 | ||||||
| Missing | 0 | 1 | 1 | ||||
| No | 1,800 | 93 | 483 | 95 | 2,283 | 94 | |
| Yes | 132 | 7 | 26 | 5 | 158 | 6 | |
| Race | .09 | ||||||
| Missing | 0 | 1 | 1 | ||||
| White | 1,802 | 92 | 487 | 95 | 2,289 | 92 | |
| Hispanic | 15 | 1 | 5 | 1 | 20 | 1 | |
| Alaskan native | 95 | 5 | 15 | 3 | 110 | 5 | |
| Black | 15 | 1 | 0 | 0 | 15 | 1 | |
| Asian/Pacific Islander | 5 | 1 | 2 | 1 | 7 | 1 | |
| Marital status | .83 | ||||||
| Missing | 206 | 86 | 292 | ||||
| Single | 64 | 4 | 19 | 5 | 83 | 4 | |
| Married | 1,373 | 80 | 328 | 77 | 1,701 | 79 | |
| Divorced | 111 | 6 | 28 | 7 | 139 | 6 | |
| Widowed | 178 | 10 | 49 | 11 | 227 | 11 | |
| Disease stage | < .001 | ||||||
| I | 973 | 50 | 181 | 36 | 1,154 | 48 | |
| II | 205 | 11 | 46 | 9 | 251 | 10 | |
| III/limited | 433 | 22 | 159 | 31 | 592 | 24 | |
| IV/extensive | 321 | 17 | 124 | 24 | 445 | 18 | |
| ECOG performance status | < .001 | ||||||
| Missing | 40 | 12 | 52 | ||||
| Fully active | 840 | 44 | 27 | 6 | 867 | 36 | |
| Light work | 913 | 48 | 223 | 45 | 1,136 | 47 | |
| Unable to work | 122 | 6 | 159 | 31 | 281 | 12 | |
| Limited self-care | 15 | 1 | 74 | 14 | 89 | 4 | |
| Disabled | 2 | 1 | 15 | 3 | 17 | 1 | |
| Smoking cessation, years | < .001 | ||||||
| Quit ≥ 10 or never smoked | 1,072 | 56 | 241 | 47 | 1,313 | 54 | |
| Quit 3-9 | 251 | 13 | 66 | 13 | 317 | 13 | |
| Quit 1-2 | 105 | 5 | 34 | 7 | 139 | 5 | |
| Quit at or after diagnosis | 414 | 21 | 125 | 25 | 539 | 22 | |
| Never quit | 90 | 5 | 44 | 8 | 134 | 6 | |
| Pack years smoked | < .001 | ||||||
| Missing | 6 | 2 | 8 | ||||
| 0 to < 20 | 697 | 36 | 114 | 22 | 811 | 33 | |
| 20 to < 40 | 431 | 23 | 99 | 20 | 530 | 22 | |
| 40 to < 60 | 407 | 21 | 150 | 29 | 557 | 23 | |
| > 60 | 391 | 20 | 145 | 29 | 536 | 22 | |
| Mean time from diagnosis, years | 1.36 | 1.39 | 1.37 | .6960 | |||
| SD | 1.26 | 1.36 | 1.28 | ||||
| Median | 0.99 | 0.85 | 0.97 | ||||
| Any other cancer | .4737 | ||||||
| No | 1,655 | 86 | 444 | 87 | 2,099 | 86 | |
| Yes | 277 | 14 | 66 | 13 | 343 | 14 | |
| Any other lung disease | .8163 | ||||||
| No | 1,463 | 76 | 389 | 76 | 1,852 | 76 | |
| Yes | 469 | 24 | 121 | 24 | 590 | 24 | |
| Any other disease | .0014 | ||||||
| No | 1,409 | 73 | 407 | 80 | 1,816 | 74 | |
| Yes | 523 | 27 | 103 | 20 | 626 | 26 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; QOL, quality of life; SD, standard deviation.
