Abstract
Background
Though adiponectin has been associated with insulin resistance and cardiovascular risk factors, the relationship between adiponectin and polycystic ovary syndrome (PCOS) remains controversial. The aim of this study was to compare adiponectin level in women with PCOS and without PCOS, and to investigate the relationship between adiponectin level and metabolic variables including insulin resistance.
Methods
60 women with PCOS were enrolled along with a control group of 80 healthy women, matched for age and body mass index (BMI). We measured hormonal and metabolic parameters, as well as the plasma adiponectin concentration of each participant. We estimated the insulin sensitivity according to the quantitative insulin sensitivity check index (QUICKI).
Results
The PCOS group displayed significantly lower level of adiponectin (P < 0.001) after adjustment for age, BMI, mean blood pressure, fasting glucose, fasting insulin, and several metabolic parameters. Adiponectin levels were positively correlated with QUICKI in the PCOS group (P < 0.001) and the control group (P = 0.03). Following step-wise multiple regression analysis, however, adiponectin level was positively correlated with QUICKI in the control group only (P = 0.03). In addition, adiponectin level was found to be independently associated with HDL-cholesterol level (P < 0.001) and BMI (P = 0.02) in the PCOS group and independently associated with HDL-cholesterol (P = 0.02) in the control group.
Conclusion
We report decreased adiponectin level in PCOS patients in relation to controls independently of insulin resistance or other metabolic factors. And adiponectin is associated with both lipid metabolism and obesity, which, in turn, is related to insulin resistance in PCOS. Further studies are needed to clarify the mechanism of adiponectin in PCOS.
Keywords: Adiponectin, Polycystic Ovary Syndrome, Insulin Resistance
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrine disorder1) among women of child-bearing age. It is characterized by chronic anovulation (oligomenorrhea or amenorrhea) hyperandrogenemia, hirsutism and acne. Recently, many studies have focused on the metabolic disorders in PCOS2,3) that lead to cardiovascular events, dyslipidemia, insulin resistance,4,5) and metabolic dysfunction is widely regarded as the leading cause of the syndrome.
Adiponectin is a cytokine produced and secreted exclusively from adipose tissue. It is believed to serve an anti-inflammatory role, as well as have anti-atherogenic and cardioprotective properties6) and an insulin-sensitizing effect.7,8) In addition, adiponectin levels are known to have an inverse relationship with insulin resistance in obesity, type 2 diabetes, dyslipidemia, hypertension and cardiovascular disease. In PCOS, however, the association between adiponectin levels and PCOS remains controversial9-18) and it is unclear so far whether adiponectin is a primary defect or occurred secondary to or in parallel with this insulin resistance.11-14,17)
The aim of this study was to compare adiponectin levels in women with PCOS to those of healthy women, and to investigate the independent relationship between plasma adiponectin concentrations and metabolic variables including insulin resistance.
METHODS
1. Subjects
We included 60 women patients with PCOS and a control group of 80 healthy women, matched for age and BMI. PCOS subjects were enrolled when they had satisfied two of the three following criteria: oligomenorrhea or amenorrhea, clinical or biochemical hyperandrogenism, and ultrasonographic polycystic ovarian morphology.19) We excluded the subjects who had weight-related amenorrhea, Cushing's syndrome, late-onset congenital adrenal hyperplasia, thyroid dysfunction, hyperprolactinaemia and androgen secreting tumours.20) Additional exclusionary criteria were diabetes, hypertension, chronic renal disease, overt proteinuria, smoking, alcohol use, and medication usage, including oral contraceptives. All of the control subjects had normal, regular menstruation, normal ovarian findings on ultrasound, and normal luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels. None displayed hirsutism. All of the test subjects provided written informed consent bearing their signatures, and the study procedure was approved by the Institutional Review Board of Severance Hospital.
2. Methods
Anthropometric measurements were performed with subjects wearing light clothing without shoes. BMI was calculated by dividing weight by square of height (kg/m2). Subjects with mild oligomenorrhea and healthy control subjects visited within the first 10 days of menstruation. In PCOS patients with severe oligomenorrhea or amenorrhea, we studied in randomized period. We had collected venous blood samples in the morning from 8 AM to 10 AM after overnight fasting in each subject. We measured the plasma concentration of LH, FSH, estradiol (E2), total testosterone, fasting insulin levels by electrochemiluminescence immunoassay (Roche, Indianapolis, IN, USA) and sex-hormone binding globulin (SHBG) level by an Immulite 2000 analyser (Diagnostic Product Co., Los Angeles, CA, USA). Fasting glucose, total cholesterol, triglyceride (TG), high-density lipoprotein (HDL)-cholesterol levels were measured using an ADVIA 1650 chemistry system (Bayer, Terrytown, NY, USA), and low-density lipoprotein (LDL)-cholesterol levels were calculated using the Friedewald equation, for serum TG levels below 400 mg/dL. Definition of clinical hyperandrogenism was presence of acne, hirsutism (Ferriman-Gallwey score ≥ 8) or alopecia. The quantitative insulin sensitivity check index (QUICKI)21) as meaning of insulin sensitivity was calculated as follow: 1 / [log (fasting insulin) + log (fasting glucose)]. Plasma adiponectin levels were measured by an enzyme immunoassay kit (AdipoGen, Seoul, Korea) and the inter- and intra-assay variability were 4.63% ± 0.82% and 2.72% ± 0.52%, respectively.
