Abstract
Purpose
Treatment outcome for black patients with cancer has been significantly worse than for their white counterparts. We determined whether recent improved treatment had narrowed the gap in outcome between black and white pediatric patients.
Patients and Methods
In a parallel comparison, we analyzed survival by disease category between black and white patients with childhood cancer registered in one of the 17 cancer registries of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program or treated at St Jude Children's Research Hospital, which provides comprehensive treatment to all patients regardless of their ability to pay, from 1992 to 2000 and from 2001 to 2007.
Results
Analysis of the SEER data indicated that in both study periods, black patients had significantly poorer rates of survival than did white patients, with the exception of a few types of cancer. Despite significantly improved treatment outcomes for patients who were treated from 2001 to 2007, the racial difference in survival has actually widened for acute myeloid leukemia and neuroblastoma. By contrast, in the cohorts treated at St Jude Children's Research Hospital, there were no significant differences in survival between black and white patients in either study period, regardless of the cancer type. Importantly, the outcome of treatment for acute lymphoblastic leukemia, acute myeloid leukemia, and retinoblastoma has improved in parallel for both races during the most recent study period.
Conclusion
With equal access to comprehensive treatment, black and white children with cancer can achieve the same high cure rates.
INTRODUCTION
Disparities in treatment and survival between black and white patients with cancer in the United States are well recognized. Despite improved overall survival rates in recent years for many cancers, such as prostate, bladder, renal cell, and endometrial cancers, as well as brain tumors, multiple myeloma, and leukemia,1 black adult patients with cancer continue to fare worse than their white counterparts.2–9 This racial disparity in survival has been variously attributed to biologic, socioeconomic, and cultural, as well as host and treatment factors. Data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program showed significantly improved 5- and 10-year cancer survival rates over three successive periods between 1975 and 1999 for black and white children up to 19 years of age.10 However, black children continued to have lower survival rates than white children, notably those with acute lymphoblastic leukemia (ALL), astrocytoma, and osteosarcoma. Two other studies based on SEER data demonstrated worse survival rates for black children compared with white children with ALL diagnosed between 1973 and 1999,11 or with germ cell tumors, hepatoblastoma, or nonrhabdomyosarcoma soft tissue sarcoma diagnosed between 1985 and 2005.12 The Children's Oncology Group (COG) also reported inferior survival for black compared with white children with acute myeloid leukemia diagnosed between 1989 and 200213 or neuroblastoma diagnosed between 2001 and 2009.14 By contrast, in our single-institution studies, we found no significant difference in survival between black and white children with any type of cancer diagnosed between the late 1970s and early 2000s, with the exception of osteosarcoma.15–17 In fact, we demonstrated that the improved treatment available in the 1990s was equally beneficial to black and white children with ALL.18
With the recent substantial improvement in survival among children with cancer, as a result of coordinated multidisciplinary protocols developed by COG and several single institutions,19–21 we asked whether this gain had narrowed the gap in outcome between black and white children treated for cancer in the United States. By using data from the SEER program, we show continued disparities in clinical outcome according to race, but these differences were not apparent in the analysis of patients treated at a single pediatric cancer center over the same 15-year period. This suggests that equal access to effective anticancer therapy and ancillary care is key to ensuring consistent treatment results for black and white patients with childhood cancer.
PATIENTS AND METHODS
Study Population and Setting
Our earlier report focused on patients diagnosed with multiple types of cancer at St Jude Children's Research Hospital (SJCRH) between 1962 and 1992.15 Here, we included patients with 19 different histologic types of cancer diagnosed between 1992 and 2007, enabling us to analyze results for two approximately equal treatment periods with adequate follow-up for both. This study was approved by the institutional review board at SJCRH. To examine parallel survival trends in the United States, we used data collected by all 17 cancer registries participating in the SEER program—Connecticut, New Jersey, Kentucky, Louisiana, Atlanta, Rural Georgia, Detroit, Iowa, Hawaii, New Mexico, Seattle-Puget Sound, Utah, San Francisco-Oakland, San Jose-Monterey, Los Angeles, Greater California, and Alaska. According to the U.S. Census Bureau Census 2000, the population covered by these 17 registries represented 26.2% of the total US population, including 23.4% of whites and 22.7% of blacks.1
SJCRH is a nonprofit tertiary care cancer center that draws patients from across the United States, with most of them (82%) from a 10-state region (Tennessee, Mississippi, Louisiana, Illinois, Arkansas, Missouri, Kentucky, Alabama, Florida, and Georgia). Patients are accepted for treatment without regard to race, ethnicity, insurance status, or financial status. All costs of treatment not covered by third-party payment (if any) are absorbed by the hospital; there are no direct charges to families. The hospital also routinely provides extensive psychosocial services, including subsidizing costs related to transportation, meals, and lodging for children and their families who must travel to Memphis to receive treatment. Long-term follow-up is a major research emphasis. Survivors who have been in remission for 5 years or more are evaluated annually in our After Completion of Therapy Clinic for monitoring and treatment of late sequelae. The Cancer Registry continues with annual follow-up of alumni survivors 18 years of age or older who have been in remission for at least 10 years and are discharged to the primary care of community physicians. Since 2007, St Jude alumni survivors have also been eligible to return for periodic cancer-related risk-based consultations as part of the St Jude Lifetime Cohort study.