QOL
Clinically significant deficits in QOL were reported by 510 patients (21%). Patients who reported a clinically significant deficit in QOL tended to be older than those who did not report a QOL deficit (59% v 53% > 65 years of age). Patients with a QOL deficit were also more likely to be current smokers (36% v 28%) and men (65% v 50%) and to have worse performance status (51% v 93%). Patients with a QOL deficit were less likely to have had surgery (54% v 74%) and less likely to have early disease stage (44% v 61%) than those who did not report a QOL deficit. The average overall QOL scores were similar among different measurement approaches: first, when QOL was assessed the first time (70.8; standard deviation [SD], 24.04); second, when QOL scores were averaged across the early 3 years of assessment for each patient (70; SD, 23.01); third, when QOL scores were averaged across 5 years of assessment (73.3; SD, 20.49); and fourth, when averaged over all assessments (68.8; SD, 22.34). For the four measurement approaches, 24% (597 of 2,442 patients), 20% (471 of 2,335), 16% (108 of 691), and 23% (553 of 2,442) of the patients, respectively, reported a clinically meaningful deficit in overall QOL (≤ 50). Subsequent analyses produced the same results for all measurement approaches. The score closest to the time of diagnosis has the most clinical utility for the cancer care practitioner, so results for the first QOL assessment are presented for the remainder of the report.
Survival Analysis
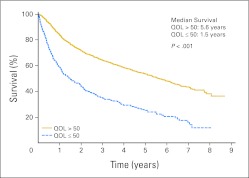
Kaplan-Meier estimate survival curves by QOL classification score are shown in Figure 1, indicating survival from the first QOL assessment between those who have a clinically deficient score (CDS) in QOL versus those who do not. Patients who reported CDS QOL had a median survival of 1.5 years, compared with 5.6 years for patients who reported a non-CDS QOL at baseline (P < .001).
Fig 1.
Kaplan-Meier survival curves for 2,442 patients with non–small-cell lung cancer by overall quality-of-life (QOL) categorization into clinically meaningful deficit versus no deficit.
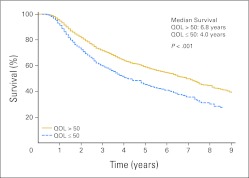
Subsequent to the univariate survival analysis, exploration for significant concomitant influences was undertaken using a Cox regression model. Association with OS was indicated for age, treatment, sex, disease stage, Eastern Cooperative Oncology Group performance score, disease recurrence/progression, having any other cancer, and time since cancer diagnosis. Modified survival curves controlling for these variables are depicted in Figure 2, indicating a persistent difference in overall survival between patients who reported CDS QOL and those who reported non-CDS QOL. After controlling for all these factors, the indication of CDS QOL at first assessment continued to demonstrate a relationship with patient survival (Table 2; hazard ratio, 1.4; P < .001). Smoking category and status at follow-up, years since quitting smoking, and years of consuming one pack every day were not contributing factors for survival in this analysis. As previously stated, these analyses were repeated using QOL scores across the first 3 years after diagnosis and across all years, with similar results (data not shown).
Fig 2.
Kaplan-Meier survival curves by first quality-of-life (QOL) assessment adjusted for age, sex, treatment, and smoking status.
Table 2.
Saturated Multivariate Cox Regression Model Survival Analysis Using First QOL Assessment
| Effect | HR | 95% CI | P |
|---|---|---|---|
| QOL (v > 50)* | |||
| ≤ 50 | 1.55 | 1.30 to 1.85 | < .001 |
| Age, years (v > 80)* | |||
| 50 to < 65 | 0.36 | 0.27 to 0.48 | < .001 |
| 65 to 80 | 0.56 | 0.43 to 0.73 | < .001 |
| ≤ 50 | 0.35 | 0.24 to 0.51 | < .001 |
| Smoker category (v current smoker)* | |||
| Never | 1.31 | 0.83 to 2.08 | .24 |
| Former | 1.22 | 0.83 to 1.8 | .32 |
| Recently quit | 1.51 | 1.14 to 1.99 | .004 |
| Treatment (v other)* | |||
| Radiation or chemotherapy only | 0.87 | 0.58 to 1.30 | .5 |
| Radiation plus chemotherapy | 0.61 | 0.40 to 0.91 | .02 |
| Surgery | 0.23 | 0.15 to 0.34 | < .001 |
| Sex (v male)* | |||
| Female | 0.83 | 0.72 to 0.975 | .015 |
| Stage (v IV)* | |||
| I | 0.39 | 0.31 to 0.48 | < .001 |
| II | 0.59 | 0.45 to 0.78 | < .001 |
| III/limited | 0.67 | 0.55 to 0.81 | < .001 |
| Recurrence and/or progression (v yes)* | |||
| No | 0.51 | 0.44 to 0.6 | < .001 |
| ECOG performance status (v 2, 3, 4)* | |||
| 0, 1 | 0.53 | 0.44 to 0.64 | < .001 |
| Smoking cessation, years (v never quit)* | |||
| Quit 1-2 | 0.73 | 0.45 to 1.19 | .21 |
| Quit 3-9 | 0.64 | 0.4 to 1.01 | .06 |
| Quit ≥ 10 or never smoked | 0.6 | 0.38 to 0.96 | .03 |
| Quit at or after diagnosis | 0.45 | 0.32 to 0.62 | < .001 |
| Pack years smoked (v > 60)* | |||
| 0 to < 20 | 0.73 | 0.57 to 0.95 | .02 |
| 20 to < 40 | 0.89 | 0.72 to 1.09 | .24 |
| 40 to < 60 | 1.14 | 0.94 to 1.38 | .18 |
| Any other cancer (v yes)* | |||
| No | 1.32 | 1.09 to 1.58 | .004 |
| Time from diagnosis, per year | 0.66 | 0.62 to 0.71 | < .001 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; QOL, quality of life.