3. Statistical Analysis
We analyzed all of the data using the SAS 9.1 (SAS Inst., Cary, NC, USA) statistical program and expressed the data as mean ± SD or median with interquartile range (IQR, 25th-75th percentile). Comparisons of the PCOS to the control group employed either the Student's t-test or the Wilcoxon rank sum test. Adiponectin levels of the PCOS and the control groups were compared using an analysis of covariance (ANCOVA) adjusted for age, BMI, mean blood pressure, fasting glucose, fasting insulin, TG, HDL-cholesterol, LDL-cholesterol, E2, LH/FSH, total testosterone and SHGB levels. Pearson's correlation coefficients were used for assessing the correlation between adiponectin levels and metabolic variables. Multivariate regression analysis was used for clarification of independent associations between adiponectin levels and other metabolic parameters.
RESULTS
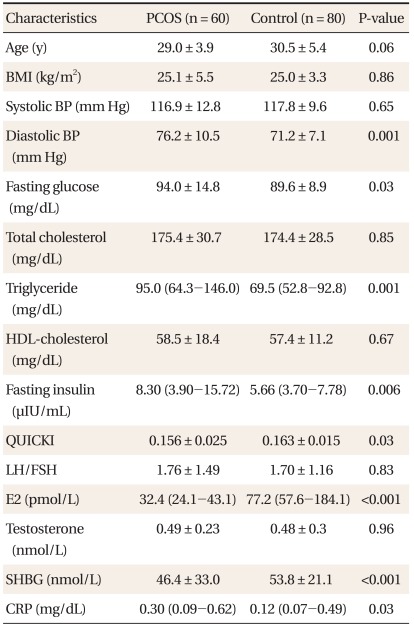
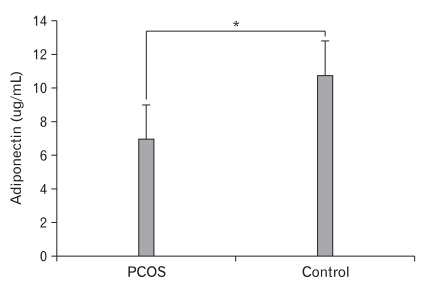
The characteristics of the PCOS and the control groups are summarized in Table 1. Diastolic blood pressure, fasting glucose, TG, fasting insulin and CRP levels were all significantly higher in the PCOS group, while QUICKI, E2 and SHBG levels were significantly lower in the PCOS group. After adjustment for age, BMI, mean blood pressure, fasting glucose, fasting insulin, TG, HDL-cholesterol, LDL-cholesterol, E2, LH/FSH, total testosterone and SHBG levels using ANCOVA, the PCOS group had significantly lower level of adiponectin (PCOS, 6.99 ± 0.50 ug/mL; Control, 10.79 ± 0.71 ug/mL; P < 0.001) (Figure 1).
Table 1.
Baseline characteristics of women with PCOS and the control groups.
Values are presented as mean ± SD or median with IQR (25th-75th percentile). P-values were calculated by the Student's t-test or Wilcoxon's rank sum test.
PCOS: polycystic ovary syndrome, BMI: body mass index, BP: blood pressure, HDL: high-density lipoprotein, QUICKI: quantative insulin sensitivity check index, LH: luteinizing hormone, FSH: follicle-stimulating hormone, E2: estradiol, SHBG: sex-hormone binding globulin, CRP: C-reactive protein.
Figure 1.
Adiponectin levels in women with the polycystic ovary syndrome (PCOS) and control groups (6.99 ± 0.50 ug/mL, 10.79 ± 0.71 ug/mL, P < 0.001, respectively). *P-value (<0.001) was calculated using ANCOVA adjusted for age, body mass index, mean blood pressure, fasting glucose, fasting insulin, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, estradiol, luteinizing hormone/follicle-stimulating hormone, total testosterone and sex-hormone binding globulin levels.
