Abstract
Background
The aim of this study was to investigate the relationship between heart rate variability (HRV), the Framingham risk score (FRS), and the 10-year risk of coronary heart disease (CHD) development among Korean adults.
Methods
The subjects were 85 healthy Korean adults recruited from a health check-up center. The FRS and 10-year risk of CHD development were calculated.
Results
The FRS in men was inversely correlated with the standard deviation of all normal to normal RR-intervals (SDNN); the root mean square successive difference (RMSSD); the percentage of successive normal cardiac inter-beat intervals greater than 20 ms, 30 ms, and 50 ms (pNN20, pNN30, pNN50); the low frequency (LF); and the high frequency (HF) (P < 0.05). There was no significant relationship between the FRS and HRV in women. Overall, in the receiver operating characteristic (ROC) analysis, the RMSSD, HF, SDNN, LF, LF/HF ratio, and pNN30 predicted an increased 10-year CHD risk. After adjusting for sex and body mass index, those with greater than one standard deviation in the RMSSD, HF, and LF had a 52-59% reduction in their 10-year risk of CHD development ≥ 10%.
Conclusion
This study therefore indicates that the HRV indices, particularly SDNN, RMSSD, pNN30, LF, and HF may be useful parameters for the assessment of CHD risk. Most notably, the usefulness of these HRV measures as indicators for CHD risk evaluation may be greater among men than among women.
Keywords: Heart Rate, Risk Assessment, Electrocardiography
INTRODUCTION
Heart rate variability (HRV), the variation in the cardiac inter-beat interval over time, has emerged as a quantitative marker of sympathetic and parasympathetic influences on the modulations of heart rate,1) and it has been used to assess autonomic imbalances. Increased sympathetic or decreased parasympathetic nervous system activity predisposes patients with coronary heart disease (CHD) to ventricular tachycardia, ventricular fibrillation, sudden cardiac death2,3) and increased mortality.4) Several studies have found that lower HRV is associated with a higher risk of coronary heart disease.5-7) Numerous studies also suggest that lower HRV is associated with traditional risk factors of cardiovascular disease8) such as hypertension,9,10) diabetes,11,12) high cholesterol,13,14) smoking tobacco,15) being overweight,16) and increasing age.17,18)
The Framingham risk score (FRS), which is a global risk assessment method comprised of factors including patient age, smoking activity, low density lipoprotein cholesterol (LDL) levels (or total cholesterol), high density lipoprotein cholesterol (HDL) levels, blood pressure (BP), and the presence of diabetes mellitus has been accepted as a tool to predict the risk of CHD development.19) Although previous work has mainly focused on the relationship between the HRV measures and individual established risk factors, little evidence is available about the relationship between HRV and the FRS.18) Therefore, we have focused on the relationships between the HRV measures and the FRS to confirm various HRV indices as predictable tools of future risk of CHD development.
METHODS
Subjects included 85 adults (55 men, 30 women, 48 ± 8 years old) recruited from the Center of Health Promotion at Inje University Busan Paik Hospital between November 2009 and December 2009. None of the subjects had a medical history of any diseases known to affect the autonomic cardiac function such as cardiovascular, neurological, or endocrine diseases, and none of the subjects were taking medication. The study protocol was approved by the Institutional Review Board of the Buan Paik Hospital.
All subjects fasted and finished their health check-up prior to the assessment of their HRV. Body weight and height were measured in subjects clothed in a light gown without shoes; these measurements were used to calculate the body mass index (BMI; kg/m2). BP measurements were performed with a standard manual sphygmomanometer while participants were in sitting position. Antecubital venous blood samples were taken from all subjects after a 12-hour overnight fast. The levels of LDL, HDL, and fasting glucose were measured using a biochemistry autoanalyzer (Toshiba 200-FR; Toshiba, Tokyo, Japan). Information about smoking habits and past medical history were obtained using a self-administrated questionnaire.