Common cancers and subtypes were grouped according to the histology and typography codes in the International Classification of Diseases for Oncology, third edition (ICD-O-3), with only minor modifications.22 We classified brain tumors into five categories: astrocytoma, including dysembryoplastic neuroepithelial tumor, ganglioglioma, glioma not otherwise specified, oligoastrocytoma, and oligodendroglioma; ependymoma; high-grade glioma, including anaplastic astrocytoma, anaplastic oligoastrocytoma, anaplastic oligodendroglioma, brainstem glioma, and glioblastoma multiforme; medulloblastoma; and other specified and unspecified CNS tumors, including primitive neuroectodermal and germ cell tumors. We considered rhabdomyosarcomas to be distinct from other soft-tissue sarcomas and classified intracranial and intraspinal germ cell tumors with brain tumors.
Statistical Analysis
Patients were included in the analyses if their self-declared race was black/African American or white/Caucasian. Survival was measured from the date of the initial diagnosis of cancer to the date of death from any cause or to the date of last contact. For the analysis of survival trends, we selected two time periods (1992 to 2000 and 2001 to 2007) that accommodated recent improvements in treatment and allowed sufficient follow-up for reliable estimates of outcome. Survival distributions were estimated by the Kaplan and Meier method, and associated SEs by the method of Peto et al23; 95% CIs of 5-year survival probabilities were calculated. Overall survival distributions by race and time period were compared by the Mantel-Haenszel statistic24; P values are for two-sided tests. The database frozen on September 12, 2011, was used for the analysis of patients treated at SJCRH; 76% of the survivors had been seen or contacted within 1 year and 89% within the past 2 years. The median follow-up time was 10.4 years (range, 0.3 to 19.5 years).
RESULTS
For the entire 15-year study period at SJCRH, 19.1% of the patients were blacks and 75.4% were whites (5.5% of the patients represented other races/ethnicities and were excluded) compared with 9.8% blacks and 57.6% whites in the SEER program (33.6% represented other races/ethnicities and were excluded). As expected from epidemiologic surveys,25 black children with nephroblastoma, osteosarcoma, retinoblastoma, nonrhabdomyosarcoma soft-tissue sarcoma, and rhabdomyosarcoma, were overrepresented whereas black children with ALL, brain tumors overall, hepatoblastoma, germ cell tumors, and especially melanoma and Ewing sarcoma were underpresented in the SEER program (Table 1). However, the proportions of black patients with ALL, astrocytoma, high-grade glioma, neuroblastoma, nephroblastoma, germ cell tumors, Ewing sarcoma, retinoblastoma, and melanoma were higher at SJCRH (Table 2) than in the SEER program (Table 1).
Table 1.
