Abstract
Purpose
Pain is prevalent among patients with cancer, yet pain management patterns in outpatient oncology are poorly understood.
Patients and Methods
A total of 3,123 ambulatory patients with invasive cancer of the breast, prostate, colon/rectum, or lung were enrolled onto this prospective study regardless of phase of care or stage of disease. At initial assessment and 4 to 5 weeks later, patients completed a 25-item measure of pain, functional interference, and other symptoms. Providers recorded analgesic prescribing. The pain management index was calculated to assess treatment adequacy.
Results
Of the 3,023 patients we identified to be at risk for pain, 2,026 (67%) reported having pain or requiring analgesics at initial assessment; of these 2,026 patients, 670 (33%) were receiving inadequate analgesic prescribing. We found no difference in treatment adequacy between the initial and follow-up visits. Multivariable analysis revealed that the odds of a non-Hispanic white patient having inadequate pain treatment were approximately half those of a minority patient after adjusting for other explanatory variables (odds ratio, 0.51; 95% CI, 0.37 to 0.70; P = .002). Other significant predictors of inadequate pain treatment were having a good performance status, being treated at a minority treatment site, and having nonadvanced disease without concurrent treatment.
Conclusion
Most outpatients with common solid tumors must confront issues related to pain and the use of analgesics. There is significant disparity in pain treatment adequacy, with the odds of undertreatment twice as high for minority patients. These findings persist over 1 month of follow-up, highlighting the complexity of these problems.
INTRODUCTION
Pain is one of the most devastating symptoms in patients with cancer. A meta-analysis of more than 50 studies revealed that more than 50% of patients with cancer in the United States experience pain and that pain is most prevalent among patients with a high disease burden.1
Although pain is prevalent among patients with cancer, many frequently receive inadequate pain treatment despite established pain treatment guidelines. More than 20 years ago, the Eastern Cooperative Oncology Group (ECOG) conducted a landmark study of pain needs in 1,308 patients in outpatient oncology, finding that 42% had inadequate analgesic prescribing.2 The findings of the ECOG study and other studies led to an increased emphasis on symptom management in cancer. Nevertheless, reducing the burden of symptoms remains challenging. In 2002, a National Institutes of Health State-of-the-Science Panel noted that “Additional research is needed on the definition, occurrence, the treatment of pain, depression, and fatigue, alone and in combination, in adequately funded prospective studies,”3 providing the impetus for this prospective observational study in oncology outpatients.
The primary objective of the present report was to assess the prevalence of pain and analgesic use in outpatient oncology practice. The secondary objectives were to identify the characteristics of patients with self-reported pain, the adequacy of opioid prescribing, and the factors associated with the undertreatment of pain.
PATIENTS AND METHODS
Study Design and Patients
From March 3, 2006, to May 19, 2008, we enrolled oncology outpatients at any point in the trajectory of their care for invasive breast, lung, prostate, or colorectal cancer. Patients were registered at 38 institutions, including six academic sites and 32 community clinics. Registering institutions were classified as minority-based if they had 40% or greater minority participation in previous clinical trials or in the current study; three academic sites and 10 community sites were coded as minority-based sites in our analysis. Patients treated in academic centers were enrolled from disease site–specific clinics. In contrast, patients treated in community clinics were enrolled from general oncology clinics. To reduce selection bias, each site devised a recruitment algorithm that was not biased toward symptom management issues and approved by the ECOG coordinating center.
In addition to a clinical diagnosis of invasive breast, lung, prostate, or colorectal cancer, patients had to be at least 18 years of age, receiving care at an ECOG-affiliated institution, willing to complete the follow-up survey, and judged by the study screener to have cognitive function adequate for completing study surveys. The protocol was approved by the institutional review boards at each registering institution. The anticipated accrual goal was 2,310 patients; however, because of the brisk accrual toward the end of the study, final accrual was 3,123 to allow all consented patients to participate. All patients provided written informed consent. The protocol and case report forms are accessible on the study web site.4
Study Procedures
Patients were recruited when they checked in for their clinic appointments, and patients' information was collected before their visit with a clinician. Patients and their treating clinicians were surveyed at the initial visit and at follow-up 28 to 35 days later.
Patients were asked to read the instructions at the beginning of each questionnaire and complete all items in terms of their experience during the preceding 24 hours. Reasons for incomplete forms were documented on the compliance form. Patients who could not complete the follow-up questionnaire because of acute illness were given the option to mail the forms to the treating clinic by day 42 after the initial visit.
Survey Measures
The initial survey was used to collect patients' basic clinical and demographic information, including cancer treatment history and current therapies. At the initial and follow-up visits, patients reported symptom intensity and functional interference using a modification of the MD Anderson Symptom Inventory (MDASI), a validated 19-item measure that is very similar to the Brief Pain Inventory in terms of structure and patient burden.5,6 Patients used the MDASI to rate the symptoms and functional interference items that they most frequently experienced in the previous 24 hours “at their worst” on an 11-point Likert scale ranging from 0 (“not present”) to 10 (“as bad as you can imagine”). Clinicians reported patients' specific medications, including those that were newly prescribed. A clinician-specific survey was used to ascertain symptom prioritization and symptom attribution.
The pain management index (PMI) is a conservative and well-validated instrument that is the most frequently used measure of pain treatment adequacy for opioid prescribing.7,8 The PMI is modeled on the WHO pain treatment paradigm, which involves a three-step analgesic ladder that progresses from nonopioid analgesics to weak opioids and then strong opioids, depending on the self-reported pain intensity.6,9 The PMI was calculated by subtracting the pain score from the analgesic score for each patient. Pain scores ranged from 0 to 3 and were based on the pain ratings patients reported on the modified MDASI. Pain scores of 0, 1, 2, and 3 corresponded to MDASI scores indicating no pain (0), mild pain (1 to 4), moderate pain (5 or 6), and severe pain (7 to 10), respectively. Analgesic scores ranged from 0 to 3 and were used to gauge the potency of the prescribed medications indicated on the medication form at each visit. If multiple analgesics were prescribed, the most potent medication was scored. The analgesic score was coded as 0 for no medications, 1 for nonopioids, 2 for weak opioids, and 3 for strong opioids. Morphine, fentanyl, oxycodone, and methadone were considered strong opioids.
Statistical Analysis
Statistical analysis focused on the dichotomization of patients based on the PMI value: undertreatment (PMI < 0) versus acceptable treatment (PMI ≥ 0). Patients who did not have pain and were not taking pain medications were not considered to be at risk for inadequate pain management and were thus excluded from the statistical analysis. Descriptive statistics were used to summarize the findings for patients at risk for inadequate pain management. Data collected at the initial assessment and data collected at the follow-up visits 4 to 5 weeks later were analyzed separately.