Reference group.
QOL Correlates
Having established the importance of QOL at the time of lung cancer diagnosis as a prognostic indicator, we explored which factors affected baseline QOL (Table 3). A deficit in patient overall QOL was associated with a considerable number of variables in a univariate model including age, sex, disease stage, smoking status, treatment type, Eastern Cooperative Oncology Group performance score, smoking cessation, and pack years smoked. Clearly, many of these variables are related to one another. When these variables were input into a stepwise logistic regression procedure, age, sex, performance status, disease stage, disease recurrence, and presence of another cancer diagnosis were selected via the modeling process as significant influences on the likelihood for the presence of CDS QOL. This model had a pseudo-R2 of 22%, indicating that overall QOL was much more than a simple amalgamation of performance status and some other demographic and clinical variables.
Table 3.
Univariate and Stepwise Logistic Regression Model Results: Variables Associated With QOL at First Assessment
| Variable | Univariate P* | Logistic Regression |
|
|---|---|---|---|
| Estimate | P | ||
| Intercept | −6.19 | < .001 | |
| Age | .02 | 0.22 | .02 |
| Sex | < .001 | 0.35 | .006 |
| Stage | < .001 | 0.13 | .03 |
| Smoking status | < .001 | −0.2 | .33 |
| Treatment | < .001 | 0.12 | .50 |
| ECOG performance status | < .001 | 0.17 | .04 |
| Recurrence and/or progression | .25 | 2.33 | < .001 |
| Smoking cessation | < .001 | 0.16 | .26 |
| Pack years smoked | < .001 | 0.15 | .06 |
| Any other cancer | .54 | 0.16 | .011 |
| Time from diagnosis | .67 | 0.01 | .81 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; QOL, quality of life.
Fisher's exact test for categorical variables; two-sample t test for continuous variables.
DISCUSSION
The importance of QOL to patients with cancer and their caregivers has been well documented. Patients with cancer care deeply about their mortality but also about their QOL during the time they have left. In this large sample of patients with lung cancer, QOL at the time of diagnosis was found to be related to mortality. Therefore, if these findings are confirmed by other investigators, perhaps QOL at the time of lung cancer diagnosis has a clinically meaningful impact both on QOL over time and on survival rates. Regardless, the strong association between deficits in overall QOL and survival observed in this large prospective sample of patients with lung cancer highlights the importance of assessing QOL of patients with lung cancer at the time of diagnosis as part of their ongoing cancer care. That this relationship remains significant even after controlling for known factors related to survival provides evidence that QOL is as important as smoking status or stage of disease in predicting survival from lung cancer.
Our findings are consistent with those of other studies that investigated the importance of QOL in patients with NSCLC, notably studies by the Radiation Therapy Oncology Group, indicating that QOL is a strong prognostic indicator in this patient population.52,53 It is possible that interventions designed and tailored for patients with lung cancer may improve both their QOL and likelihood of survival. These findings add to the ever-growing research literature that points toward an important and crucial role for assessing QOL in clinical practice and hold the potential for identifying subsets of patients who are experiencing deficits in QOL and may therefore benefit from specific attention to these expressed QOL needs.54–57
Although the study had a high enrollment and adherence rate, the interplay of many forces makes interpreting longitudinal QOL results challenging. The data presented here came from a set of patients with lung cancer able and willing to respond to the mailed questionnaires and do not accurately represent our overall cohort of patients with lung cancer, potentially introducing bias. A recently reported study conducted by our group restricted to long-term lung cancer survivors with QOL assessments within 3 and beyond 5 years after diagnosis did demonstrate worsening QOL over time (Yang et al, manuscript submitted for publication). The cohort in the current study did not include long-term survivors exclusively; rather, it was a group of survivors observed longitudinally beginning at diagnosis and continuing to death.