To limit the influence of diurnal and environmental variations, the HRV measurements were taken while the patients were in a sitting position after resting for 20 minutes. The measurements were taken in the morning and at the same room by one trained research assistant according to a standardized method. The HRV measurement was taken twice for each subject with a short-term interval in-between. HRV recordings were obtained in each subject using a standard electrocardiogram (ECG) recorder implemented in SA6000 (Medicore Co. Ltd, Seoul, Korea). From the ECG of each subject, the HRV was extracted with an R-peak detection algorithm using differential ECG signals after preprocessing procedures, such as low-pass filtering and detrending. Recordings were excluded in the event of a failure to extract HRV from the ECG due to noise. Recordings with non-sinus beats that were more than 1% of the total number of beats were also excluded. Premature beats and artifacts were carefully eliminated automatically and manually by visual inspection of all RR intervals.
Using the time domain analysis of HRV, the mean length of all normal to normal RR intervals (MEAN), the standard deviation of all normal to normal R-R intervals (SDNN), the square root of the mean squared differences of successive normal sinus intervals (RMSSD), and the percentage of successive RR interval differences whose absolute value exceeds x ms (pNNx) were computed according to standardized procedures.20,21) In the frequency domain analysis, the total power (TP), the very low frequency (VLF) spectral power component, the low frequency (LF) spectral power component, and the high frequency (HF) spectral component were calculated. The TP was defined as the total area under the power spectral density (PSD) curve, VLF as the power between 0.00 and 0.04 Hz bands, LF as the power between 0.04 and 0.15 Hz, and HF as the power between 0.15 and 0.4 Hz bands under the PSD curve. The LF to HF ratio (LF/HF) and the normalized LF [LFnu = LF/(TP-VLF) × 100] and HF [HFnu = HF/(TP-VLF)] were computed.22-24) The intra-class correlation coefficient for SDNN, RMSSD, pNN10, pNN20, pNN30, pNN50, LF, HF, LF/HF, LFnu, and HFnu were 0.59, 0.68, 0.84, 0.91, 0.84, 0.77, 0.56, 0.66, 0.76, 0.73, and 0.66, respectively (P < 0.001).
The FRS and 10-year risk of CHD development were determined by calculating the number of Framingham points assigned to each risk factor such as age, LDL, HDL, BP (regardless of the use of antihypertensive medications), cigarette smoking, and diabetes mellitus (having a diagnosis, fasting glucose ≥ 126 mg/dL, or hemoglobin A1C > 6.5).19)
1. Statistical Analyses
