Abstract
Purpose
A randomized, placebo-controlled study based on preclinical and clinical data that supports the potential role of vascular endothelial growth factor in prostate cancer was performed to evaluate the addition of bevacizumab to standard docetaxel and prednisone therapy in patients with metastatic castration-resistant prostate cancer (mCRPC).
Patients and Methods
Patients with chemotherapy-naive progressive mCRPC with Eastern Cooperative Oncology Group performance status ≤ 2 and adequate bone marrow, hepatic, and renal function were randomly assigned to receive docetaxel 75 mg/m2 intravenously (IV) over 1 hour for 21 days plus prednisone 5 mg orally twice per day (DP) with either bevacizumab 15 mg/kg IV every 3 weeks (DP + B) or placebo. The primary end point was overall survival (OS), and secondary end points were progression-free survival (PFS), 50% decline in prostate-specific antigen, objective response (OR), and toxicity.
Results
In total, 1,050 patients were randomly assigned. The median OS for patients given DP + B was 22.6 months compared with 21.5 months for patients treated with DP (hazard ratio, 0.91; 95% CI, 0.78 to 1.05; stratified log-rank P = .181). The median PFS time was superior in the DP + B arm (9.9 v 7.5 months, stratified log-rank P < .001) as was the proportion of patients with OR (49.4% v 35.5%; P = .0013). Grade 3 or greater treatment-related toxicity was more common with DP + B (75.4% v 56.2%; P ≤ .001), as was the number of treatment-related deaths (4.0% v 1.2%; P = .005).
Conclusion
Despite an improvement in PFS and OR, the addition of bevacizumab to docetaxel and prednisone did not improve OS in men with mCRPC and was associated with greater toxicity.
INTRODUCTION
Vascular endothelial growth factor (VEGF) plays a critical role in the pathogenesis and progression of human prostate cancer.1,2 In human prostate cancer cells, the expression of VEGF (Flk-1/KDR) receptors correlates with poorly differentiated tumors and poor prognosis.1 VEGF is present in both localized and metastatic prostate tumors, and increasing plasma concentration of VEGF correlates with metastatic disease progression.3–5 In patients with metastatic castration-resistant prostate cancer (mCRPC), both plasma and urine VEGF levels are independent predictors of overall survival (OS).6,7 These data supported the role of VEGF in prostate cancer progression and the hypothesis that inhibition of VEGF may enhance current therapies in metastatic prostate cancer. To test this hypothesis, a multicenter, cooperative group phase II study of bevacizumab (Avastin; Genentech/Roche Pharmaceuticals, San Francisco, CA), a humanized immunoglobulin G monoclonal antibody to all the isoforms of VEGF-A, was combined with estramustine phosphate, prednisone, and docetaxel in 77 patients with mCRPC by the Cancer and Leukemia Group B (CALGB) in study 90006.8 A 50% or greater post-therapy decline in prostate-specific antigen (PSA) was observed in 75% of the patients, and complete or partial regression of measurable disease was achieved in 59% of the patients.8 The observed median progression-free survival (PFS) time and median OS time were 8 months and 24 months, respectively. These results were encouraging when compared with historical outcomes of patients with mCRPC treated in a series of other CALGB studies that used the docetaxel backbone. Following US Food and Drug Administration approval of docetaxel and prednisone for mCRPC, a double-blind, placebo-controlled phase III trial was conducted to determine whether the addition of bevacizumab to docetaxel and prednisone would increase OS compared with docetaxel and prednisone alone.
PATIENTS AND METHODS
Study Population
Eligible patients were required to have progressive adenocarcinoma of the prostate despite castrate levels of testosterone following antiandrogen withdrawal, as defined by Prostate-Specific Antigen Working Group 1 (PSAWG1) criteria.9 Patients were required to have an available Gleason score, a baseline PSA ≥ 5 ng/mL for patients with bone only disease, and no therapy with prior cytotoxics or antiangiogenic agents; they were also required to be 4 weeks or more from major surgery and prior radiotherapy and 8 weeks from prior radioisotope therapy. Patients were allowed to be taking a bisphosphonate if the dose was stable for at least 4 weeks before protocol treatment. Study exclusion criteria included evidence of brain metastasis, congestive heart failure (New York Heart classification II, III, or IV), uncontrolled hypertension, a GI bleed within the past 12 months, history of an arterial thrombotic event within the past 12 months, serious nonhealing ulcers or wounds, or grade ≥ 2 peripheral neuropathy. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≥ 2, absolute neutrophil count ≥ 1,500/μL, platelet count ≥ 100,000 μL, creatinine ≤ 1.5 × upper limit of normal (ULN), bilirubin ≤ 1.5 × ULN, AST ≤ 1.5 × ULN, and serum testosterone level ≤ 50 ng/dL. Stable doses of anticoagulants or antiplatelet therapy were allowed, but all herbal or alternative medications had to be discontinued before treatment. The protocol was approved by the local ethics committees of all participating centers, and written informed consent was obtained from all patients.
