Abstract
Wilson's disease is an autosomal recessive disorder affecting copper transport; it results in the accumulation of copper in the liver, brain, and other organs. Wilson's disease is the most common inherited liver disease with more than 500 cases reported in Korea. An impairment in biliary excretion process leads to copper accumulation in the liver, which progressively damages the liver, leading to cirrhosis. Since effective treatment is available for this disease, early and correct diagnosis is very important. Here, we report a case of Wilson's disease with mildly elevated liver enzyme levels in a 29-year-old breast-feeding woman with weight loss.
Keywords: Wilson's Disease, Hepatitis, Copper, Diagnosis
INTRODUCTION
Chronic hepatitis is defined as an inflammatory disease of the liver lasting for more than 6 months. Mild forms of the liver disease are non-progressive or show slow progression, whereas other more severe forms are associated with architectural disorganization, which can lead to cirrhosis.1) Chronic hepatitis often leads to nonspecific symptoms such as malaise, fatigue, and weakness, or no symptoms at all. It is commonly identified by blood tests performed either for screening or for evaluating nonspecific symptoms. Hepatitis B and C are the most common causes of chronic hepatitis.2,3) Drug-induced, alcoholic, and autoimmune hepatitis are other common causes of chronic hepatitis. In many cases, physicians can correctly diagnose the condition underlying by using clinical and laboratory findings. In some cases, physicians may misdiagnose, because they do not consider the hidden causes of chronic hepatitis.
Wilson's disease (WD), a genetic disorder involving biliary copper excretion, can cause elevated liver enzyme levels, lasting for more than 6 months in asymptomatic patients.4,5) While the prevalence of WD is very low, it is a treatable liver disease and therefore needs to be properly identified.6) Here, we report a case of WD with mildly elevated liver enzymes in a 29-year-old breast-feeding woman with weight loss.
CASE REPORT
A 29-year-old breast-feeding mother was admitted to our hospital with a weight loss of approximately 20 kg during the preceding 8 months. She weighed 53 kg before pregnancy and gained 10 kg during pregnancy; this totaled to 63 kg. She lost 20 kg within 8 months of delivery but complained of fatigue. Her appetite was good but she told us that she did not have enough time to eat. She was a non-smoker and non-drinker. She had no history of medical treatment, except for hepatotonic administration for 3 months because she had elevated liver enzyme level. Family history did not yield any remarkable findings. On physical examination, the patient's blood pressure was 96/60 mmHg; pulse, 76 beats/minute; temperature, 37℃; and respiratory rate, 16 breaths/minute. Her weight was 42.2 kg, and her body mass index was 15.8 kg/m2. Her tongue and skin showed no signs of dehydration. Auscultation of the heart and lung were normal, and the abdomen was soft. Neurological examination yielded normal findings.
A normal hemogram was obtained during further examination. The levels of serum electrolytes, blood sugar, and creatinine were also normal. Liver enzyme assay revealed the levels of AST, ALT, ALP, and total bilirubin to be 52 U/L, 55 U/L, 323 IU/L, and 0.6 mg/dL. We, therefore, checked for anti-HAV IgM, HBsAg, anti-HBc IgM, and anti-HCV antibodies, but the results were negative. She had no history of any medicines or herbs. Thyroid function test, chest X-ray, and gastrofiberscopy showed no abnormalities. Abdominal ultrasonography revealed no remarkable findings except for a small hepatic cyst.
We advised her to increase her food intake. Next, we planned to recheck liver function tests on the basis of the elevated levels of liver enzymes. After 1 week, she had gained about 1 kg and showed improvement in subjective health status. The levels of liver enzymes had decreased, i.e., AST level decreased to 46 U/L and ALT, to 52 U/L. Therefore, we planned to monitor her progress.
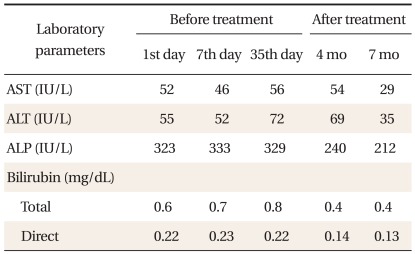
After 1 month, her weight remained unchanged at 42.7 kg. Her AST level increased to 56 U/L, and ALT level, 72 U/L (Table 1). Further examination for chronic hepatitis was conducted. Her serum ceruloplasmin level had reduced to 4 mg/dL (normal, 17.9 to 53.3 mg/dL), and copper level had reduced to 59.2 µg/dL (normal, 75 to 145 µg/dL), whereas after 24 hours, the rate of urinary copper excretion increased to 291.5 µg/day (normal, 15 to 60 µg/day). Ophthalmological examination did not show the presence of Kayser-Fleischer ring.