To identify predictors of pain treatment adequacy, we performed univariable and multivariable logistic regression analyses of patients' demographic and clinical data as well as clinician response data. The logistic regression analysis modeled the probability of undertreatment. Because patients receiving care at the same clinic are likely to be treated more similarly than patients receiving care at different clinics, we treated the data as clustered and used generalized linear models with generalized estimating equations to account for the intracluster correlation. In both the univariable and multivariable logistic regression models, patients for whom the values of any of the variables were missing were excluded from data analysis. Explanatory variables in the univariable analysis revealed to be significant (P ≤ .10) for the response variable (ie, pain treatment adequacy) were included in the multivariable analysis. Any nonsignificant predictors (P > .10) were trimmed from the multivariable model. This procedure was repeated until all predictors in the model met the criteria of P ≤ .10. Finally, a model of interactions between any of the main effects in the multivariable analysis was fitted. SAS statistical software (version 9.2; SAS Institute, Cary, NC) was used for all data analyses.
RESULTS
Pain, Analgesic Prescribing, and Symptom Attributes at Initial Assessment
We identified 3,023 patients at risk for pain; of these patients, 2,026 (67%) had pain or were receiving analgesics and were included in the statistical analysis (Fig 1). Of these 2,026 patients, 1,356 (67%) had adequate pain management. Patient demographics and disease characteristics at the initial assessment of the 2,026 patients at risk for pain are presented in Table 1. Most of these 2,026 patients had breast cancer (1,009 patients; 50%); 427 patients (21%) had colorectal cancer, 385 patients (19%) had lung cancer, and 205 patients (10%) had prostate cancer. At the initial assessment, 78% of these patients were receiving cancer therapy. Patients' median age was 60 years (range, 18 to 93 years), and 75% of the patients were between 45 and 75 years of age. The median time from initial disease diagnosis to study registration was 16 months (range, 0 to 627 months). Most patients were women (71%), white (86%), and had an ECOG performance status level of 0 (50%). Approximately one fourth of the patients analyzed were minority patients (9% Hispanic or Latino, 12% black, 1% Asian, and 1% other minority).
Fig 1.
Flow diagram of patient enrollment.
Table 1.
Patient Demographics and Disease Characteristics at Baseline for All Potentially Analyzable Patients (N = 3,106)
| Characteristic | Potentially Analyzable Patients |
Patients in PMI Analysis at Initial Assessment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients Not in PMI Analysis at Initial Assessment |
Patients in PMI Analysis at Initial Assessment |
Breast |
Disease Site Colorectal Prostate |
Lung |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 1,080 | 35 | 2,026 | 65 | 1,009 | 50 | 427 | 21 | 205 | 10 | 385 | 19 |
| Time from diagnosis, months | 1,069 | 1,987 | 985 | 422 | 198 | 382 | ||||||
| Mean | 35 | 35 | 44 | 23 | 59 | 15 | ||||||
| Median | 14 | 16 | 20 | 12 | 42 | 7 | ||||||
| Range | 0-308 | 0-627 | 0-627 | 0-197 | 0-269 | 0-149 | ||||||
| Age, years | 1,080 | 2,026 | 1,009 | 427 | 205 | 385 | ||||||
| Mean | 62 | 60 | 57 | 60 | 70 | 64 | ||||||
| Median | 63 | 60 | 57 | 59 | 71 | 64 | ||||||
| Range | 28-92 | 18-93 | 18-91 | 23-89 | 47-93 | 35-88 | ||||||
| < 45 | 92 | 8 | 221 | 11 | 154 | 15 | 51 | 12 | 0 | 0 | 16 | 4 |
| 45-60 | 343 | 32 | 741 | 37 | 432 | 43 | 163 | 38 | 35 | 17 | 111 | 29 |
| 60-75 | 460 | 43 | 771 | 38 | 342 | 34 | 148 | 35 | 93 | 45 | 188 | 49 |
| ≥ 75 | 185 | 17 | 293 | 14 | 81 | 8 | 65 | 15 | 77 | 38 | 70 | 18 |
| Sex | ||||||||||||
| Male | 340 | 31 | 596 | 29 | 3 | 0 | 215 | 50 | 205 | 100 | 173 | 45 |
| Female | 740 | 69 | 1,430 | 71 | 1,006 | 100 | 212 | 50 | 0 | 0 | 212 | 55 |
| Race | ||||||||||||
| White | 926 | 87 | 1,722 | 86 | 862 | 86 | 357 | 84 | 171 | 86 | 332 | 87 |
| Black | 127 | 12 | 237 | 12 | 116 | 12 | 58 | 14 | 26 | 13 | 37 | 10 |
| Asian | 11 | 1 | 19 | 1 | 13 | 1 | 3 | 1 | 0 | 0 | 3 | 1 |
| Native Hawaiian | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Native American | 3 | 0 | 14 | 1 | 5 | 1 | 3 | 1 | 0 | 0 | 6 | 2 |
| Indian | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient refusal | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Multiracial | 2 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 0 |
| Unknown | 10 | — | 27 | — | 11 | — | 4 | — | 6 | — | 6 | — |
| Ethnicity | ||||||||||||
| Hispanic or Latino | 116 | 12 | 169 | 9 | 68 | 7 | 53 | 13 | 23 | 13 | 25 | 7 |
| Non-Hispanic | 879 | 88 | 1,693 | 91 | 858 | 93 | 356 | 87 | 157 | 87 | 322 | 92 |
| Patient refusal | 4 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 |
| Institution refusal | 1 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Unknown | 80 | — | 158 | — | 81 | — | 17 | — | 25 | — | 35 | — |
| Race/ethnicity | ||||||||||||
| Minority | 248 | 25 | 434 | 23 | 197 | 21 | 117 | 29 | 51 | 27 | 69 | 20 |
| White and non-Hispanic | 753 | 75 | 1,440 | 77 | 735 | 79 | 292 | 71 | 135 | 73 | 278 | 80 |