Treatment
All patients were administered docetaxel 75 mg/m2 intravenously on day 1 every 21 days and prednisone 5 mg orally daily starting on day 1 (DP). Patients were premedicated with dexamethasone 8 mg orally 12, 3, and 1 hour before the administration of docetaxel. Patients were randomly assigned with equal probability by the CALGB Statistical Center to receive either bevacizumab 15 mg/kg intravenously every 21 days (DP + B) or intravenous placebo on the same schedule (DP). A stratified random block design was used with randomization stratified by 24-month survival probability as predicted by a validated nomogram 10 (< 10%, 10% to 29.9%, ≥ 30%), age (< 65 years, ≥ 65 years), and prior history of arterial events (yes, no). Treatment was continued until disease progression or unacceptable toxicity for a maximum of 2 years. PSA progression was assessed by PSAWG1 criteria.9 Patients were required to receive a minimum of three cycles of therapy before response assessment. Appropriate use of growth factor support according to American Society of Clinical Oncology guidelines was permitted,11 and aspirin 81 mg daily in those patients who could tolerate it was encouraged. Treatment with a luteinizing hormone-releasing hormone agonist was continued for all patients who had not undergone a bilateral orchiectomy. Docetaxel dose was held if absolute neutrophil count was less than 1,500/μL or platelet count was less than 100,000/μL. The dose of docetaxel was decreased by 10 mg/m2 for grade 3 or 4 neutropenia for more than 1 week, febrile neutropenia, or grade 3 or 4 hepatic, neurologic, or GI toxicities. Patients could have a maximum of two docetaxel dose reductions. Bevacizumab or placebo was held for uncontrolled hypertension (≥ 160/90 mmHg), and if hypertension was not controlled with antihypertensive agents for more than one cycle, the bevacizumab or placebo was discontinued. Bevacizumab or placebo was discontinued for grade 4 hypertension, reversible posterior leukencephalopathy syndrome, recurrent arterial thrombotic events, grade ≥ 2 arterial thrombotic events, grade 3 hemorrhage or bleeding from any cause, GI perforation, wound dehiscence, or nephrotic syndrome. Patients who could not tolerate docetaxel therapy were encouraged to continue with bevacizumab or placebo alone, if tolerated. Prednisone dosage could be modified for toxicity as clinically indicated as by the treating physician.
Study Procedures
Baseline assessment included history or physical examination, CBC, liver function tests, serum testosterone, urinalysis, bone scan, magnetic resonance imaging or computed tomography scan of the abdomen and pelvis, and imaging of the chest. Patients had a repeat CBC, liver function tests, and urinalysis every cycle and a repeat bone scan and computed tomography scan of the abdomen/pelvis every 3 months until progression was confirmed for a maximum of 5 years after registration. Adverse events were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0.
Study Design and Data Analysis
The primary end point was OS with a target sample size of 1,050 patients. With 748 deaths, the log-rank test has 86% power to detect a 21% decrease in hazard of death (equivalent to an increase in median OS from 19 months in the DP arm to 24 months in the DP + B arm), assuming a two-sided type I error rate of 0.05. The following assumptions were made to achieve the target 748 deaths: an accrual rate of 29 patients per month over a 35-month enrollment period, 25-month follow-up after study closure, and OS time following an exponential distribution. Secondary end points were the proportion of patients who experienced at least a 50% post-therapy PSA decline, PFS, biochemical (PSA) PFS, objective response for patients with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST), and toxicity. OS was defined as the time from date of random assignment to date of death due to any cause. PFS was defined from the date of random assignment to date of progression or death due to any cause, whichever occurred first. Progression was defined by using PSAWG1 criteria with the exception that more than two new bone lesions were required for bone progression on a bone scan. Patients who discontinued treatment for reasons other than progression were followed for disease progression or death.