Table 1.
Disease course in this case.
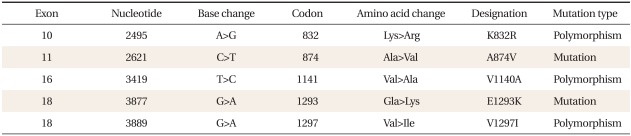
We performed ultrasound-assisted percutaneous liver biopsy. The liver copper content was slightly elevated at 45.68 µg/g dry weight (normal, 10 to 35 µg/g). We proceeded to perform mutation analysis of the ATP7B gene. Upon sequencing, we detected compound heterozygosity for c.2621C>T (p.A874V) and c.3877G>A (E1293K) (Table 2). The c.2621C>T (p.A874V) mutation is the most commonly detected mutation in WD. Trientine therapy was initiated (250 mg, four times/day). At a follow-up 7 months later, her liver enzyme level was normalized (AST 29 U/L, ALT 35 U/L, ALP 212 U/L). At present she is on a maintenance dose of 750 mg (250 mg, three times/day).
Table 2.
ATP7B gene mutation in this case.
DISCUSSION
WD is an autosomal recessive disorder caused by mutation in the ATPB7 gene, which encodes a membrane-bound copper-transporting ATPase. An impairment in biliary excretion leads to the accumulation of copper, initially in the liver, and then in the other tissues.7,8) The domestic frequency of WD is approximately 1 case in 94,000 individuals in most populations, and approximately 1 case in 156,000 individuals in populations older than 15 years of age.6) Most patients present in their mid to late teenage years, although the age of presentation is quite broad and extends into the fifth decade of life.
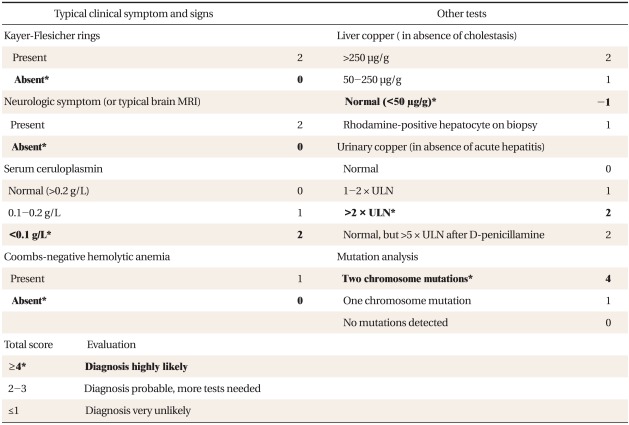
There is no single specific test for the diagnosis of WD. In a nation-wide survey of WD, low serum ceruloplasmin (<20 mg/dL), high 24-h urine copper level (>100 g), high hepatic copper content (>250 µg/g of dry liver), and Kayser-Fleischer rings were found in 96%, 86%, 88%, and 73% cases, respectively.6) A combination of any 2 of the above 4 laboratory findings forms a strong support for the diagnosis of WD.9-13) Genetic testing to confirm the diagnosis is a reasonable option, although a specific mutation cannot be identified in all patients. Recently, a scoring system based on the clinical and biochemical parameters for the diagnosis of WD was proposed at the eighth International Conference on Wilson Disease and Menkes Disease (Table 3). In this case, she had a diagnostic score of 7 (Table 3, shown in bold).
Table 3.
Scoring system developed at the 8th International Meeting on Wilson Disease, Leipzig, 2001.
ULN: upper limit of normal range.
*Diagnostic scoring of Wilson's disease in this case.
Episodes of hepatitis can occur with elevated liver enzymes, and then, show spontaneous regression. WD leads to liver cirrhosis without adequate therapy. Hepatitis often reoccurs, and most of these patients eventually develop cirrhosis.14) In a study of 439 patients in Korea, 75% of the adult WD patients with hepatitis were diagnosed with liver cirrhosis because of delayed diagnosis. The neurological manifestations of WD typically occur in patients in their early twenties. Neurological symptoms such as dystonia, incoordination, and tremor have been observed along with psychiatric symptoms such as depression, hyperactivity, or loss of sexual inhibition.15)
In symptomatic WD patients, chelation therapy is an effective treatment strategy, wherein D-penicillamine and trientine were the most commonly used drugs. Patients are informed to avoid copper-rich nutrients such as shellfish, organ meats, nuts, and chocolate.16-18)
In the present case, it was difficult to make an early diagnosis, because even with a history of increased liver enzyme levels, more precise examinations were not prescribed, and the patient was prescribed hepatotonics in the local clinic. This patient did not have any other complications except hepatitis. Although her main complaint was weight loss during breast feeding, her enzyme levels were elevated, and we diagnosed WD by using the results of additional tests. We suggest that she lost her weight due to breast feeding and difficulty in childcare.