Comparison of Treatment Outcome by Disease Category in Black and White Children in the SEER Program
| Diagnosis | 5-Year Survival |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1992-2000 |
2001-2007 |
|||||||||||||
| No. | Black Patients(%) | 95% CI | No. | White Patients (%) | 95% CI | P | No. | Black Patients (%) | 95% CI | No. | White Patients (%) | 95% CI | P | |
| ALL | 233 | 72.8 | 67.1 to 78.5 | 1,931 | 85.9 | 84.3 to 87.5 | < .01 | 358 | 82.1 | 75.4 to 88.8 | 2,700 | 89.0 | 87.0 to 91.0 | < .01 |
| AML | 78 | 48.7 | 37.7 to 59.7 | 377 | 49.9 | 44.8 to 55.0 | .81 | 146 | 46.1 | 33.9 to 58.3 | 566 | 66.6 | 60.5 to 72.7 | < .01 |
| Hodgkin's lymphoma | 125 | 88.8 | 83.3 to 94.3 | 844 | 94.8 | 93.2 to 96.4 | .03 | 194 | 94.3 | 88.0 to 100.0 | 1,124 | 96.8 | 95.2 to 98.4 | .03 |
| NHL | 88 | 75.0 | 66.0 to 84.0 | 467 | 80.0 | 76.3 to 83.7 | .03 | 186 | 78.2 | 68.4 to 88.0 | 704 | 84.4 | 80.3 to 88.5 | .03 |
| Astrocytoma | 140 | 76.3 | 69.2 to 83.4 | 942 | 86.3 | 84.1 to 88.5 | .01 | 186 | 76.8 | 66.4 to 87.2 | 1,197 | 86.4 | 83.5 to 89.3 | < .01 |
| Ependymoma | 23 | 56.5 | 36.9 to 76.1 | 124 | 66.8 | 58.4 to 75.2 | 1.00 | 39 | 60.1 | 38.5 to 81.7 | 183 | 74.4 | 64.4 to 84.4 | .30 |
| Medulloblastoma | 30 | 76.0 | 60.7 to 91.3 | 244 | 74.4 | 68.9 to 79.9 | .64 | 40 | 62.8 | 39.3 to 86.3 | 344 | 71.6 | 64.0 to 79.2 | .08 |
| High-grade glioma | 76 | 31.6 | 21.4 to 41.8 | 277 | 50.9 | 45.0 to 56.8 | < .01 | 103 | 41.7 | 27.0 to 56.4 | 471 | 52.1 | 45.0 to 59.2 | .14 |
| Other CNS tumors | 44 | 56.8 | 42.5 to 71.1 | 218 | 57.1 | 50.4 to 63.8 | .33 | 49 | 50.7 | 32.1 to 69.3 | 297 | 65.0 | 56.8 to 73.2 | .11 |
| Neuroblastoma | 96 | 65.1 | 55.3 to 74.9 | 521 | 68.2 | 64.1 to 72.3 | .53 | 148 | 66.7 | 54.2 to 79.2 | 739 | 78.8 | 74.1 to 83.5 | < .01 |
| Nephroblastoma | 108 | 89.8 | 84.1 to 95.5 | 396 | 91.1 | 88.2 to 94.0 | .62 | 112 | 91.6 | 83.8 to 99.4 | 469 | 89.8 | 85.5 to 94.1 | .61 |
| Germ cell tumors | 86 | 84.8 | 77.2 to 92.4 | 603 | 92.3 | 90.1 to 94.5 | < .01 | 117 | 89.3 | 80.7 to 97.9 | 928 | 93.5 | 91.1 to 95.9 | .24 |
| Osteosarcoma | 63 | 57.1 | 45.1 to 69.1 | 245 | 67.3 | 61.4 to 73.2 | .05 | 120 | 67.7 | 55.9 to 79.5 | 374 | 70.5 | 63.4 to 77.6 | .44 |
| Rhabdomyosarcoma | 74 | 64.9 | 54.1 to 75.7 | 253 | 66.2 | 60.3 to 72.1 | .68 | 104 | 58.5 | 42.8 to 74.2 | 380 | 67.5 | 60.1 to 74.9 | .19 |
| NRSTS | 54 | 61.1 | 48.4 to 73.8 | 227 | 75.7 | 70.0 to 81.4 | .02 | 98 | 54.3 | 39.2 to 69.4 | 323 | 73.4 | 66.0 to 80.8 | < .01 |
| Ewing sarcoma | 7 | 71.4 | 42.0 to 100.0 | 251 | 64.4 | 58.5 to 70.3 | .90 | 13 | 57.7 | 26.3 to 89.1 | 406 | 69.9 | 63.2 to 76.6 | .09 |
| Retinoblastoma | 41 | 97.5 | 92.8 to 100.0 | 178 | 98.8 | 97.2 to 100.0 | .24 | 58 | 88.5 | 75.4 to 100.0 | 206 | 98.4 | 95.5 to 100.0 | .02 |
| Melanoma | 4 | 100.0 | 246 | 88.4 | 84.3 to 92.5 | .43 | 8 | 71.4 | 28.3 to 100.0 | 455 | 94.9 | 91.8 to 98.0 | < .01 | |
| Hepatoblastoma | 10 | 46.7 | 17.3 to 76.1 | 79 | 59.0 | 48.0 to 70.0 | .79 | 15 | 51.3 | 2.3 to 100.0 | 122 | 71.6 | 58.7 to 84.5 | .06 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin's lymphoma; NRSTS, nonrhabdomyosarcoma soft tissue sarcoma; SEER, Surveillance, Epidemiology, and End Results.