This trial was monitored by the CALGB Data and Safety Monitoring Board for efficacy and safety. Superiority and futility analyses were conducted for the OS end point. The Lan-Demets analog of the Emerson-Fleming sequential boundary was used to maintain the overall significance level of α = .05 while conducting interim analyses on OS. The final analysis was performed when 748 deaths had been observed. An intention-to-treat approach was used in the analysis for all the clinical end points with the exception of toxicity. The primary analysis of the OS end point was based on a two-sided stratified log-rank test for treatment effect, adjusting for the stratification factors. The proportional hazards model was used to assess the importance of the treatment effect after adjustment for stratification variables in subset analysis. The Kaplan-Meier product limit method was used to estimate the OS and PFS distributions. The χ2 and the Fisher exact tests were used to compare the two arms on objective response rates (ORRs) in patients with measurable disease, post-therapy decline in PSA from baseline, and adverse events between the two treatment groups. All analyses were performed by using SAS, version 9.1 (SAS Institute, Cary, NC). The study was designed by the CALGB, endorsed by the ECOG, and approved by the NCI Cancer Therapy Evaluation Program (CTEP). The CALGB Statistical Center performed registration, data collection, and statistical analyses.
As part of the CALGB quality assurance program, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Statistical Center staff and the study chair reviewed the data; the CALGB Audit Committee reviewed records on-site for 141 (13%) of 1,050 patients enrolled on the study.
RESULTS
Patients
Between May 2005 and December 2007, 1,050 patients were randomly assigned. The baseline clinical characteristics between the two arms were well balanced for known prognostic variables (Table 1). The majority of the patients were white, more than 60% of the patients were older than age 65 years, and 47% of the patients had a more than 30% probability of being alive at 24 months.10 Half the patient had measurable disease, and 35% of patients in each arm were taking opioid analgesics.
Table 1.
Baseline and Clinical Characteristics of 1,050 Patients by Treatment Arm
| Variable | DP + B(%; n = 524) | DP(%; n = 526) | Total(%; N = 1,050) | |||
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 88 | 87 | 88 | |||
| Age, years | ||||||
| < 65 | 34 | 33 | 33 | |||
| ≥ 65 | 66 | 67 | 67 | |||
| Median | 68.8 | 69.3 | 69.0 | |||
| Interquartile range | 63.0-74.4 | 62.4-75.6 | 62.7-75.2 | |||
| Prior history of arterial events | ||||||
| Yes | 7 | 8 | 7 | |||
| No | 93 | 92 | 93 | |||
| 24-month predicted survival probability, % | ||||||
| < 10 | 18 | 18 | 18 | |||
| 10-29.9 | 35 | 35 | 35 | |||
| ≥ 30 | 47 | 47 | 47 | |||
| ECOG performance status | ||||||
| 0 | 57 | 55 | 56 | |||
| 1 | 39 | 40 | 40 | |||
| 2 | 4 | 5 | 4 | |||
| Measurable disease | 47 | 53 | 50 | |||
| Sites of metastases* | ||||||
| Bone | 87 | 84 | 86 | |||
| Liver | 7 | 7 | 6 | |||
| Lung | 11 | 10 | 10 | |||
| Lymph node | 41 | 44 | 43 | |||
| Visceral | 17 | 14 | 16 | |||
| Other | 13 | 16 | 14 | |||
| Alkaline phosphatase, U/L | ||||||
| Median | 117 | 118.5 | 118 | |||
| Interquartile range | 84-220 | 81-230 | 82-226 | |||
| Hemoglobin, g/dL | ||||||
| Median | 12.9 | 12.6 | 12.8 | |||
| Interquartile range | 11.8-13.9 | 11.6-13.7 | 11.6-13.8 | |||
| LDH, U/L | ||||||
| Median | 202.5 | 206.0 | 205.0 | |||
| Interquartile range | 165.0-287.0 | 166.0-300.0 | 166.0-297.0 | |||
| PSA, ng/mL | ||||||
| Median | 88 | 82 | 85 | |||
| Interquartile range | 31-237 | 31-239 | 31-239 | |||
Abbreviations: DP, docetaxel 75 mg/m2 intravenously over 1 hour for 21 days plus prednisone 5 mg orally twice per day; DP + B, DP plus bevacizumab 15 mg/kg intravenously every 3 weeks; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PSA, prostate-specific antigen.
Not mutually exclusive.