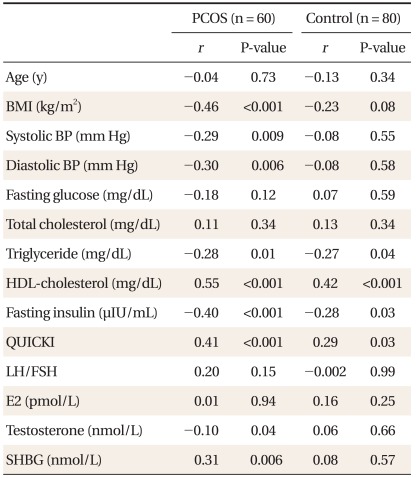
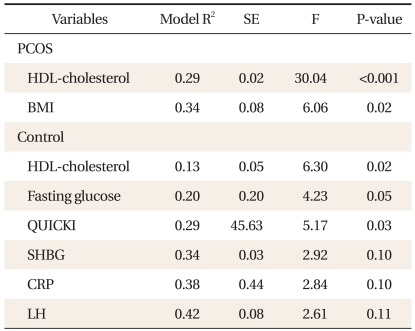
Adiponetin level was significantly negatively correlated with BMI, systolic blood pressure, diastolic blood pressure, TG, fasting insulin, testosterone and positively correlated with HDL-cholesterol in the PCOS group (Table 2). QUICKI had positive association with adiponectin level in the PCOS group and the control group (P < 0.001; P = 0.03, respectively). However, after step-wise multiple regression analysis, adiponectin level was positively correlated with QUICKI in the control group only (P = 0.03). In addition, adiponectin level was found to be independently associated with HDL-cholesterol level (P < 0.001) and BMI (P = 0.02) in the PCOS group and independently associated with HDL-cholesterol level (P = 0.02) in the control group, as shown in Table 3.
Table 2.
Correlation of adiponectin level and metabolic factors in women with the PCOS and the control groups.
Coefficients (r) and P-values were calculated using the Pearson's correlation model.
PCOS: polycystic ovary syndrome, BMI: body mass index, BP: blood pressure, HDL: high-density lipoprotein, QUICKI: quantative insulin sensitivity check index, LH: luteinizing hormone, FSH: follicle-stimulating hormone, E2: estradiol, SHBG: sex-hormone binding globulin.
Table 3.
Step-wise multiple linear regression analysis to identify clinical variables associated with adiponectin level as a dependant variable.
P-values of all models were calculated by the multiple regression analysis adjusted for age and BMI.
PCOS: polycystic ovary syndrome, HDL: high-density lipoprotein, BMI: body mass index, QUICKI: quantative insulin sensitivity check index, SHBG: sex-hormone binding globulin, CRP: C-reactive protein, LH: luteinizing hormone.
DISCUSSION
PCOS is a complex hormonal and metabolic syndrome, com prising hyperandrogenemia, reproductive abnormalities, glucose intolerance, atherogenic lipid profile, obesity and insulin resistance.22-24) The pathogenesis is not clear, but it is conceivably due to immature ovarian cysts and dysregulation of insulin signaling in theca cells resulting from genetic defects, extrinsic exposures, or both.25) Adiponectin, a 247 amino acid polypeptide, secreted predominantly from adipose tissues, is inversely related with obesity, metabolic syndrome and insulin resistance.7,8,20,26,27) The metabolic mechanism of adiponectin is not fully understood, but regulation of glucose and lipid metabolism via stimulation of fatty acid oxidation, suppression of hepatic glucose output and increased insulin sensitivity in liver and skeletal muscles are known to be key roles of adiponectin.28,29)
In previous studies addressing the topic, the relationship between adiponectin and PCOS has been controversial, though a large proportion of such studies9-13) have shown that low adiponectin levels were found in the PCOS group when compared to the control group. And it is unclear so far whether adipo nectin is a primary defect or occurred secondary to or in parallel with this insulin resistance.11-14,17) In our study, the PCOS group had significantly lower adiponectin when compared to the non-PCOS group independently of insulin resistance or other metabolic factors. In addition, we found adiponectin correlated with insulin levels and QUICKI in both PCOS patients and control subjects, therefore, the association between adiponectin and insulin resistance indices does not represent a typical finding of PCOS status. Besides, our multivariate model shows that independent association between adiponectin and QUICKI was found only in the control group not in the PCOS group. This implies an alternative explanation other than insulin resistance should be needed with respect to those relationship. An additional intriguing finding from our study is the fact that serum lipid profiles and BMI were independently correlated with adiponectin level in the PCOS group. Because regulation of fatty acid metabolism and obesity is closely associated with insulin resistance,30) adiponectin might affect insulin resistance and metabolic abnormalities in PCOS through dysregulated lipid metabolism and obesity, although the exact pathway is unclear.
The salient limitation of our study was that we did not consider body fat composition. Body fat distribution indicators such as visceral fat ratio and waist hip ratio are also confounding variables in the relationship of adiponectin to insulin resistance in PCOS. Moreover, ours being a cross-sectional study, we could not distinguish whether adiponectin was a cause or a consequence of PCOS pathogenesis.