Table 2.
Comparison of Treatment Outcome by Disease Category in Black and White Children Treated at St Jude Children's Research Hospital
| Diagnosis | 5-Year Survival |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1992-2000 |
2001-2007 |
|||||||||||||
| No. | Black Patients (%) | 95% CI | No. | White Patients (%) | 95% CI | P | No. | Black Patients (%) | 95% CI | No. | White Patients (%) | 95% CI | P | |
| ALL | 82 | 81.6 | 73.2 to 90.0 | 370 | 85.7 | 82.2 to 89.2 | .58 | 76 | 89.4 | 81.4 to 97.4 | 380 | 93.2 | 90.1 to 96.3 | .41 |
| AML | 30 | 40.0 | 23.1 to 56.9 | 111 | 47.7 | 38.5 to 56.9 | .55 | 44 | 57.9 | 39.5 to 76.3 | 167 | 66.7 | 55.3 to 78.1 | .17 |
| Hodgkin's lymphoma | 38 | 94.5 | 87.2 to 100.0 | 181 | 92.0 | 88.1 to 95.9 | .53 | 42 | 87.6 | 75.1 to 100.0 | 118 | 96.3 | 92.4 to 100.0 | .06 |
| NHL | 31 | 85.9 | 73.0 to 98.8 | 126 | 78.6 | 71.5 to 85.7 | .70 | 39 | 81.5 | 66.2 to 96.8 | 81 | 81.1 | 71.5 to 90.7 | .89 |
| Astrocytoma | 53 | 86.8 | 77.4 to 96.2 | 177 | 89.8 | 85.3 to 94.3 | .92 | 26 | 100.0 | 131 | 93.3 | 88.2 to 98.4 | .16 | |
| Ependymoma | 7 | 57.1 | 25.7 to 88.5 | 65 | 81.3 | 71.7 to 90.9 | .04 | 12 | 83.3 | 59.8 to 100.0 | 62 | 79.7 | 68.3 to 91.1 | .68 |
| Medulloblastoma | 16 | 75.0 | 55.4 to 94.6 | 90 | 75.5 | 66.7 to 84.3 | .61 | 9 | 55.6 | 24.2 to 87.0 | 56 | 71.1 | 58.6 to 83.6 | .41 |
| High-grade glioma | 25 | 24.0 | 8.5 to 39.5 | 73 | 16.4 | 8.2 to 24.6 | .90 | 37 | 16.2 | 3.3 to 29.1 | 119 | 14.9 | 6.5 to 23.3 | .61 |
| Other CNS tumors | 15 | 73.3 | 53.7 to 92.9 | 48 | 70.6 | 57.3 to 83.9 | .66 | 8 | 75.0 | 39.7 to 100.0 | 73 | 48.9 | 35.2 to 62.6 | .24 |
| Neuroblastoma | 18 | 44.4 | 22.8 to 66.0 | 91 | 62.0 | 51.8 to 72.2 | .07 | 25 | 72.0 | 54.0 to 90.0 | 61 | 65.6 | 51.3 to 79.9 | .94 |
| Nephroblastoma | 50 | 92.0 | 84.6 to 99.4 | 80 | 92.4 | 86.5 to 98.3 | .55 | 24 | 100.0 | 41 | 92.7 | 83.5 to 100.0 | .19 | |
| Germ cell tumors | 16 | 100.0 | 35 | 87.9 | 76.3 to 99.5 | .12 | 9 | 83.3 | 46.1 to 100.0 | 21 | 90.0 | 75.7 to 100.0 | .84 | |
| Osteosarcoma | 20 | 60.0 | 40.4 to 79.6 | 74 | 64.2 | 53.2 to 75.2 | .73 | 20 | 75.0 | 53.4 to 96.6 | 43 | 73.6 | 58.7 to 88.5 | .98 |
| Rhabdomyosarcoma | 23 | 65.2 | 46.4 to 84.0 | 57 | 64.9 | 52.7 to 77.1 | .78 | 16 | 62.5 | 37.0 to 88.0 | 39 | 79.2 | 65.1 to 93.3 | .17 |
| NRSTS | 13 | 69.2 | 45.7 to 92.7 | 33 | 60.6 | 44.3 to 76.9 | .54 | 4 | 0.0 | 16 | 75.0 | 49.5 to 100.0 | < .01 | |