As the distribution of HRV parameters and FRS (and 10 year CHD risk) were skewed, median and interquartile ranges were used. A natural logarithmic transformation was applied to normalize the distribution of LF and HF.24) The Spearman correlation coefficients between the HRV measures and the CHD risk scores (FRS and 10 year CHD risk) were calculated for the total population of subjects and for each gender. The HRV measures were compared between those with a high 10-year CHD risk (≥ 10%) and those with a low 10-year CHD risk (< 10%) using Mann-Whitney test. The predictive ability of each HRV measure with respect to higher 10-year CHD risk was analyzed using the receiver operating characteristic (ROC) curves and logistic regression analyses. ROC curve calculated the area under the ROC curve (AUC) to identify higher 10-year CHD risk scores. Logistic regression analyses were conducted for the z-score of each HRV measure after adjustment for sex and BMI. These analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
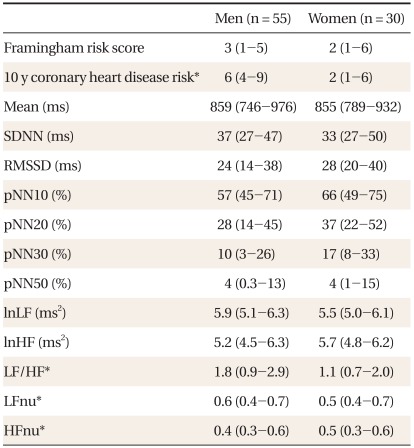
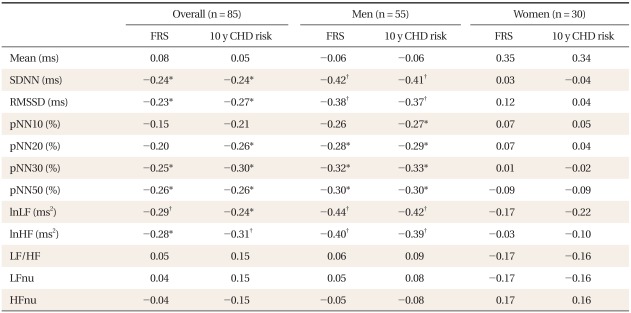
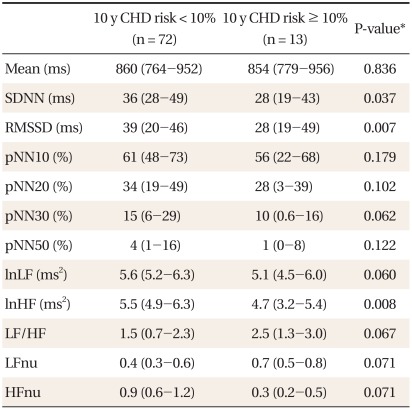
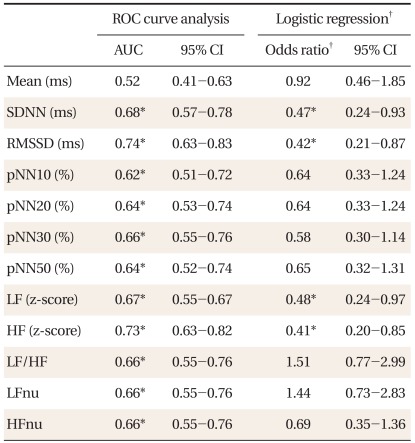
Table 1 shows the characteristics of subjects. Compared to women, men had significantly higher 10-year CHD risk, LF to HF ratios, LFnu, and lower HFnu. The correlations between the FRS and the HRV measures are presented in Table 2. Overall, the FRS was significantly and inversely correlated with SDNN, RMSSD, pNN30, pNN50, LF, and HF, although the magnitude of the correlations was moderate to weak. When the relationships were compared by gender, these significant inverse correlations were found only in men. When the HRV measures were compared between those with high 10-year CHD risk and those with low 10-year CHD risk, SDNN, RMSSD, and HF were significantly lower in those with high 10-year CHD risk (Table 3). To determine the predictive ability of the HRV measures for high 10-year CHD risk, the ROC comparison and logistic regression analyses were conducted, and the results are shown in Table 4. The RMSSD had the greatest AUC among the HRV measures, followed by the AUC of HF, SDNN, LF, LF/HF, and pNN30, which were significant. In logistic regression models for high 10-year CHD risk, the odds ratios were significant for SDNN, RMSSD, LF, and HF after adjustment for sex and BMI. An increase in these valued by one standard deviation attenuated the risk of having a high 10-year CHD risk by 52-59%.
Table 1.
Characteristics of subjects.
Values are presented as median (interquartile range).
SDNN: standard deviation of all normal RR intervals, RMSSD: root mean square of successive differences, pNN10, 20, 30, and 50: percentage of differences between adjacent RR intervals that are greater than 10 ms, 20 ms, 30 ms, and 50 ms respectively, ln: natural logarithm, LF: low frequency power, HF: high frequency power, LFnu: normalized low frequency power, HFnu: normalized high frequency power.
*P < 0.05 by Mann-Whitney test.
Table 2.
The correlations between HRV and the CHD risk score.