Treatment Exposure
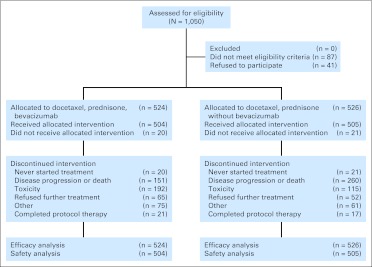
A total of 524 patients were randomly assigned to DP + B and 526 to DP (Fig 1). Patients who were given DP + B received a median of eight cycles of therapy (range, 0 to 37 cycles), and patients who were administered DP received a median of eight cycles of therapy (range, 0 to 40 cycles). Overall, more patients on the DP arm than on the experimental arm were removed from the study (49% v 29%). However, fewer patients were removed from the DP arm than from the DP + B arm due to toxicity (22% v 37%).
Fig 1.
CONSORT diagram.
Sixty-eight percent of the patients on the DP + B arm and 66% of the patients on the DP arm subsequently received second-line systemic therapy after they completed or were removed from the CALGB study. Forty-seven percent of the patients on the DP + B and DP arms received additional cytotoxic chemotherapy, with a majority receiving a docetaxel-based regimen.
Efficacy
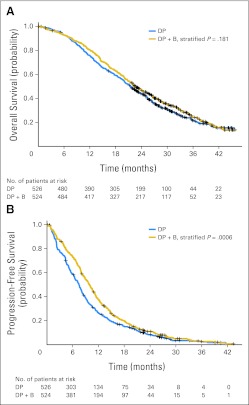
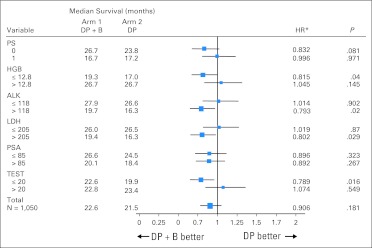
The median OS time was 22.6 months (95% CI, 21.1 to 24.5 months) for patients treated with DP + B compared with 21.5 months (95% CI, 20.0 to 23.0 months) for patients treated with DP with an estimated hazard ratio of 0.91 (95% CI, 0.7 to 1.05; stratified log-rank P = .181; Fig 2A). Patients treated with DP + B had longer PFS compared with patients treated with DP (median, 9.9 months [95% CI, 9.0 to 10.6 months] v 7.5 months [95% CI, 6.8 to 8.0 months]; hazard ratio, 0.80; 95% CI, 0.71 to 0.91; stratified log-rank P < .001; Fig 2B). More patients treated with DP + B achieved ≥ 50% post-therapy PSA declines compared with patients treated with DP (69.5% v 57.9%; P < .001). More patients treated on the bevacizumab arm achieved an objective response in measurable disease by RECIST (49.4% v 35.5%; P = .0013). Exploratory subset analysis suggested that patients with markers of high tumor burden or more advanced disease (low hemoglobin, increased serum alkaline phosphatase, or high lactate dehydrogenase) as well as patients with low testosterone levels (< 20 ng/dL) had an improved OS with the addition of bevacizumab to docetaxel and prednisone (Fig 3).
Fig 2.
Kaplan-Meier plot for (A) overall survival and (B) progression-free survival by treatment arm. DP, docetaxel 75 mg/m2 intravenously over 1 hour for 21 days plus prednisone 5 mg orally twice per day; DP + B, DP plus bevacizumab 15 mg/kg intravenously every 3 weeks.
Fig 3.
Forest plot of overall survival in select subgroups. ALK, alkaline phosphatase; DP, docetaxel 75 mg/m2 intravenously over 1 hour for 21 days plus prednisone 5 mg orally twice per day; DP + B, DP plus bevacizumab 15 mg/kg intravenously every 3 weeks; HGB, hemoglobin; HR, hazard ratio; LDH, lactate dehydrogenase; PS, [Eastern Cooperative Oncology Group] performance status; PSA, prostate-specific antigen; TEST, testosterone. (*) HRs are based on the proportional hazards model adjusting for the stratification factors.
Safety
Table 2 presents selected grade ≥ 3 adverse events that are possibly, probably, or definitely related to treatment. Higher rates of grade ≥ 3 neutropenia, fatigue, leukopenia, hypertension, GI hemorrhage and perforation, mucositis, and pneumonitis were reported in the bevacizumab arm; however, venous thrombosis and pulmonary embolism were less frequent in the DP + B arm. There was an overall greater number of maximum hematologic and nonhematologic adverse events associated with the administration of bevacizumab compared with DP (75.4% v 56.2%; P ≤ .001). The number of treatment-related deaths (4.0% v 1.2%; Fisher's exact test P = .005) was greater in the DP + B arm. The major cause of treatment-related deaths was related to infectious complications.
Table 2.