| Unknown | 79 | — | 152 | — | 77 | — | 18 | — | 19 | — | 38 | — |
| ECOG PS* | ||||||||||||
| 0 | 744 | 69 | 1,011 | 50 | 628 | 63 | 192 | 45 | 81 | 40 | 110 | 29 |
| 1 | 298 | 28 | 807 | 40 | 310 | 31 | 198 | 47 | 94 | 46 | 205 | 53 |
| ≥ 2 | 34 | 3 | 197 | 10 | 64 | 6 | 36 | 8 | 28 | 14 | 69 | 18 |
| Unknown | 4 | — | 11 | — | 7 | — | 1 | — | 2 | — | 1 | — |
| Weight loss in previous 6 months | ||||||||||||
| < 5% | 967 | 91 | 1,664 | 83 | 879 | 89 | 320 | 75 | 176 | 87 | 289 | 75 |
| ≥ 5% | 101 | 9 | 338 | 17 | 113 | 11 | 105 | 25 | 26 | 13 | 94 | 25 |
| Unknown | 12 | — | 24 | — | 17 | — | 2 | — | 3 | — | 2 | — |
| Current status of disease | ||||||||||||
| CR | 512 | 48 | 645 | 32 | 471 | 47 | 118 | 27 | 21 | 10 | 35 | 9 |
| PR | 43 | 4 | 104 | 5 | 34 | 3 | 16 | 4 | 18 | 9 | 36 | 9 |
| SD | 406 | 38 | 930 | 46 | 371 | 37 | 216 | 51 | 111 | 54 | 232 | 61 |
| PD | 108 | 10 | 338 | 17 | 128 | 13 | 76 | 18 | 55 | 27 | 79 | 21 |
| Unknown | 11 | — | 9 | — | 5 | — | 1 | — | 0 | — | 3 | — |
| NED | 578 | 54 | 754 | 37 | 532 | 53 | 138 | 33 | 36 | 18 | 48 | 13 |
| Locoregional | 221 | 20 | 368 | 18 | 160 | 16 | 51 | 12 | 29 | 14 | 128 | 33 |
| Metastatic | 233 | 22 | 726 | 36 | 275 | 27 | 197 | 46 | 114 | 56 | 140 | 36 |
| Locoregional and metastatic | 44 | 4 | 171 | 9 | 39 | 4 | 39 | 9 | 25 | 12 | 68 | 18 |
| Unknown | 4 | — | 7 | — | 3 | — | 2 | — | 1 | — | 1 | — |
| Prior chemo/immuno/hormonal therapy | ||||||||||||
| No | 460 | 43 | 735 | 36 | 320 | 32 | 160 | 37 | 73 | 36 | 182 | 47 |
| Yes | 620 | 57 | 1,290 | 64 | 689 | 68 | 267 | 63 | 132 | 64 | 202 | 53 |
| Unknown | 0 | — | 1 | — | 0 | — | 0 | — | 0 | — | 1 | — |
| Prior radiation therapy | ||||||||||||
| No | 661 | 62 | 1,121 | 56 | 511 | 51 | 314 | 74 | 86 | 42 | 210 | 55 |
| Yes | 411 | 38 | 886 | 44 | 489 | 49 | 109 | 26 | 117 | 58 | 171 | 45 |
| Unknown | 8 | — | 19 | — | 9 | — | 4 | — | 2 | — | 4 | — |
| Currently receiving cancer treatment | ||||||||||||
| No | 359 | 33 | 448 | 22 | 194 | 19 | 106 | 25 | 49 | 24 | 99 | 26 |
| Yes | 721 | 67 | 1,578 | 78 | 815 | 81 | 321 | 75 | 156 | 76 | 286 | 74 |
| Metastatic sites | ||||||||||||
| No site | 754 | 70 | 1,080 | 53 | 676 | 67 | 175 | 41 | 66 | 32 | 163 | 42 |
| Single site | 198 | 18 | 487 | 24 | 150 | 15 | 130 | 30 | 101 | 49 | 106 | 28 |
| Multiple sites | 128 | 12 | 459 | 23 | 183 | 18 | 122 | 29 | 38 | 19 | 116 | 30 |
| Disease stage and current treatment status | ||||||||||||
| Advanced stage and currently treated | 243 | 23 | 800 | 40 | 297 | 30 | 210 | 50 | 124 | 61 | 169 | 44 |
| Advanced stage and not currently treated | 34 | 3 | 97 | 5 | 17 | 2 | 26 | 6 | 15 | 7 | 39 | 10 |
| Nonadvanced stage and currently treated | 477 | 44 | 774 | 38 | 516 | 51 | 111 | 26 | 31 | 15 | 116 | 30 |
| Nonadvanced stage and not currently treated | 322 | 30 | 348 | 17 | 176 | 17 | 78 | 18 | 34 | 17 | 60 | 16 |
| Unknown | 4 | — | 7 | — | 3 | — | 2 | — | 1 | — | 1 | — |
| Institution type | ||||||||||||
| Academic | 101 | 9 | 202 | 10 | 75 | 7 | 41 | 10 | 43 | 21 | 43 | 11 |
| Community | 979 | 91 | 1,824 | 90 | 934 | 93 | 386 | 90 | 162 | 79 | 342 | 89 |
| Clinic practice type | ||||||||||||
| Majority based | 783 | 73 | 1,541 | 76 | 787 | 78 | 302 | 71 | 140 | 68 | 312 | 81 |
| Minority based | 297 | 27 | 485 | 24 | 222 | 22 | 125 | 29 | 65 | 32 | 73 | 19 |
Abbreviations: CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; NED, no evidence of disease; PD, progressive disease; PMI, pain management index; PR, partial response.
ECOG PS, where a level of 0 indicates full activity, without restriction. Higher levels indicate greater impairment in function.
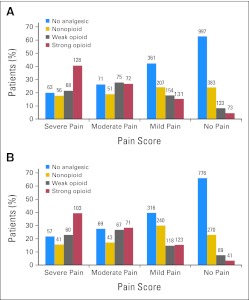
The analgesics prescribed in relation to pain severity at initial assessment and follow-up are summarized in Figure 2. At initial assessment, 404 patients (13%) were being treated with strong opioids, and 584 patients (19%) had moderate or severe pain. Of the patients with moderate or severe pain, 241 (41%) were not receiving an opioid analgesic. Twenty percent of the patients in severe pain were not receiving any analgesic. The most commonly prescribed nonopioids were acetaminophen (35%) and nonsteroidal anti-inflammatory agents (19%). The most prevalent moderate or severe nonpain symptoms at initial assessment were fatigue (35%), sleep disturbance (27%), and drowsiness (23%). More than one third of patients (40%) reported having at least three moderate or severe symptoms. Clinicians attributed symptoms at least moderately to cancer or cancer treatment in 51% of patients. One or more factors associated with an increased risk of pain (neuropathic pain syndrome, incidental pain, psychological distress, addiction behavior, or cognitive impairment)10 were noted in 49% of patients. On the basis of the clinicians' reports, 3% of patients had a history of addiction behavior, 3% had partial cognitive impairment, 28% had psychological distress, and 31% had incidental pain. Clinicians judged pain to be the top-ranked symptom in 22% of patients and one of the top three symptoms in 35% of patients.
Fig 2.
Analgesic prescribing in relation to pain severity at (A) initial assessment and (B) follow-up 28 to 35 days later. The numbers of patients are displayed according to the WHO category of analgesic prescribing and their self-reported level of pain intensity.