HRV: heart rate variability, CHD: coronary heart disease, FRS: Framingham risk score, SDNN: standard deviation of all normal RR intervals, RMSSD: root mean square of successive differences, pNN10, 20, 30, and 50: percentage of differences between adjacent RR intervals that are greater than 10 ms, 20 ms, 30 ms, and 50 ms respectively, ln: natural logarithm, LF: low frequency power, HF: high frequency power, LFnu: normalized low frequency power, HFnu, normalized high frequency power.
*P < 0.05. †P < 0.01.
Table 3.
Comparison of the HRV measures between high and low CHD risk.
HRV: heart rate variability, CHD: coronary heart disease, FRS: Framingham risk score, SDNN: standard deviation of all normal RR intervals, RMSSD: root mean square of successive differences, pNN10: 20, 30, and 50: percentage of differences between adjacent RR intervals that are greater than 10 ms, 20 ms, 30 ms, and 50 ms respectively, ln: natural logarithm, LF: low frequency power, HF: high frequency power, LFnu: normalized low frequency power, HFnu: normalized high frequency power.
*By Mann-Whitney test.
Table 4.
ROC curve analysis and logistic regression of each heart rate variability measure (transformed to z-score) for high (≥ 10%) 10 y CHD risk.
ROC: receiver operating characteristic, CHD: coronary heart disease, AUC: area under the ROC curve, SDNN: standard deviation of all normal RR intervals, RMSSD: root mean square of successive differences, pNN10, 20, 30, and 50: percentage of differences between adjacent RR intervals that are greater than 10 ms, 20 ms, 30 ms, and 50 ms respectively, ln: natural logarithm, LF: low frequency power, HF: high frequency power, LFnu: normalized low frequency power, HFnu: normalized high frequency power.
*P < 0.05. †Adjusted for sex and body mass index.
DISCUSSION
In this study, we found that low HRV measures were significantly associated with the FRS, which is comprised of traditional CV risk factors. Low HRV measures were also associated with the 10-year risk of CHD development. Significant relationships were found for SDNN, RMSSD, LF, and HF whether they were applied as continuous or discrete variables. In contrast, LF/HF, LFnu, and HFnu were not significantly associated with either the FRS or the 10-year risk of CHD development.
The associations between autonomic imbalance and coronary arteriosclerosis and its risk factors have been proposed and current result is comparable to previous findings.5,6,18) In population-based studies, lower HRV has been associated with an increased risk of CHD development. Prior studies also have shown a lower vagal tone in individuals with hypertension than in healthy individuals.9,10) A lower vagal tone has also been reported in those diagnosed with diabetes compared to those without diabetes.11,12) LDL levels, or total cholesterol, were inversely associated with 24-hour HRV13) or the RMSSD.14) A low vagal tone was also associated with both acute and chronic smoking15) and increasing age.17) However, the relationship between HRV measures and a global risk assessment tool for CHD, such as the FRS, has not been clearly documented.
The SDNN physiologically reflects the parasympathetic activity corresponding to fast variations in heart rate and the sympathetic activity controlling slow modulation of heart function. The RMSSD values are also commonly used HRV measures derived from interval differences. All of these measures of short-term variation estimate the high-frequency modulation in the heart rate, and they are highly correlated.24) The LF component has been proposed as an index of sympathetic modulation, whereas HF, which is a measure of respiratory sinus arrhythmia, is also used as an index of vagal activity.22,24) Non-significant associations with LF/HF, LFnu, and HFnu could be expected because the ratio and proportion measures attenuate the information derived from LF and HF, which are significantly associated with the FRS.5)
Because lower HRV has been indicated as a predictor for the development of CHD5-7) and the FRS is considered an accepted assessment tool to estimate the risk of CHD development,19) our findings may confirm HRV as a plausible tool to evaluate the future risk of CHD development. However, because previous finding regarding the association between lower HRV and the development of CHD were significant after adjusting for traditional CV risk factors,5) the causal pathway between HRV and the FRS cannot be determined using these data. It is likely that reduced HRV may reflect subclinical coronary atherosclerosis, and thus it may be a simple marker similar to the FRS, which can be used to predict the 10-year risk of CHD development.