Grade 3 and Higher Adverse Events by Treatment Arm
| Adverse Event | Arm* | Grade of Adverse Event |
|||||
|---|---|---|---|---|---|---|---|
| 3 (severe) |
4 (life threatening) |
5 (lethal) |
|||||
| No. | % | No. | % | No. | % | ||
| Hematologic | |||||||
| Anemia | DP + B | 15 | 3 | 3 | 1 | 0 | 0 |
| DP | 15 | 3 | 1 | 0 | 0 | 0 | |
| Leukocytes (total WBC) | DP + B | 48 | 10 | 35 | 7 | 0 | 0 |
| DP | 43 | 9 | 23 | 5 | 0 | 0 | |
| Low neutrophils/granulocytes | DP + B | 40 | 8 | 112 | 22 | 0 | 0 |
| DP | 46 | 9 | 77 | 15 | 0 | 0 | |
| Nonhematologic | |||||||
| Cardiac ischemia/infarction | DP + B | 6 | 1 | 5 | 1 | 1 | 0 |
| DP | 1 | 0 | 1 | 0 | 1 | 0 | |
| Left ventricular diastolic dysfunction | DP + B | 0 | 0 | 0 | 0 | 0 | 0 |
| DP | 1 | 0 | 0 | 0 | 0 | 0 | |
| Left ventricular systolic dysfunction | DP + B | 1 | 0 | 0 | 0 | 0 | 0 |
| DP | 1 | 0 | 0 | 0 | 0 | 0 | |
| Hypertension | DP + B | 34 | 7 | 2 | 0 | 0 | 0 |
| DP | 6 | 1 | 1 | 0 | 0 | 0 | |
| Thrombosis/thrombus/embolism | DP + B | 8 | 2 | 11 | 2 | 0 | 0 |
| DP | 15 | 3 | 19 | 4 | 0 | 0 | |
| Fatigue | DP + B | 81 | 16 | 8 | 2 | 0 | 0 |
| DP | 49 | 10 | 4 | 1 | 0 | 0 | |
| Weight loss | DP + B | 4 | 1 | 0 | 0 | 0 | 0 |
| DP | 1 | 0 | 0 | 0 | 0 | 0 | |
| Anorexia | DP + B | 20 | 4 | 1 | 0 | 0 | 0 |
| DP | 8 | 2 | 0 | 0 | 0 | 0 | |
| Nausea | DP + B | 18 | 4 | 0 | 0 | 0 | 0 |
| DP | 7 | 1 | 0 | 0 | 0 | 0 | |
| GI perforation | DP + B | 9 | 2 | 7 | 1 | 2 | 0 |
| DP | 3 | 1 | 0 | 0 | 0 | 0 | |
| Dehydration | DP + B | 26 | 5 | 0 | 0 | 0 | 0 |
| DP | 14 | 3 | 0 | 0 | 0 | 0 | |
| Mucositis/stomatitis (functional/symptomatic) | DP + B | 14 | 3 | 0 | 0 | 0 | 0 |
| DP | 1 | 0 | 0 | 0 | 0 | 0 | |
| Genitourinary hemorrhage | DP + B | 5 | 1 | 0 | 0 | 0 | 0 |
| DP | 4 | 1 | 0 | 0 | 0 | 0 | |
| GI hemorrhage | DP + B | 25 | 5 | 4 | 1 | 1 | 0 |
| DP | 11 | 2 | 1 | 0 | 0 | 0 | |
| CNS cerebrovascular ischemia | DP + B | 3 | 1 | 4 | 1 | 0 | 0 |
| DP | 0 | 0 | 1 | 0 | 0 | 0 | |
| Dyspnea (shortness of breath) | DP + B | 12 | 2 | 2 | 0 | 0 | 0 |
| DP | 8 | 2 | 2 | 0 | 0 | 0 | |
| Pneumonitis/pulmonary infiltrates | DP + B | 6 | 1 | 3 | 1 | 2 | 0 |
| DP | 1 | 0 | 1 | 0 | 0 | 0 | |
| Proteinuria | DP + B | 8 | 2 | 2 | 0 | 0 | 0 |
| DP | 2 | 0 | 1 | 0 | 0 | 0 | |
| Infection | |||||||
| Colitis, infectious (eg, Clostridium difficile) | DP + B | 0 | 0 | 0 | 0 | 0 | 0 |
| DP | 1 | 0 | 1 | 0 | 0 | 0 | |
| Febrile neutropenia | DP + B | 31 | 6 | 5 | 1 | 1 | 0 |
| DP | 20 | 4 | 2 | 0 | 0 | 0 | |
| Infection (documented clinically) | DP + B | 32 | 6 | 4 | 1 | 2 | 0 |
| DP | 15 | 3 | 6 | 1 | 1 | 0 | |
| Infection, other | DP + B | 13 | 3 | 2 | 0 | 1 | 0 |
| DP | 5 | 1 | 0 | 0 | 0 | 0 | |
| Infection with normal ANC or grade 1 or 2 neutropenia | DP + B | 14 | 3 | 1 | 0 | 2 | 0 |
| DP | 7 | 1 | 1 | 0 | 0 | 0 | |
| Infection with unknown ANC | DP + B | 8 | 2 | 1 | 0 | 0 | 0 |
| DP | 4 | 1 | 0 | 0 | 0 | 0 | |
| Maximum overall adverse events | DP + B | 208 | 41 | 152 | 30 | 20 | 4 |
| DP | 158 | 31 | 120 | 24 | 6 | 1 | |
Abbreviations: DP, docetaxel 75 mg/m2 intravenously over 1 hour for 21 days plus prednisone 5 mg orally twice per day; DP + B, DP plus bevacizumab 15 mg/kg intravenously every 3 weeks; ANC, absolute neutrophil count.