Follow-Up Assessment of Pain, Analgesic Prescribing, and Pain Treatment Adequacy
Table 2 tabulates changes in pain and analgesia between initial and follow-up assessments. Among the 1,457 patients who had both PMI values available at initial and follow-up assessments, the McNemar test revealed no significant changes in treatment adequacy between the two visits (across disease sites or by disease site). Of the 406 patients with undertreatment at initial assessment, only 31% received acceptable pain treatment by the follow-up visit. Moreover, 10% of the 1,051 patients with acceptable treatment at baseline became undertreated by the time of follow-up.
Table 2.
Tabulation of Pain and Analgesia at Initial and Follow-Up Assessments
| Pain at Baseline* | Pain at Follow-Up |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing |
No Pain |
Mild Pain |
Moderate Pain |
Severe Pain |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| No pain | 152 | 9 | 1,048 | 65 | 319 | 20 | 56 | 3 | 40 | 3 | 1,615 |
| Mild pain | 81 | 9 | 211 | 25 | 416 | 48 | 89 | 10 | 66 | 8 | 863 |
| Moderate pain | 34 | 12 | 43 | 16 | 77 | 28 | 62 | 22 | 60 | 22 | 276 |
| Severe pain | 45 | 14 | 35 | 11 | 63 | 20 | 63 | 20 | 113 | 35 | 319 |
| Analgesia at Baseline† | Analgesia at Follow-Up |
Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Missing |
No Analgesic |
Non-Opioid |
Weak Opioid |
Strong Opioid |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| No analgesic | 240 | 16 | 1,159 | 77 | 64 | 4 | 24 | 2 | 23 | 1 | 1,510 |
| Nonopioid | 102 | 15 | 45 | 6 | 524 | 75 | 17 | 2 | 13 | 2 | 701 |
| Weak opioid | 40 | 9 | 34 | 8 | 25 | 6 | 312 | 72 | 23 | 5 | 434 |
| Strong opioid | 57 | 14 | 25 | 6 | 15 | 4 | 9 | 2 | 302 | 74 | 408 |
All potentially analyzable patients with pain score reported (regardless of the availability of the analgesic score) at initial assessment were analyzed.
All potentially analyzable patients with analgesic score reported (regardless of the availability of the pain score) at initial assessment were analyzed.
Predictors of Pain Treatment Adequacy
Multivariable analysis of the initial and follow-up data revealed that non-Hispanic white patients, patients treated at sites with mostly non-Hispanic white patients, patients with poor performance status, and patients with advanced cancer who were receiving cancer treatment were least likely to receive inadequate pain treatment in terms of opioid prescribing.
The odds ratios (ORs) for inadequate pain treatment from the univariable and multivariable logistic regression analyses at initial and follow-up assessments are summarized in Table 3.
Table 3.
Univariable and Multivariable Logistic Regression Analyses for Undertreatment of Pain at Initial Assessment and Follow-Up
| Predictor and Level | Initial Assessment |
Follow-Up Assessment |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Undertreatment (No.) | Univariable (n = 1,874-2,026) |
Multivariable (n = 1,836) |
% Undertreatment (No.) | Univariable (n = 1,565-1,708) |
Multivariable (n = 1,461) |
|||||
| P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | |||
| Disease site | ||||||||||
| Colorectal | 35 (149/427) | .037 | 0.98 (0.74 to 1.30) | 36 (135/373) | .635 | 1.04 (0.79 to 1.38) | ||||
| Prostate | 32 (65/205) | 0.85 (0.53 to 1.35) | 30 (47/155) | 0.80 (0.43 to 1.48) | ||||||
| Lung | 26 (99/385) | 0.63 (0.46 to 0.86) | 32 (101/320) | 0.85 (0.61 to 1.17) | ||||||
| Breast | 35 (357/1009) | 1.00 | 35 (303/860) | 1.00 | ||||||
| Age, years | ||||||||||
| 45-60 | 33 (242/741) | .537 | 0.89 (0.65 to 1.21) | 34 (214/625) | .802 | 0.86 (0.58 to 1.27) | ||||
| 60-75 | 32 (247/771) | 0.86 (0.65 to 1.15) | 34 (227/663) | 0.86 (0.61 to 1.21) | ||||||
| ≥ 75 | 35 (103/293) | 0.99 (0.68 to 1.45) | 32 (76/237) | 0.78 (0.48 to 1.26) | ||||||
| < 45 | 35 (78/221) | 1.00 | 38 (69/183) | 1.00 | ||||||
| Sex | ||||||||||
| Female | 33 (471/1430) | .880 | 0.98 (0.75 to 1.28) | 35 (419/1209) | .704 | 1.05 (0.80 to 1.39) | ||||
| Male | 33 (199/596) | 1.00 | 34 (167/499) | 1.00 | ||||||
| ECOG PS* | ||||||||||
| 1 | 33 (263/807) | .001 | 0.86 (0.62 to 1.18) | .012 | 0.93 (0.68 to 1.26) | 36 (232/653) | < .001 | 0.97 (0.69 to 1.36) | .004 | 1.16 (0.83 to 1.62) |
| ≥ 2 | 20 (40/197) | 0.45 (0.31 to 0.67) | 0.55 (0.36 to 0.83) | 18 (28/158) | 0.38 (0.25 to 0.57) | 0.45 (0.27 to 0.73) | ||||
| 0 | 36 (364/1011) | 1.00 | 1.00 | 36 (322/886) | 1.00 | 1.00 | ||||
| Race/ethnicity | ||||||||||
| White and non-Hispanic | 29 (411/1440) | .010 | 0.38 (0.24 to 0.61) | .002 | 0.51 (0.37 to 0.70) | 30 (371/1229) | .018 | 0.41 (0.25 to 0.68) | .001 | 0.50 (0.35 to 0.70) |
| Minority | 51 (221/434) | 1.00 | 1.00 | 511 (180/352) | 1.00 | 1.00 | ||||
| Disease stage and treatment | ||||||||||
| Advanced stage and currently treated | 24 (195/800) | .001 | 0.39 (0.29 to 0.53) | .002 | 0.40 (0.29 to 0.54) | 26 (179/685) | .002 | 0.41 (0.30 to 0.58) | .005 | 0.41 (0.30 to 0.57) |
| Advanced stage and not currently treated | 309 (29/97) | 0.52 (0.33 to 0.81) | 0.55 (0.34 to 0.87) | 30 (24/80) | 0.50 (0.28 to 0.91) | 0.60 (0.34 to 1.09) | ||||
| Nonadvanced stage and currently treated | 37 (285/774) | 0.71 (0.56 to 0.89) | 0.69 (0.55 to 0.88) | 38 (256/666) | 0.73 (0.56 to 0.95) | 0.68 (0.52 to 0.90) | ||||