The relationship between QOL and survival also has a possible psychosocial connection that warrants investigation. Adherence to cancer care is important for positive health outcomes. Perhaps, given their poor functioning, individuals with poor QOL demonstrate poor adherence to their medical treatment.58,59 If a patient with lung cancer is not feeling well, optimistic, or motivated, it would seem possible that he or she may be less likely to keep medical appointments, attend treatment sessions, or adhere to medication plans. Future investigators should include measures of adherence to cancer treatment when examining QOL deficits in patients with lung cancer.
The use of a single-item assessment for identifying deficits in QOL is naturally appealing for its simplicity, although it naturally is unable to describe the precise nature of the deficit observed. Herein lie the complementary roles that single- and multiple-item QOL assessments can play in cancer research and clinical practice, as described by Sloan et al.37,38 The single-item QOL assessment has the advantage of covering any and all domains that the patient defines as important to his or her QOL, which a predefined multiple-item scale might not include. However, once these are identified, the role of the multiple-item scale in describing the precise nature of the deficit and identifying potential follow-up interventions is obvious.
We have begun to use brief QOL assessments routinely at baseline in North Central Cancer Treatment Group clinical trials and during clinical oncology visits at the Mayo Clinic to begin incorporating this information into patient care decisions. Preliminary findings indicate that as many as 20% of oncology practice patients report a clinically significant deficit in QOL, with scores ≤ 2 on a scale of 0 to 10. Furthermore, between 20% and 50% of our patients with cancer report clinically deficient QOL in domains that can be ameliorated with relatively simple and established interventions, such as antidepressants for fatigue, pharmacologic solutions for erectile dysfunction, and counseling for social and financial aspects of QOL.
In summary, this prospective study of a large cohort of patients with lung cancer adds to the growing evidence that patient-reported QOL outcomes at the time of cancer diagnosis can identify vulnerable subpopulations. Over and above performance status and key clinical and demographic variables, a QOL patient-reported outcome assessment can identify deficits that are independently associated with abbreviated survival. The next step in this line of research is to develop and test interventions to apply when QOL deficits occur to improve patient QOL and survival.
Footnotes
Supported by National Institutes of Health Research Grants No. CA77118, CA80127, and CA84354 (P.Y.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jeff A. Sloan, Matthew M. Clark, Ping Yang
Collection and assembly of data: Jeff A. Sloan, Jason Wampfler,Ping Yang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR. 21. J Clin Oncol. 2006;24:3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 2.Bottomley A, Efficace F, Thomas R, et al. Health-related quality of life in non–small-cell lung cancer: Methodologic issues in randomized controlled trials. J Clin Oncol. 2003;21:2982–2992. doi: 10.1200/JCO.2003.01.203. [DOI] [PubMed] [Google Scholar]
- 3.Lilenbaum RC, Cashy J, Hensing TA, et al. Prevalence of poor performance status in lung cancer patients: Implications for research. J Thorac Oncol. 2008;3:125–129. doi: 10.1097/JTO.0b013e3181622c17. [DOI] [PubMed] [Google Scholar]
- 4.Broberger E, Sprangers M, Tishelman C. Do internal standards of quality of life change in lung cancer patients? Nurs Res. 2006;55:274–282. doi: 10.1097/00006199-200607000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Brundage MD, Davidson JR, Mackillop WJ. Trading treatment toxicity for survival in locally advanced non-small cell lung cancer. J Clin Oncol. 1997;15:330–340. doi: 10.1200/JCO.1997.15.1.330. [DOI] [PubMed] [Google Scholar]
- 6.Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: A modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer. 1994;30A:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 7.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55:265–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 8.Eton DT, Fairclough DL, Cella D, et al. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: Results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21:1536–1543. doi: 10.1200/JCO.2003.07.128. [DOI] [PubMed] [Google Scholar]
- 9.Efficace F, Bottomley A, Smit EF, et al. Is a patient's self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Ann Oncol. 2006;17:1698–1704. doi: 10.1093/annonc/mdl183. [DOI] [PubMed] [Google Scholar]