DP +B, n = 504; DP, n = 505.
DISCUSSION
The addition of bevacizumab to docetaxel and prednisone did not significantly prolong median survival in men with mCRPC, the primary end point in this study. However, the addition of bevacizumab to docetaxel and prednisone did have a statistically significant impact on PFS (9.9 v 7.5 months; P < .001), ORR (49.4% v 35.5%; P = .0013), and PSA decline ≥ 50% (69.5% v 57.9%; P < .001), suggesting that VEGF signaling may play a role in mCRPC. The observed dissociation between OS and other outcomes, such as PFS, post-therapy PSA decline, and ORR, is complex and may be related to several factors, including stage migration, trial design issues, impact of postprotocol treatment, or a modest correlation between clinical end points and OS. The observed median OS time in the docetaxel arm in this study was longer at 21.5 months than what was assumed in the design of the trial (median OS, 19.2 months based on the pivotal TAX 327 study12). This may represent stage migration in patients with mCRPC treated with docetaxel chemotherapy and is consistent with the relatively high proportion of good-risk patients enrolled onto the study. This undoubtedly will prove to be even more of a confounding factor in future studies with the earlier initiation of therapy and as more active agents are approved for the treatment of mCRPC.
In considering trial design issues, this study did not allow for the continuation of bevacizumab therapy beyond progression. Although not proven, it has been hypothesized that prolonged VEGF inhibition is needed to show a clinical benefit. Burger et al13 from the Gynecologic Oncology Group showed the therapeutic impact of concurrent and maintenance bevacizumab administration with standard chemotherapy in patients with advanced ovarian cancer. CALGB 90401 was not designed to evaluate the role of maintenance bevacizumab therapy. Although patients were allowed to continue on single modality bevacizumab or placebo treatment if the docetaxel therapy was not tolerated, few patients and physicians elected to continue monotherapy with bevacizumab or placebo.
Two thirds of all patients received additional therapy after protocol treatment was completed or the patient was removed from the study. The majority received additional docetaxel-based chemotherapy as a second-line chemotherapy, although the clinical benefit of this approach has not been well defined. Since the completion of CALGB 90401, several agents have been shown to prolong survival in patients with mCRPC, including sipuleucel-T, cabazitaxel, and abiraterone acetate, that could also account for longer OS, although these agents were largely unavailable to the patients on this trial. Whether the observed OS on this study was affected by subsequent docetaxel or other treatments cannot be definitively determined.
In addition, the population of elderly patients with prostate cancer may respond to bevacizumab differently from younger populations such as the populations with colon or lung cancer who experienced the clinical benefit of bevacizumab added to standard chemotherapy.14–17 The population studied in CALGB 90401 was approximately a decade older than those populations and had castrate testosterone levels with bone-predominant disease. In the breast, colon, and lung cancer studies, patients were younger and most had metastatic disease confined to soft tissues (nonosseous).14–17 In the Bevacizumab Regimens' Investigation of Treatment Effects (BRiTE) observational cohort of metastatic colon cancer, elderly patients who received bevacizumab with first-line chemotherapy demonstrated treatment benefit. However, there was a diminished median survival with increasing age.18 Similarly, in an ECOG trial for metastatic breast cancer, 14,15 the effect of bevacizumab significantly declined when age was evaluated as a continuous variable, with the 65- to 85-year-old group having the least benefit. Although the safety profiles were similar in all age cohorts in these studies, these trends suggest that elderly patients may have different biologic effects from the administration of bevacizumab or similar VEGF-targeted therapies. It has recently been demonstrated that docetaxel clearance is increased by approximately 100% in castrate men.19 Little is known about the difference in drug distribution in bone versus soft tissues and, although it is unlikely, castration may have a subtle impact on bevacizumab pharmacokinetics.