| Nonadvanced stage and not currently treated | 45 (157/348) | 1.00 | 1.00 | 46 (124/269) | 1.00 | 1.00 | ||||
| Type of site | ||||||||||
| Minority based | 49 (235/485) | .019 | 2.39 (1.41 to 4.04) | .026 | 1.64 (1.10 to 2.44) | 48 (196/412) | .037 | 2.11 (1.22 to 3.64) | .059 | 1.53 (1.00 to 2.33) |
| Majority based | 28 (435/1541) | 1.00 | 1.00 | 30 (390/1296) | 1.00 | 1.00 | ||||
| rESS risk status† | ||||||||||
| Poor risk | 32 (378/1183) | .309 | 0.88 (0.69 to 1.12) | 33 (297/900) | .403 | 0.88 (0.66 to 1.18) | ||||
| Good risk | 35 (287/826) | 1.00 | 36 (238/665) | 1.00 | ||||||
| Pain mechanism | ||||||||||
| Nociceptive | 29 (249/857) | .073 | 0.74 (0.53 to 1.01) | 31 (214/690) | .277 | 0.74 (0.53 to 1.05) | ||||
| Neuropathic | 35 (87/246) | 0.98 (0.74 to 1.30) | 33 (62/188) | 0.81 (0.57 to 1.16) | ||||||
| Insufficient information to classify | 48 (14/29) | 1.68 (0.71 to 3.97) | 42 (8/19) | 1.20 (0.40 to 3.61) | ||||||
| No pain syndrome | 36 (314/878) | 1.00 | 38 (252/668) | 1.00 | ||||||
| Incidental pain | ||||||||||
| Presence of incidental pain | 32 (242/769) | .579 | 0.89 (0.68 to 1.16) | 33 (201/606) | .641 | 0.94 (0.66 to 1.33) | ||||
| Insufficient information to classify | 35 (24/68) | 1.06 (0.55 to 2.04) | 41 (17/42) | 1.29 (0.71 to 2.32) | ||||||
| Absence of incidental pain | 34 (400/1174) | 1.00 | 35 (321/928) | 1.00 | ||||||
| Distress/addiction | ||||||||||
| Psych distress alone present | 31 (193/618) | .314 | 0.87 (0.67 to 1.13) | 34 (149/442) | .133 | 0.96 (0.71 to 1.29) | ||||
| Addiction alone present | 43 (9/21) | 1.44 (0.65 to 3.20) | 33 (5/15) | 0.94 (0.30 to 2.92) | ||||||
| Both present | 23 (10/43) | 0.58 (0.29 to 1.16) | 11 (3/28) | 0.23 (0.07 to 0.74) | ||||||
| Insufficient information to classify | 32 (11/34) | 0.92 (0.37 to 2.27) | 45 (21/47) | 1.52 (0.78 to 2.99) | ||||||
| Neither present | 34 (445/1300) | 1.00 | 35 (362/1045) | 1.00 | ||||||
| Cognitive function | ||||||||||
| Partial impairment | 33 (28/84) | .999 | 1.01 (0.57 to 1.81) | 30 (24/80) | .577 | 0.82 (0.52 to 1.30) | ||||
| Insufficient information to classify | 33 (2/6) | 1.01 (0.16 to 6.53) | 43 (3/7) | 1.43 (0.45 to 4.56) | ||||||
| No impairment | 33 (638/1927) | 1.00 | 34 (512/1490) | 1.00 | ||||||
| Clinician-judged difficulties attributed to cancer | ||||||||||
| The other choices | 28 (242/853) | .007 | 0.69 (0.53 to 0.89) | 31 (180/587) | .057 | 0.78 (0.61 to 1.00) | ||||
| Not at all/a little bit | 37 (424/1162) | 1.00 | 36 (358/991) | 1.00 | ||||||
| Clinician-judged difficulties attributed to cancer treatment | ||||||||||
| The other choices | 32 (288/900) | .494 | 0.92 (0.72 to 1.17) | 36 (244/678) | .155 | 1.16 (0.95 to 1.42) | ||||
| Not at all/a little bit | 34 (377/1114) | 1.00 | 33 (293/898) | 1.00 | ||||||
| Clinician-judged pain as top 1 area | ||||||||||
| Yes | 29 (169/579) | .041 | 0.78 (0.62 to 0.98) | 31 (145/472) | .119 | 0.80 (0.62 to 1.03) | ||||
| No | 35 (499/1440) | 36 (394/1108) | ||||||||
| Clinician-judged pain as top 3 areas | ||||||||||
| Yes | 30 (284/943) | .092 | 0.78 (0.58 to 1.03) | 31 (239/778) | .042 | 0.78 (0.58 to 1.03) | .067 | 0.77 (0.59, 0.99) | ||
| No | 36 (384/1076) | 37 (300/802) | ||||||||
| Clinician-judged financial problems as top 1 area | ||||||||||
| Yes | 40 (10/25) | .498 | 1.35 (0.58 to 3.14) | 42 (8/19) | .546 | 1.41 (0.48 to 4.11) | ||||
| No | 33 (658/1994) | 34 (531/1561) | ||||||||
| Clinician-judged financial problems as top 3 areas | ||||||||||
| Yes | 36 (47/130) | .387 | 1.16 (0.85 to 1.57) | 40 (36/89) | .201 | 1.33 (0.90 to 1.99) | ||||
| No | 33 (621/1889) | 34 (503/1491) | ||||||||
| Pain discrepancy‡ | ||||||||||
| Yes | 35 (270/780) | .252 | 1.12 (0.93 to 1.35) | 31 (188/599) | .162 | 0.82 (0.63 to 1.07) | ||||
| No | 32 (398/1239) | 36 (351/981) | ||||||||
NOTE. Undertreatment is being modeled in the analysis model. The level bolded in each predictor is the reference group in the logistic regression model.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; OR, odds ratio; rESS, revised Edmonton Staging System.
ECOG PS, where a level of 0 indicates full activity, without restriction. Higher levels indicate greater impairment in function.
rESS refers to the revised Edmonton Staging System for pain. Poor–risk patients have at least one high-risk indicator (neuropathic pain, incident pain, psychological distress or addictive behavior, or cognitive impairment).
Pain discrepancy refers to mismatch between the clinician and patient regarding pain perception. If the patient assigned a score ≥ 5 (moderate/severe) to the pain item at initial assessment and the clinician ranked pain as the top 3 areas or the patient assigned a score < 5 (not present/mild) to the pain item and the clinician did not rank pain as the top 3 areas, no discrepancy was noted.
Multivariable analysis revealed that across registering institutions (minority-based or majority-based), the odds of a non-Hispanic white patient having inadequate pain treatment at both the initial assessment and follow-up were approximately half those of a minority patient after adjusting for other explanatory variables (OR, 0.51; 95% CI, 0.37 to 0.70; P = .002). There were no significant pairwise interactions in the final model.