In conclusion, lower adiponectin level in the PCOS group was observed and adiponectin was associated with lipid metabolism and obesity both, which, in turn, is related to insulin resistance in PCOS. Further studies are needed to clarify the mechanism of adiponectin in PCOS.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2009-0075404).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Shayya R, Chang RJ. Reproductive endocrinology of adolescent polycystic ovary syndrome. BJOG. 2010;117:150–155. doi: 10.1111/j.1471-0528.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 2.Park HR, Choi Y, Lee HJ, Oh JY, Hong YS, Sung YA. The metabolic syndrome in young Korean women with polycystic ovary syndrome. Diabetes Res Clin Pract. 2007;77(Suppl 1):S243–S246. doi: 10.1016/j.diabres.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 3.Baranova A, Tran TP, Birerdinc A, Younossi ZM. Systematic review: association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;33:801–814. doi: 10.1111/j.1365-2036.2011.04579.x. [DOI] [PubMed] [Google Scholar]
- 4.Sung YA. Insulin resistance in polycystic ovary syndrome. Korean Diabetes J. 2008;32:1–6. [Google Scholar]
- 5.Stanley T, Misra M. Polycystic ovary syndrome in obese adolescents. Curr Opin Endocrinol Diabetes Obes. 2008;15:30–36. doi: 10.1097/MED.0b013e3282f41d55. [DOI] [PubMed] [Google Scholar]
- 6.Krentz AJ, von Muhlen D, Barrett-Connor E. Adipocytokines, sex hormones, and cardiovascular risk factors in postmenopausal women: factor analysis of the Rancho Bernardo study. Horm Metab Res. 2009;41:773–777. doi: 10.1055/s-0029-1224104. [DOI] [PubMed] [Google Scholar]
- 7.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 8.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 9.Sepilian V, Nagamani M. Adiponectin levels in women with polycystic ovary syndrome and severe insulin resistance. J Soc Gynecol Investig. 2005;12:129–134. doi: 10.1016/j.jsgi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Wickham EP, 3rd, Cheang KI, Clore JN, Baillargeon JP, Nestler JE. Total and high-molecular weight adiponectin in women with the polycystic ovary syndrome. Metabolism. 2011;60:366–372. doi: 10.1016/j.metabol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz M, Bukan N, Demirci H, Ozturk C, Kan E, Ayvaz G, et al. Serum resistin and adiponectin levels in women with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25:246–252. doi: 10.1080/09513590802653833. [DOI] [PubMed] [Google Scholar]
- 12.Ardawi MS, Rouzi AA. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1708–1716. doi: 10.1016/j.fertnstert.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 13.Sharifi F, Hajihosseini R, Mazloomi S, Amirmogaddami H, Nazem H. Decreased adiponectin levels in polycystic ovary syndrome, independent of body mass index. Metab Syndr Relat Disord. 2010;8:47–52. doi: 10.1089/met.2009.0036. [DOI] [PubMed] [Google Scholar]
- 14.Spranger J, Mohlig M, Wegewitz U, Ristow M, Pfeiffer AF, Schill T, et al. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;61:738–746. doi: 10.1111/j.1365-2265.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 15.Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1790–1796. doi: 10.1093/humrep/deg353. [DOI] [PubMed] [Google Scholar]
- 16.Olszanecka-Glinianowicz M, Kuglin D, Dabkowska-Huc A, Skalba P. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;154:51–56. doi: 10.1016/j.ejogrb.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Gulcelik NE, Aral Y, Serter R, Demir Y, Culha C. Adiponectin is an independent determinant of insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2006;22:511–515. doi: 10.1080/09513590600917943. [DOI] [PubMed] [Google Scholar]
- 18.Kim YA, Noh JH, Kim DJ, Um TH, Cho CR, Jang NY, et al. Serum adiponectin, TNF-alpha, IL-6 and insulin resistance in women with polycystic ovary syndrome. J Korean Diabetes Assoc. 2006;30:104–111. [Google Scholar]
- 19.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 21.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 22.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 23.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–3242. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 24.Gambineri A, Pelusi C, Manicardi E, Vicennati V, Cacciari M, Morselli-Labate AM, et al. Glucose intolerance in a large cohort of mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes. 2004;53:2353–2358. doi: 10.2337/diabetes.53.9.2353. [DOI] [PubMed] [Google Scholar]
- 25.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 26.Hulthe J, Hulten LM, Fagerberg B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism. 2003;52:1612–1614. doi: 10.1016/s0026-0495(03)00313-5. [DOI] [PubMed] [Google Scholar]
- 27.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Coronary artery disease: association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 28.Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34:12–18. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin: a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 30.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]