Any possible clinical benefit from the addition of bevacizumab to docetaxel and prednisone needs to be tempered by the increased morbidity and toxicities observed with bevacizumab treatment in this trial. Patients treated with bevacizumab experienced more severe neutropenia, fatigue, leukopenia, hypertension, GI hemorrhage and perforation, mucositis, and pneumonitis. The incidence and severity of the adverse events were similar to those reported in other diseases.14–17 A particular concern was the increased number of treatment-related deaths (4.0% v 1.2%), which were mostly related to infectious complications which may reflect an increased likelihood of neutropenia in an elderly population.
The initial hypothesis of this trial was that inhibiting VEGF by adding bevacizumab to docetaxel and prednisone would provide a survival advantage to patients with mCRPC. The results of this study failed to support this hypothesis. However, there was a prolongation of PFS in patients treated with bevacizumab, and similar PFS improvements supported the approval of this drug in breast and renal cell carcinoma.14,20,21 Exploratory analyses were undertaken to try to identify the characteristics of those patients with CRPC who were more likely to benefit from this therapy and have an improvement in both PFS and OS. These analyses have generated the hypothesis that a targeted subpopulation of poor-risk patients with mCRPC (defined by either high tumor burden or disease progression in the setting of low testosterone levels) would experience greater clinical benefit from VEGF inhibition. This study further illustrates that the success of future phase III programs in mCRPC is dependent on identifying critical biomarkers that enrich the study population for the targeted therapy and to better understand the associations between PFS, PSA response, and objective tumor response as an intermediate marker for OS in this patient population. Other ongoing phase III trials with aflibercept and tasquinimod may help further define the clinical benefit of vascular targeting agents in patients with mCRPC.
Supplementary Material
Acknowledgment
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Appendix
The following institutions (with supporting Grant No. in parentheses) participated in this study: Board of Regents of the University of Oklahoma, Oklahoma City, OK, Shubham Pant, MD (CA37447); Christiana Care Health Services Community Clinical Oncology Program (CCOP), Wilmington, DE, Stephen Grubbs, MD (CA45418); Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD (CA32291); Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH, Konstantin Dragnev, MD (CA04326); Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD (CA47577); Georgetown University Medical Center, Washington, DC, Minetta C. Liu, MD (CA77597); Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, MD (CA29165); Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY, Jeffrey Kirshner, MD (CA45389); Illinois Oncology Research Association, Peoria, IL, John W. Kugler, MD (CA35113); Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO, Rakesh Gaur, MD; Long Island Jewish Medical Center, Lake Success, NY, Kanti R. Rai, MD (CA35279); Massachusetts General Hospital, Boston, MA, Jeffrey W. Clark, MD (CA32291); Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford A. Hudis, MD (CA77651); Missouri Baptist Medical Center, St Louis, MO, Alan P. Lyss, MD (CA114558-02); Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, MD (CA45564); Mount Sinai School of Medicine, New York, NY, Lewis R. Silverman, MD (CA04457); Nevada Cancer Research Foundation CCOP, Las Vegas, NV, John A. Ellerton, MD (CA35421); New Hampshire Oncology-Hematology PA, Concord, NH, Douglas J. Weckstein, MD; North Shore-Long Island Jewish Health System, New Hyde Park, NY, Daniel Budman, MD (CA35279); North Shore University Health System CCOP, Evanston, IL, David L. Grinblatt, MD; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN, Rafat Ansari, MD (CA86726); Rhode Island Hospital, Providence, RI, William Sikov, MD (CA08025); Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD (CA59518); Sibley Memorial Hospital, Washington, DC, Frederick Barr, MD; Southeast Cancer Control Consortium CCOP, Goldsboro, NC, James N. Atkins, MD (CA45808); State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD (CA21060); The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD (CA77658); University of California at San Diego, San Diego, CA, Barbara A. Parker, MD (CA11789); University of California at San Francisco, San Francisco, CA, Charles J. Ryan, MD (CA60138); University of Chicago, Chicago, IL, Hedy L. Kindler, MD (CA41287); University of Illinois MBCCOP, Chicago, IL, David J. Peace, MD (CA74811); University of Iowa, Iowa City, IA, Daniel A. Vaena, MD (CA47642); University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD (CA31983); University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD (CA16450); University of Nebraska Medical Center, Omaha, NE, Anne Kessinger, MD (CA77298); University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD (CA47559), University of Vermont, Burlington, VT, Steven M. Grunberg, MD (CA77406); Washington University School of Medicine, St Louis, MO, Nancy Bartlett, MD (CA77440); Weill Medical College of Cornell University, New York, NY, John Leonard, MD (CA07968); Western Pennsylvania Cancer Institute, Pittsburgh, PA, John Lister, MD; Yale University, New Haven, CT, Lyndsay N. Harris, MD (CA16359).