Minority patients and patients with less care complexity (less weight loss, analgesic use, pain severity, lower stage, and fewer metastatic sites) were more likely than their counterparts to have incomplete follow-up data at the second time-point for the PMI (Appendix Table A1, online only).
DISCUSSION
The present study shows that in the United States, pain is as prevalent in ambulatory oncology patients with common solid tumors as it was more than 20 years ago, despite the fact that opioid prescribing in the United States has increased more than 10-fold since 1990.11 It is appropriate that a recent Institute of Medicine report has called for coordinated, national efforts to create a cultural transformation in the way the nation understands and approaches pain management and prevention.12 In the present study, two thirds of the patients who were determined to be at risk of pain reported having pain or receiving analgesic treatment, and one third of the patients who had pain or used analgesics received inadequate treatment for their pain. Furthermore, we found that the odds of inadequate analgesic prescribing are twice as high for minority patients compared with non-Hispanic white patients.
Pain control remains a serious issue in patients with cancer throughout the world, as rates of undertreatment have also been reported in studies from industrialized nations such as Canada and some European countries.13–15 That the magnitude and scope of pain treatment inadequacy has not decreased substantially in the past two decades in the United States despite a long-standing awareness of this problem is surprising. In the early 1990s, nearly 900 ECOG clinicians were surveyed about pain treatment barriers; approximately 50% of respondents believed their patients had good pain control, and a number of the surveyed clinicians cited concerns about pain assessment, opioid adverse effects, and the reluctance of patients to report pain and take medications.16 Several observational and survey-based studies from various oncology care settings in the United States and Europe17–19 have since confirmed the results of the earlier ECOG survey, namely, that pain is not a primary concern for many patients and that patients and clinicians have misgivings about the adverse effects of pain medications; the costs associated with opioids and the medications used to mitigate their adverse effects; and the perceived dangers of driving, operating machinery, or caring for children while using certain pain medications.
In contrast to the 1994 ECOG pain study,2 the present study did not reveal age and sex to be significant factors for pain treatment adequacy. Our analysis also included individual race and ethnicity variables, as well as follow-up data (data that were not collected in the earlier ECOG pain study). The present study corroborates others' findings about the inequality of pain treatment adequacy between minority patients and non-Hispanic white patients and shows that these findings persist at short-term follow-up. This finding of pain treatment disparity has also been observed across a variety of noncancer settings.20,21 Minority patient factors, such as beliefs about the value of stoicism, concerns about opioid addiction and adverse effects, and reluctance to report pain or request analgesics putatively influenced this disparity.21–23 Some studies have suggested that communication difficulties between non-Hispanic white physicians and minority patients are common and may lower mutual trust and thus quality of care.22,24–26 When communication and trust between minority patients and their physicians are problematic, concerns about opioid-associated deaths, opioid diversion problems, and recreational opioid use may exacerbate disparities in pain treatment adequacy. Of note, the observation that white patients were also more likely to be undertreated at minority sites suggests that system factors (eg, opioid availability) could also be contributing to the disparity.
The complexity of care and symptom burden that patients with cancer experience throughout the trajectory of their care pose particular concerns. In the present study, 40% of patients seen in outpatient oncology settings at any point in their illness had at least three concurrent moderate or severe symptoms. Cancer survivors, like patients actively being treated for their disease, have complex and often unmet needs, and pain assessment and treatment are poorly understood in this population.27 Patients with nonadvanced cancer who were not receiving cancer-directed treatment were especially likely to be undertreated for pain. This disparity may be explained in part by the fact that nearly 50% of these patients experienced symptoms that oncologists believed were not attributable to cancer or cancer therapy and thus were not treated aggressively. This potential gap in pain treatment could be bridged with improved coordination of care between oncologists and nononcology providers. For example, Temel et al28 described the benefits of the early integration of palliative care specialists for patients with lung cancer receiving initial chemotherapy, and the Indiana Cancer Pain and Depression trial29 demonstrated the value of symptom management collaboration between oncologists and other providers. Finally, the availability of effective pain medication that is not perceived to interfere with driving, work performance, social interactions, or bowel habits could improve adherence to pain treatment.
This study is the largest prospective evaluation of pain and other symptoms in outpatient oncology in the United States. The distribution of the four solid tumors is typical for outpatient cancer care, including the low relative proportion of patients with prostate cancer. This study had several potential limitations. First, these findings can be generalized to patients with common solid tumors who receive care at sites associated with a US clinical cooperative group, yet a significant number of ambulatory patients with cancer have less common solid tumors or hematologic malignancies and/or receive care at sites outside the cooperative group system. In addition, we did not collect data on patients' comorbidities, insurance status, or socioeconomic status or clinicians' attributes (eg, age, race, and sex); these factors may influence pain management practice. Also, 28% of the patients in the present study did not have complete PMI data at follow-up for treatment adequacy assessment. These data were not missing at random, as these patients tended to be healthier and were likely to be minority patients or patients enrolled at minority-based sites. This pattern of missing data is itself a unique observation with potential utility for planning future research. Yet the patterns of pain expression and analgesic prescribing at the initial assessment and follow-up as revealed by multivariable analysis were remarkably similar. Finally, although the PMI is the best available and most widely used instrument to measure pain treatment adequacy, it remains only a gross indicator of pain treatment adequacy because it focuses on opioid analgesic prescribing categories and does not reflect the dosing of opioids or use of nonopioid pain interventions.
In conclusion, pain remains a significant concern in ambulatory oncology. Non-Hispanic white patients and patients with the most obvious burden of illness are most likely to receive adequate cancer pain management. Innovative pain treatments and refined measures of pain management adequacy as well as the better integration of nononcology clinical resources into the oncology setting all hold promise for improving outcomes in outpatient care of patients with cancer. Improved communication between providers and all patients about pain and pain treatment holds promise to help formulate the most appropriate patient-centered treatment goals and maximize health outcomes.
Acknowledgment
We thank Joseph Munch, Kate Newberry, and Joann Aaron for editorial assistance.