| Ewing sarcoma | 1 | 100.0 | 32 | 74.2 | 58.7 to 89.7 | —* | 5 | 60.0 | 22.8 to 97.2 | 40 | 84.1 | 70.4 to 97.8 | .09 | |
| Retinoblastoma | 12 | 83.3 | 63.7 to 100.0 | 30 | 96.7 | 90.4 to 100.0 | .03 | 29 | 100.0 | 59 | 100.0 | 1.0 | ||
| Melanoma | 1 | 100.0 | 23 | 47.8 | 28.2 to 67.4 | —* | 3 | 66.7 | 13.8 to 100.0 | 21 | 100.0 | .13 | ||
| Hepatoblastoma | 2 | 50.0 | 1.0 to 99.0 | 17 | 82.4 | 65.0 to 99.8 | —* | 1 | 0.0 | 5 | 60.0 | 9.0 to 100.0 | —* | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin's lymphoma; NRSTS, nonrhabdomyosarcoma soft tissue sarcoma.
Too few patients for statistical analysis.
Analysis of the SEER data showed significantly inferior outcomes for black patients in the vast majority of disease categories, especially in the recent study period (Table 1); there were too few patients with Ewing sarcoma, hepatoblastoma, and melanoma to draw firm conclusions for these three disease categories. These findings contrast sharply with the similar 5-year survival rates for black and white children with any cancer treated at SJCRH, with the possible exceptions of retinoblastoma and ependymoma treated during the earlier period and nonrhabdomyosarcoma soft-tissue sarcoma treated during the recent period (Table 2).
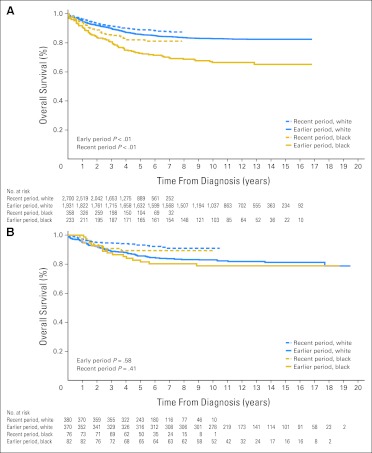
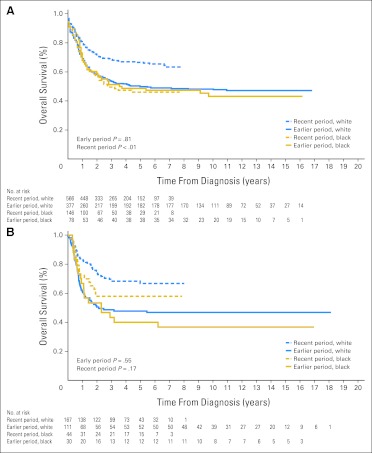
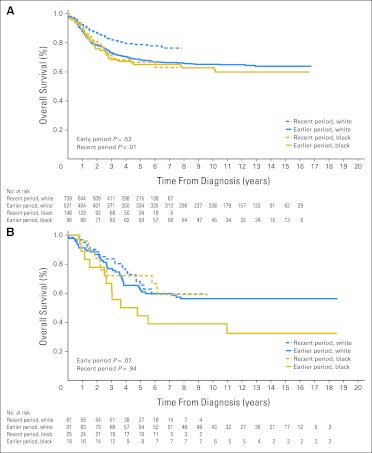
Despite significant improvement in survival rates for several types of cancer within the SEER database, including ALL, acute myeloid leukemia, Hodgkin's lymphoma, neuroblastoma, and melanoma, from the earlier to the recent period (Appendix Table A1, online only), the previous disparities in outcome generally persisted between blacks and whites (Table 1). Although the gap in survival narrowed for ALL (Fig 1A) and Hodgkin's lymphoma, it widened for acute myeloid leukemia (Fig 2A) and neuroblastoma (Fig 3A), because most of the improvement for these two cancers occurred mainly in white patients. Patients treated at SJCRH for ALL, acute myeloid leukemia, retinoblastoma, melanoma, and neuroblastoma also showed higher survival rates from the earlier to the recent period (Appendix Table A2, online only), but the result for neuroblastoma lacked statistical significance (P = .3). In contrast to the SEER results, these positive findings extended equally to black and white patients (Figs 1B, 2B, and 3B).