In many cases, if the findings of viral hepatitis tests are normal, we prescribe conservative treatment for patients with increased liver enzyme levels, because of the high frequency of viral- and alcohol-induced hepatitis in the Korean population. However, if the blood level of liver enzymes is consistently higher than the upper normal limit, further tests are necessary. WD can occur at various ages; therefore, early diagnosis and treatment are very important. Diagnosis may include procedures. WD patients lead healthy lives if they undergo proper treatment in the early stages of the disease. Therefore, it is imperative that the first physician does not fail to diagnose WD.
Footnotes
This case was supported by a grant from Pusan National University Yangsan Hospital 2011.
References
- 1.Hoofnagle JH, Di Bisceglie AM. Serologic diagnosis of acute and chronic viral hepatitis. Semin Liver Dis. 1991;11:73–83. doi: 10.1055/s-2008-1040426. [DOI] [PubMed] [Google Scholar]
- 2.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582–592. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith AD, Rockey DC. Viral hepatitis C. Lancet. 2004;363:661. doi: 10.1016/S0140-6736(04)15609-2. [DOI] [PubMed] [Google Scholar]
- 4.Seo JK. Wilson disease: an update. Korean J Hepatol. 2006;12:333–363. [PubMed] [Google Scholar]
- 5.Gitlin N. Wilson's disease: the scourge of copper. J Hepatol. 1998;28:734–739. doi: 10.1016/s0168-8278(98)80302-4. [DOI] [PubMed] [Google Scholar]
- 6.Seo JK, Kim YS, Hahn CJ, Baik SK. A nationwide survey for prevalence and clinical characteristics of Wilson disease in Korea. Korean J Hepatol. 2004;10(Suppl):S5–S15. [Google Scholar]
- 7.Davies K. Cloning the menkes disease gene. Nature. 1993;361:98. doi: 10.1038/361098a0. [DOI] [PubMed] [Google Scholar]
- 8.Fatemi N, Sarkar B. Structural and functional insights of wilson disease copper-transporting ATPase. J Bioenerg Biomembr. 2002;34:339–349. doi: 10.1023/a:1021245902195. [DOI] [PubMed] [Google Scholar]
- 9.Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of wilson disease. Liver Int. 2003;23:139–142. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaffney D, Fell GS, O'Reilly DS. ACP best practice no 163. Wilson's disease: acute and presymptomatic laboratory diagnosis and monitoring. J Clin Pathol. 2000;53:807–812. doi: 10.1136/jcp.53.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuttall KL, Palaty J, Lockitch G. Reference limits for copper and iron in liver biopsies. Ann Clin Lab Sci. 2003;33:443–450. [PubMed] [Google Scholar]
- 12.Harada M. Wilson disease. Med Electron Microsc. 2002;35:61–66. doi: 10.1007/s007950200007. [DOI] [PubMed] [Google Scholar]
- 13.Pfeil SA, Lynn DJ. Wilson's disease: copper unfettered. J Clin Gastroenterol. 1999;29:22–31. doi: 10.1097/00004836-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Walshe JM. Wilson's disease presenting with features of hepatic dysfunction: a clinical analysis of eighty-seven patients. Q J Med. 1989;70:253–263. [PubMed] [Google Scholar]
- 15.Dening TR, Berrios GE. Wilsons disease: psychiatric symptoms in 195 cases. Arch Gen Psychiatry. 1989;46:1126–1134. doi: 10.1001/archpsyc.1989.01810120068011. [DOI] [PubMed] [Google Scholar]
- 16.Roberts EA, Schilsky ML Division of Gastroenterology and Nutrition, Hospital for Sick Children, Toronto, Ontario, Canada. A practice guideline on Wilson disease. Hepatology. 2003;37:1475–1492. doi: 10.1053/jhep.2003.50252. [DOI] [PubMed] [Google Scholar]
- 17.Martins da Costa C, Baldwin D, Portmann B, Lolin Y, Mowat AP, Mieli-Vergani G. Value of urinary copper excretion after penicillamine challenge in the diagnosis of wilson's disease. Hepatology. 1992;15:609–615. doi: 10.1002/hep.1840150410. [DOI] [PubMed] [Google Scholar]
- 18.Walshe JM. Penicillamine: the treatment of first choice for patients with Wilson's disease. Mov Disord. 1999;14:545–550. doi: 10.1002/1531-8257(199907)14:4<545::aid-mds1001>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]