Appendix
Lead physicians at participating sites: Joseph A. Sparano, MD, Albert Einstein College of Medicine; Bruce J. Averbrook, MD, FACS, Case Western-MetroHealth Medical Center; Al B. Benson III, MD, FACP, Northwestern University; James Lockhart, MD, St Francis Hospital: Oklahoma CCOP; Jim Cleary, MD, University of Wisconson; Matthias Weiss, MD, PhD, Marshfield Clinic; Paul Gilman, MD, Lankenau Hospital: Main Line Health CCOP; Eduardo Pajon, MD, West Michigan Cancer Center: Kalamazoo CCOP; Raymond Lord, MD, West Michigan Cancer Center: Kalamazoo CCOP; J. Philip Kuebler, MD, PhD, Columbus CCOP; Stephen Grubbs, MD, Christiana Care CCOP; James Wade, MD, Decatur Memorial Hospital: Central Illinois CCOP; Robert Veith, MD, Louisiana State University Medical Center MBCCOP; Jyotsna Fuloria, MD, Ochsner Clinic; Janice Dutcher, MD, OLM Cancer Center MBCCOP; Jose Bufill, MD, Memorial Hospital of South Bend: Northern Indiana CRC CCOP; Fa-Chyi Lee, MD, University of New Mexico MBCCOP; Gerald Bayer, MD, St Vincent Hosp Regional Cancer Center CCOP; Witold B. Rybka, MD, Penn State Cancer Institute; Thomas Lad, John H. Stroger Hospital of Cook County MBCCOP; Luis Baez-Diaz, MD, FACP, San Juan Minority Based CCOP; David L. Grinblat, MD, Evanston CCOP-NorthShore University; Martin Wiesenfeld, MD, Cedar Rapids Oncology Associates; Daniel Nikcevich, MD, PhD, Duluth Clinic; Robert Behrens, MD, Iowa Oncology Research Assoc.: Iowa CCOP; John Kugler, MD, Illinois Oncology Research Associates CCOP; Shaker Dakhil, MD, FACP, Wichita CCOP; Rex Mowat, MD, Toledo Community Hosp Oncology Program CCOP; Philip Stella, MD, Michigan Cancer Research Consortium CCOP; Gamini Soori, MD, MBA, FACP, FRCP, CPE, Missouri Valley Cancer Consortium; Miroslaw Mazurczak, MD, Sanford Cancer Center: Sioux Community CCOP; Steven N. Wolff, MD, Meharry Medical College; Anand Jillella, MD, Georgia Health Sciences University: Medical College of Georgia MBCCOP; David J. Peace, MD, University of Illinois at Chicago MBCCOP; Lucile Adams Campbell, MD, Howard University Cancer Center; Marianne N. Prout, MD, MPH, Boston University Schools of Medicine & Public Health; Carlos Vallejos, MD, Grupo de Estudios Clinical Oncology del Peru.
Table A1.
Patient Demographics and Disease Characteristics by PMI Data Completeness (N = 2,026)
| Characteristic | PMI Data Completeness |
Total |
P* | ||||
|---|---|---|---|---|---|---|---|
| No (n = 569) |
Yes (n = 1,457) |
||||||
| No. | % | No. | % | No. | % | ||
| Time from diagnosis, months† | |||||||
| Median | 16 | 15 | 16 | ||||
| Range | 0-368 | 0-627 | 0-627 | ||||
| Age, years | |||||||
| Median | 61 | 60 | 60 | ||||
| Range | 24-90 | 18-93 | 18-93 | ||||
| < 45 | 63 | 11 | 158 | 11 | 221 | 11 | |
| 45-60 | 211 | 37 | 530 | 36 | 741 | 37 | |
| 60-75 | 202 | 36 | 569 | 39 | 771 | 38 | |
| ≥ 75 | 93 | 16 | 200 | 14 | 293 | 14 | |
| Disease site | |||||||
| Breast | 277 | 49 | 732 | 50 | 1,009 | 50 | |
| Colorectal | 116 | 20 | 311 | 22 | 427 | 21 | |
| Prostate | 71 | 13 | 134 | 9 | 205 | 10 | |
| Lung | 105 | 18 | 280 | 19 | 385 | 19 | |
| Sex | |||||||
| Male | 174 | 31 | 422 | 29 | 596 | 29 | |
| Female | 395 | 69 | 1,035 | 71 | 1,430 | 71 | |
| Race/ethnicity | .0225 | ||||||
| Minority | 142 | 27 | 292 | 22 | 434 | 23 | |
| White and non-Hispanic | 390 | 73 | 1,050 | 78 | 1,440 | 77 | |
| Unknown | 37 | — | 115 | — | 152 | — | |
| ECOG PS‡ | |||||||
| 0 | 270 | 48 | 741 | 51 | 1,011 | 50 | |
| 1 | 247 | 43 | 560 | 39 | 807 | 40 | |
| ≥ 2 | 51 | 9 | 146 | 10 | 197 | 10 | |
| 999 | 1 | — | 10 | — | 11 | — | |
| Weight loss | .0011 | ||||||
| < 5% | 490 | 88 | 1,174 | 81 | 1,664 | 83 | |
| ≥ 5% | 70 | 12 | 268 | 19 | 338 | 17 | |
| Unknown | 9 | — | 15 | — | 24 | — | |
| Analgesic score at baseline | < .0001 | ||||||
| No analgesic | 226 | 40 | 269 | 19 | 495 | 25 | |
| Nonopioid | 167 | 29 | 530 | 36 | 697 | 34 | |
| Weak opioid | 84 | 15 | 346 | 24 | 430 | 21 | |
| Strong opioid | 92 | 16 | 312 | 21 | 404 | 20 | |
| Pain score at baseline | .0126 | ||||||
| No pain | 147 | 26 | 442 | 30 | 589 | 29 | |
| Mild pain (1-4) | 269 | 47 | 584 | 40 | 853 | 42 | |
| Moderate pain (5-6) | 78 | 14 | 191 | 13 | 269 | 13 | |
| Severe pain (7-10) | 75 | 13 | 240 | 17 | 315 | 16 | |
| Current stage of disease | .0010 | ||||||
| No evidence of disease | 236 | 41 | 518 | 36 | 754 | 37 | |
| Locoregional | 117 | 21 | 251 | 17 | 368 | 18 | |
| Metastatic | 166 | 29 | 560 | 39 | 726 | 36 | |
| Locoregional and metastatic | 49 | 9 | 122 | 8 | 171 | 9 | |
| Unknown | 1 | — | 6 | — | 7 | — | |
| Currently receiving cancer treatment | < .0001 | ||||||
| No | 160 | 28 | 288 | 20 | 448 | 22 | |
| Yes | 409 | 72 | 1,169 | 80 | 1,578 | 78 | |
| Metastatic sites | .0014 | ||||||
| No sites of metastatic disease | 339 | 60 | 741 | 51 | 1,080 | 53 | |
| Single site | 124 | 22 | 363 | 25 | 487 | 24 | |
| Multiple sites | 106 | 18 | 353 | 24 | 459 | 23 | |
| Advanced stage and currently treated | 189 | 33 | 611 | 42 | 800 | 40 | |
| Advanced stage and not currently treated | 26 | 5 | 71 | 5 | 97 | 5 | |
| Nonadvanced stage and currently treated | 220 | 39 | 554 | 38 | 774 | 38 | |
| Nonadvanced stage and not currently treated | 133 | 23 | 215 | 15 | 348 | 17 | |
| Unknown | 1 | — | 6 | — | 7 | — | |
| Institution type | < .0001 | ||||||
| Academic | 102 | 18 | 100 | 7 | 202 | 10 | |
| Community | 467 | 82 | 1,357 | 93 | 1,824 | 90 | |
| Clinic practice type | .0393 | ||||||
| Majority based | 415 | 73 | 1,126 | 77 | 1,541 | 76 | |
| Minority based | 154 | 27 | 331 | 23 | 485 | 24 | |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PMI, pain management index.