- 10.Langendijk H, Aaronson NK, de Jong JM, et al. The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol. 2000;55:19–25. doi: 10.1016/s0167-8140(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 11.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non–small-cell lung cancer receiving chemotherapy: A prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 12.Nowak AK, Stockler MR, Byrne MJ. Assessing quality of life during chemotherapy for pleural mesothelioma: Feasibility, validity, and results of using the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire and Lung Cancer Module. J Clin Oncol. 2004;22:3172–3180. doi: 10.1200/JCO.2004.09.147. [DOI] [PubMed] [Google Scholar]
- 13.Claassens L, van Meerbeeck J, Coens C, et al. Health-related quality of life in non–small-cell lung cancer: An update of a systematic review on methodologic issues in randomized controlled trials. J Clin Oncol. 2011;29:2104–2120. doi: 10.1200/JCO.2010.32.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley ME. Symptoms in adults with lung cancer: A systematic research review. J Pain Symptom Manage. 2000;19:137–153. doi: 10.1016/s0885-3924(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 15.Dales RE, Bélanger R, Shamji FM, et al. Quality-of-life following thoracotomy for lung cancer. J Clin Epidemiol. 1994;47:1443–1449. doi: 10.1016/0895-4356(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 16.Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 17.Finkelstein DM, Cassileth BR, Bonomi PD, et al. A pilot study of the Functional Living Index-Cancer (FLIC) scale for the assessment of quality of life for metastatic lung cancer patients: An Eastern Cooperative Oncology Group Study. Am J Clin Oncol. 1988;11:630–633. doi: 10.1097/00000421-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ganz PA, Lee JJ, Siau J. Quality of life assessment: An independent prognostic variable for survival in lung cancer. Cancer. 1991;67:3131–3135. doi: 10.1002/1097-0142(19910615)67:12<3131::aid-cncr2820671232>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 20.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncology. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 21.Efficace F, Therasse P, Piccart MJ, et al. Health-related quality of life parameters as prognostic factors in a nonmetastatic breast cancer population: An international multicenter study. J Clin Oncol. 2004;22:3381–3388. doi: 10.1200/JCO.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 22.Montazeri A, Milroy R, Hole D. Quality of life in lung cancer patients: As an important prognos tic factor. Lung Cancer. 2001;31:233–240. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 23.Mauer M, Stupp R, Taphoorn MJ, et al. The prognostic value of health-related quality-of-life data in predicting survival in glioblastoma cancer patients: Results from an international randomised phase III EORTC Brain Tumour and Radiation Oncology Groups, and NCIC Clinical Trials Group study. Br J Cancer. 2007;97:302–307. doi: 10.1038/sj.bjc.6603876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan AD, Novotny PJ, Kaur JS, et al. A patient-level meta-analytic investigation of the prognostic significance of baseline quality of life (QOL) for overall survival (OS) among 3,704 patients participating in 24 North Central Cancer Treatment Group (NCCTG) and Mayo Clinic Cancer Center (MC) oncology clinical trials. J Clin Oncol. 2008;26(suppl):505s. abstr 9515. [Google Scholar]
- 25.Qi Y, Schild SE, Mandrekar SJ, et al. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. Thorac Oncol. 2009;4:1075–1082. doi: 10.1097/JTO.0b013e3181ae27f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarna L, Padilla G, Holmes C, et al. Quality of life of long-term survivors of non–small-cell lung cancer. J Clin Oncol. 2002;20:2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Farmer M, Case D, Lesser G, et al. A phase III double blind placebo controlled prospective randomized trial on the effect of megestrol acetate on weight and health-related quality of life in lung cancer and head/neck cancer patients receiving definitive radiation therapy. Int J Radiat Oncol Biol Physics. 2005;63:S77. abstr. [Google Scholar]
- 29.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 30.Geddes DM. Quality of life in lung cancer. Respir Med. 1991;85(suppl B):7–11. doi: 10.1016/s0954-6111(06)80162-9. discussion 33-37. [DOI] [PubMed] [Google Scholar]
- 31.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 32.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: Experience at Mayo Clinic from 1997-2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 33.Clark MM, Novotny PJ, Patten CA, et al. Motivational readiness for physical activity and quality of life in long-term lung cancer survivors. Lung Cancer. 2008;61:117–122. doi: 10.1016/j.lungcan.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimura H, Yang P. Long-term survivorship in lung cancer: A review. Chest. 2006;129:1088–1097. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 35.Hollen PJ, Gralla RJ, Kris MG, et al. Normative data and trends in quality of life from the Lung Cancer Symptom Scale (LCSS) Support Care Cancer. 1999;7:140–148. doi: 10.1007/s005200050244. [DOI] [PubMed] [Google Scholar]