In summary, our findings extend previous knowledge, confirming that hypoactive parasympathetic activity tends to predict a higher 10-year risk of CHD. This study therefore indicates that the HRV indices, particularly SDNN, RMSSD, pNN30, LF, and HF, may be useful parameters for CHD risk assessment. Most notably, the usefulness of these HRV measures as indicators for the evaluation of CHD risk may be greater among men than among women.
However, some limitations are worth noting. The small sample size of women may dampen the statistical power, and future studies designed to evaluate gender differences in these relationships among a larger sample size of women should therefore be conducted.
ACKNOWLEDGEMENTS
This study was supported by a grant from Ministry of Knowledge Economy of Korea. (budget number 10033321).
References
- 1.Malik M, Camm AJ. Components of heart rate variability: what they really mean and what we really measure. Am J Cardiol. 1993;72:821–822. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 2.Podrid PJ, Fuchs T, Candinas R. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation. 1990;82(2 Suppl):I103–I113. [PubMed] [Google Scholar]
- 3.Pruvot E, Thonet G, Vesin JM, van-Melle G, Seidl K, Schmidinger H, et al. Heart rate dynamics at the onset of ventricular tachyarrhythmias as retrieved from implantable cardioverter-defibrillators in patients with coronary artery disease. Circulation. 2000;101:2398–2404. doi: 10.1161/01.cir.101.20.2398. [DOI] [PubMed] [Google Scholar]
- 4.Hayano J, Sakakibara Y, Yamada M, Ohte N, Fujinami T, Yokoyama K, et al. Decreased magnitude of heart rate spectral components in coronary artery disease. Its relation to angiographic severity. Circulation. 1990;81:1217–1224. doi: 10.1161/01.cir.81.4.1217. [DOI] [PubMed] [Google Scholar]
- 5.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower heart rate variability is associated with the development of coronary heart disease in individuals with diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes. 2002;51:3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 7.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, et al. Atherosclerosis Risk In Communities. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 8.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 11.Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, et al. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study) Am J Cardiol. 2000;86:309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 12.Carnethon MR, Fortmann SP, Palaniappan L, Duncan BB, Schmidt MI, Chambless LE. Risk factors for progression to incident hyperinsulinemia: the Atherosclerosis Risk in Communities Study, 1987-1998. Am J Epidemiol. 2003;158:1058–1067. doi: 10.1093/aje/kwg260. [DOI] [PubMed] [Google Scholar]
- 13.Christensen JH, Toft E, Christensen MS, Schmidt EB. Heart rate variability and plasma lipids in men with and without ischaemic heart disease. Atherosclerosis. 1999;145:181–186. doi: 10.1016/s0021-9150(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 14.Kupari M, Virolainen J, Koskinen P, Tikkanen MJ. Short-term heart rate variability and factors modifying the risk of coronary artery disease in a population sample. Am J Cardiol. 1993;72:897–903. doi: 10.1016/0002-9149(93)91103-o. [DOI] [PubMed] [Google Scholar]
- 15.Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, et al. Short- and long-term effects of cigarette smoking on heart rate variability. Am J Cardiol. 1990;65:84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- 16.Karason K, Mølgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 17.Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Suh YS, Kim DH. The relationship of Framingham risk score and heart rate variability in non-obese males. J Korean Acad Fam Med. 2008;29:330–335. [Google Scholar]
- 19.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and Physio-Net: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 21.Mietus JE, Peng CK, Henry I, Goldsmith RL, Goldberger AL. The pNNx files: re-examining a widely used heart rate variability measure. Heart. 2002;88:378–380. doi: 10.1136/heart.88.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi F. Chaos theory, heart rate variability, and arrhythmic mortality. Circulation. 2000;101:8–10. doi: 10.1161/01.cir.101.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]