This P value reported was from a χ2 test, not adjusted for multiple comparisons.
n = 556 for patients without PMI complete data; n = 1,431 for patients with PMI complete data.
ECOG PS, where a level of 0 indicates full activity, without restriction. Higher levels indicate greater impairment in function.
Footnotes
See accompanying editorial on page 1907 and article on page 1974; listen to the podcast by Dr Von Roenn at www.jco.org/podcasts
Supported in part by Public Health Service Grants No. CA37604, CA23318, CA026582, and CA17145 and grants from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services.
Presented in part at the 47th Annul Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. M.J.F. had full access to all of the data in the study and made the final decision to submit them for publication.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00303914.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Michael J. Fisch, Lynne I. Wagner, David Cella, Judith B. Manola, Lori M. Minasian, Tito R. Mendoza, Charles S. Cleeland
Administrative support: Judith B. Manola
Collection and assembly of data: Matthias Weiss, Lynne I. Wagner
Data analysis and interpretation: Michael J. Fisch, Ju-Whei Lee, Matthias Weiss, Lynne I. Wagner, Victor T. Chang, David Cella, Lori M. Minasian, Worta McCaskill-Stevens
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 2.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 3.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer—Pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst Monogr. 2004:9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 4.Eastern Cooperative Oncology Group: SOAPP (Symptom Outcomes and Practice Patterns): A Survey of Disease and Treatment-Related Symptoms in Patients With Invasive Cancer of the Breast, Prostate, Lung, or Colon/Rectum. http://www.ecogsoapp.com.
- 5.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Zech DF, Grond S, Lynch J, et al. Validation of the World Health Organization guidelines for cancer pain relief: A 10-year prospective study. Pain. 1995;63:65–76. doi: 10.1016/0304-3959(95)00017-M. [DOI] [PubMed] [Google Scholar]
- 7.de Wit R, van Dam F, Abu-Saad HH, et al. Empirical comparison of commonly used measures to evaluate pain treatment in cancer patients with chronic pain. J Clin Oncol. 1999;17:1280–1287. doi: 10.1200/JCO.1999.17.4.1280. [DOI] [PubMed] [Google Scholar]
- 8.Deandrea S, Montanari M, Moja L, et al. Prevalence of undertreatment in cancer pain: A review of published literature. Ann Oncol. 2008;19:1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventafridda V, Tamburini M, Caraceni A, et al. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850–856. doi: 10.1002/1097-0142(19870215)59:4<850::aid-cncr2820590432>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Fainsinger RL, Nekolaichuk CL, Lawlor PG, et al. A multicenter study of the revised Edmonton Staging System for classifying cancer pain in advanced cancer patients. J Pain Symptom Manage. 2005;29:224–237. doi: 10.1016/j.jpainsymman.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 12.Relieving Pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. p. 350. [PubMed] [Google Scholar]
- 13.Apolone G, Corli O, Caraceni A, et al. Pattern and quality of care of cancer pain management: Results from the Cancer Pain Outcome Research Study Group. Br J Cancer. 2009;100:1566–1574. doi: 10.1038/sj.bjc.6605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breivik H, Cherny N, Collett B, et al. Cancer-related pain: A pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433. doi: 10.1093/annonc/mdp001. [DOI] [PubMed] [Google Scholar]
- 15.Mitera G, Fairchild A, DeAngelis C, et al. A multicenter assessment of the adequacy of cancer pain treatment using the pain management index. J Palliat Med. 2010;13:589–593. doi: 10.1089/jpm.2009.0342. [DOI] [PubMed] [Google Scholar]
- 16.Von Roenn JH, Cleeland CS, Gonin R, et al. Physician attitudes and practice in cancer pain management: A survey from the Eastern Cooperative Oncology Group. Ann Intern Med. 1993;119:121–126. doi: 10.7326/0003-4819-119-2-199307150-00005. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS, Janjan N, Scott CB, et al. Cancer pain management by radiotherapists: A survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47:203–208. doi: 10.1016/s0360-3016(99)00276-x. [DOI] [PubMed] [Google Scholar]
- 18.Sun V, Borneman T, Piper B, et al. Barriers to pain assessment and management in cancer survivorship. J Cancer Surviv. 2008;2:65–71. doi: 10.1007/s11764-008-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein SM, Romanus D, Lepisto EM, et al. Documentation of pain in comprehensive cancer centers in the United States: A preliminary analysis. J Natl Compr Canc Netw. 2004;2:173–180. doi: 10.6004/jnccn.2004.0015. [DOI] [PubMed] [Google Scholar]
- 20.Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537–1539. [PubMed] [Google Scholar]
- 21.Anderson KO, Richman SP, Hurley J, et al. Cancer pain management among underserved minority outpatients: Perceived needs and barriers to optimal control. Cancer. 2002;94:2295–2304. doi: 10.1002/cncr.10414. [DOI] [PubMed] [Google Scholar]
- 22.Im EO, Guevara E, Chee W. The pain experience of Hispanic patients with cancer in the United States. Oncol Nurs Forum. 2007;34:861–868. doi: 10.1188/07.ONF.861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surbone A. Cultural competence in oncology: Where do we stand? Ann Oncol. 2010;21:3–5. doi: 10.1093/annonc/mdp546. [DOI] [PubMed] [Google Scholar]
- 24.Gordon HS, Street RL, Jr, Sharf BF, et al. Racial differences in trust and lung cancer patients' perceptions of physician communication. J Clin Oncol. 2006;24:904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 25.Gordon HS, Street RL, Jr, Sharf BF, et al. Racial differences in doctors' information-giving and patients' participation. Cancer. 2006;107:1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 26.Street RL, Jr, Gordon H, Haidet P. Physicians' communication and perceptions of patients: Is it how they look, how they talk, or is it just the doctor? Soc Sci Med. 2007;65:586–598. doi: 10.1016/j.socscimed.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton AW, Fanciullo GJ, Beasley RD, et al. Chronic pain in the cancer survivor: A new frontier. Pain Med. 2007;8:189–198. doi: 10.1111/j.1526-4637.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 28.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Zhong X, Theobald D, et al. Somatic symptoms in patients with cancer experiencing pain or depression: Prevalence, disability, and health care use. Arch Intern Med. 2010;170:1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]