Figure 4.
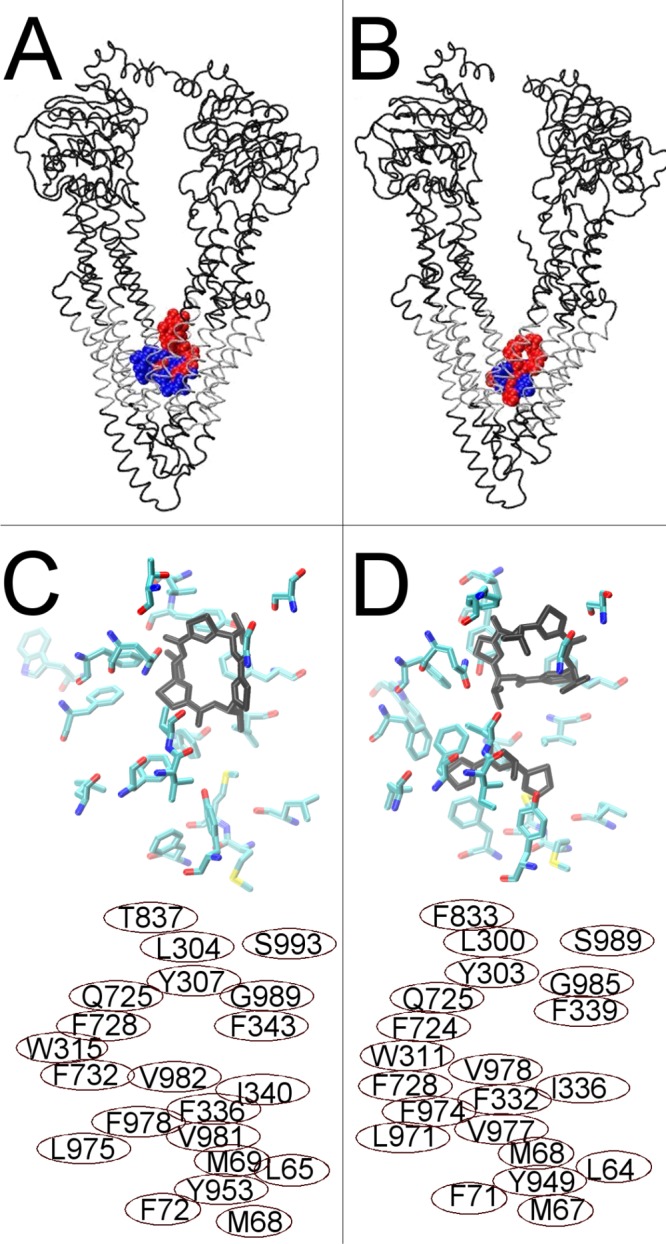
Docking of cyclic inhibitor analogues, QZ59-RRR (blue) and QZ59-SSS (red), to fully opened inward Pgp conformations. (A) Human Pgp showing the preferred docking clusters, the most reproducible drug docking clusters for two simulation frames at the fully opened inward conformation, superposed. (B) Mouse Pgp structures from ref (17) with the QZ59-RRR (blue) and QZ59-SSS (red) cyclic inhibitors bound. Transmembrane regions of both proteins are colored silver. (C) The top portion shows the results for docking of the sulfur analogue of the QZ59-SSS stereoisomer to the fully opened inward conformation of human Pgp. Residues within 3.5 Å of the three most reproducible docking clusters are shown as licorice colored by atom type. The most reproducibly docked QZ59-SSS analogue is shown bound as black licorice. (D) The top portion shows the mouse Pgp structure from ref (17) with QZ59-SSS bound (black). Amino acids identified by Aller et al.17 to be involved in drug binding are shown as licorice colored by atom type. The bottom portions of panels C and D identify the residue numbers of the amino acids shown in the top panel (C, human numbering, and D, mouse numbering). Note that each residue shown in either panel has its homologue shown in the same relative position in the other panel.