Footnotes
Written on behalf of the Cancer and Leukemia Group B and Eastern Cooperative Oncology Group.
Supported in part by Grants No. CA31946 from the National Cancer Institute to the Cancer and Leukemia Group B (CALGB) and No. CA33601 to the CALGB Statistical Center and by Roche-Genentech.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00110214.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Nicholas J. Vogelzang, US Oncology (C) Consultant or Advisory Role: William Kevin Kelly, sanofi-aventis (C), Genentech (C); Michael Carducci, sanofi-aventis (C), Genentech (C); Daniel George, sanofi-aventis (C), Genentech (C); Walter M. Stadler, Genentech (C); Michael Morris, sanofi-aventis (U), Millennium Pharmaceuticals (C) Stock Ownership: None Honoraria: Susan Halabi, sanofi-aventis; J. Paul Monk, sanofi-aventis; Nicholas J. Vogelzang, Genentech, sanofi-aventis, Celgene, OncoGenex Pharmaceuticals, Teva Pharmaceuticals, Johnson & Johnson, Dendreon Research Funding: William Kevin Kelly, sanofi-aventis, Genentech; Susan Halabi, sanofi-aventis; Walter M. Stadler, Genentech; Michael Morris, sanofi-aventis, Algeta, Genta, Johnson & Johnson, Agensys, GlaxoSmithKline, Hollis-Eden Pharmaceuticals, Bristol-Myers Squibb; Nicholas J. Vogelzang, Tokai Pharmaceuticals, Progenix, Algeta, Bayer Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: William Kevin Kelly, Susan Halabi, Michael Carducci, Daniel George, Walter M. Stadler, Philip Kantoff, Nicholas J. Vogelzang, Eric J. Small
Provision of study materials or patients: William Kevin Kelly, Michael Carducci, Daniel George, John F. Mahoney, Walter M. Stadler, Michael Morris, Philip Kantoff, J. Paul Monk, Nicholas J. Vogelzang, Eric J. Small
Collection and assembly of data: William Kevin Kelly, Susan Halabi, Ellen Kaplan, Eric J. Small
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ferrer FA, Miller LJ, Lindquist R, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 2.Weidner N, Carroll PR, Flax J, et al. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrer FA, Miller LJ, Andrawis RI. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: In situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997;157:2329–2333. [PubMed] [Google Scholar]
- 4.Duque JL, Loughlin KR, Adam RM, et al. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999;54:523–527. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 5.Duque JL, Loughlin KR, Adam RM, et al. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: Relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61:401–408. doi: 10.1590/s1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 6.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 7.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from Cancer and Leukemia Group B 9480. Clin Cancer Res. 2005;11:1815–1820. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 8.Picus J, Halabi S, Kelly WW, et al. A phase II study of estramustine, docetaxel, and bevacizumab in men with castration resistant prostate cancer: Results of Cancer and Leukemia Group B (CALGB) 90006. Cancer. 2011;117:526–533. doi: 10.1002/cncr.25421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 10.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 11.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 12.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 13.Burger RA, Brady MF, Bookman MA, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): A Gynecologic Oncology Group study. J Clin Oncol. 2010;28(suppl):6s. doi: 10.1016/j.ygyno.2013.07.100. abstr LBA1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.Gray R, Bhattacharya S, Bowden C, et al. Independent review of E2100: A phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1416–1423. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 18.Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: Results from the BRiTE observational cohort study. Oncology. 2010;78:329–339. doi: 10.1159/000320222. [DOI] [PubMed] [Google Scholar]
- 19.Franke RM, Carducci MA, Rudek MA, et al. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J Clin Oncol. 2010;28:4562–4567. doi: 10.1200/JCO.2010.30.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 21.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.