- 36.Hollen PJ, Gralla RJ, Kris MG, et al. A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptom Scale (LCSS): Does format affect patient ratings of symptoms and quality of life? Qual Life Res. 2005;14:837–847. doi: 10.1007/s11136-004-0833-8. [DOI] [PubMed] [Google Scholar]
- 37.Sloan JA, Dueck A, Frost MH, et al. Applying QOL assessments: Solutions for oncology clinical practice and research, part 1. Curr Prob Cancer. 2005;29:267–351. [Google Scholar]
- 38.Sloan JA, Dueck A, Frost MH, et al. Applying QOL assessments: Solutions for oncology clinical practice and research, part 2. Curr Probl Cancer. 2006;30:235–331. [Google Scholar]
- 39.Huschka MM, Mandrekar SJ, Schaefer PL, et al. A pooled analysis of quality of life measures and adverse events data in North Central Cancer Treatment Group lung cancer clinical trials. Cancer. 2007;109:787–795. doi: 10.1002/cncr.22444. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan DR, O'Mara AM, Kelaghan JW, et al. Quality-of-life assessment in the symptom management trials of the National Cancer Institute–supported community clinical oncology program. J Clin Oncol. 2005;23:591–598. doi: 10.1200/JCO.2005.12.181. [DOI] [PubMed] [Google Scholar]
- 41.Giorgi F, Cellerino R, Gramazio A, et al. Assessing quality of life in patients with cancer: A comparison of a visual-analogue and a categorical model. Am J Clin Oncol. 1996;19:394–399. doi: 10.1097/00000421-199608000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Ballatori E, Porzio G, Roila F, et al. Is there still a role for the uniscale assessment of quality of life? Tumori. 2007;93:78–81. doi: 10.1177/030089160709300114. [DOI] [PubMed] [Google Scholar]
- 43.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute–sponsored clinical trials networks. J Clin Oncol. 2007;25:5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 44.Locke DE, Decker PA, Sloan JA, et al. Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage. 2007;34:628–638. doi: 10.1016/j.jpainsymman.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginns P, Barrie S. Reliability of single-item ratings of quality in higher education: A replication. Psychol Rep. 2004;95:1023–1030. doi: 10.2466/pr0.95.3.1023-1030. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerman M, Ruggero CJ, Chelminski I, et al. Developing brief scales for use in clinical practice: The reliability and validity of single-item self-report measures of depression symptom severity, psychosocial impairment due to depression, and quality of life. J Clin Psychiatry. 2006;67:1536–1541. doi: 10.4088/jcp.v67n1007. [DOI] [PubMed] [Google Scholar]
- 47.Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35:20–30. doi: 10.1016/j.jpainsymman.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 48.Belsey DA, Kuh E, Welsch RE. New York, NY: John Wiley & Sons; 1980. Regression Diagnostics. [Google Scholar]
- 49.Sun Z, Aubry MC, Deschamps C, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: An analysis of 5,018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg. 2006;131:1014–1020. doi: 10.1016/j.jtcvs.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 50.Visbal AL, Williams BA, Nichols FC, 3rd, et al. Gender differences in non-small-cell lung cancer survival: An analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–215. doi: 10.1016/j.athoracsur.2003.11.021. discussion 215. [DOI] [PubMed] [Google Scholar]
- 51.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 52.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non–small-cell lung cancer: An analysis of RTOG 9801. J Clin Oncol. 2009;27:5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacot W, Colinet B, Bertrand D, et al. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol. 2008;19:1458–1464. doi: 10.1093/annonc/mdn064. [DOI] [PubMed] [Google Scholar]
- 54.Bernhard J, Hürny C, Bacchi M, et al. Initial prognostic factors in small-cell lung cancer patients predicting quality of life during chemotherapy: Swiss Group for Clinical Cancer Res (SAKK) Br J Cancer. 1996;74:1660–1667. doi: 10.1038/bjc.1996.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooley ME. Quality of life in persons with non-small cell lung cancer: A concept analysis. Cancer Nurs. 1998;21:151–161. doi: 10.1097/00002820-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Fergusson RJ, Cull A. Quality of life measurement for patients undergoing treatment for lung cancer. Thorax. 1991;46:671–675. doi: 10.1136/thx.46.9.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 59.Reimer T, Gerber B. Quality-of-life considerations in the treatment of early-stage breast cancer in the elderly. Drugs Aging. 2010;27:791–800. doi: 10.2165/11584700-000000000-00000. [DOI] [PubMed] [Google Scholar]