Fig 1.
Kaplan-Meier estimates of survival for black compared with white children with acute lymphoblastic leukemia in the earlier (1992-2000) and recent (2001-2007) study periods. Patients were either (A) registered in the SEER program or (B) treated at the St Jude Children's Research Hospital (SJCRH). Survival was worse for black children during both the earlier (P < .01) and recent periods (P < .01) in the SEER program, but there were no significant race-related differences in survival during either period at SJCRH (P = .58 and P = .41, respectively). Numbers of patients still at risk are shown beneath the graph.
Fig 2.
Kaplan-Meier estimates of survival for black compared with white children with acute myeloid leukemia in the earlier (1992-2000) and recent (2001-2007) study periods. Patients were either (A) registered in the SEER program or (B) treated at the St Jude Children's Research Hospital (SJCRH). Survival did not differ between the two races during the earlier period (P = .81) but was worse for black children during the recent period (P < .01) in the SEER program. There were no significant race-related differences in survival during either period (P = .55 and P = .17, respectively) at SJCRH. Numbers of patients still at risk are shown beneath the graph.
Fig 3.
Kaplan-Meier estimates of survival for black compared with white children with neuroblastoma in the earlier (1992-2000) and recent (2001-2007) study periods. Patients were either (A) registered in the SEER program or (B) treated at the St Jude Children's Research Hospital (SJCRH). Survival did not differ between the two races during the earlier period (P = .53) but was worse for black children during the recent period (P < .01) in the SEER program. Significant race-related differences in survival were not apparent during either the earlier (P = .07) or recent period (P = .94) at SJCRH. Numbers of patients still at risk are shown beneath the graph.
Data on the socioeconomic status of our patients' families are not available because information on income and education is not routinely requested on admission to the hospital. We therefore assessed insurance status as an indirect indicator of financial status. Among patients for whom insurance information was available, 54 (8%) of the 678 blacks as compared with 287 (11.8%) of the 2,425 whites were uninsured (P < .001). For those with insurance, the types of coverage differed significantly by race (P < .001). Black patients were more likely than white patients to be recipients of public insurance (73.2% v 37.2%) and less likely to have private insurance (26.8% v 62.9%), suggesting lower proportions of black patients in the middle and upper socioeconomic classes.
DISCUSSION
The 5-year cancer survival rate for all children 0 to 19 years old who were diagnosed between 2001 and 2007 has increased to 82.6% in the United States, as estimated by the SEER program.1 Nonetheless, we found continued disparities in survival between black and white patients registered in the SEER program, beginning in an earlier treatment period (1992 to 2000) and persisting through a recent period (2001 to 2007). This trend appears to include all childhood cancers, with the exception of nephroblastoma, germ cell tumors, osteosarcoma, rhabdomyosarcoma, and certain brain tumors (Table 1). By contrast, the same analysis applied to data from our pediatric cancer center indicated similar survival rates for black and white patients, regardless of treatment period and cancer type, possibly excluding ependymoma and retinoblastoma in the earlier period and nonrhabdomyosarcoma soft tissue sarcoma in the recent period (Table 2).
The well-recognized lower incidence of ALL, brain tumors, germ cell tumors, hepatoblastoma, and especially Ewing sarcoma and melanoma in black compared with white children25 was readily apparent in the racial distribution by diagnosis in the SEER data and in our own patient population (Tables 1 and 2). Less clear was the higher proportion of black children in certain diagnostic categories within our cohort compared with the SEER database. One explanation is that SJCRH has a different catchment area and is a tertiary referral center with a policy of accepting patients regardless of their ability to pay. Any comparison of outcome data between the SEER program and SJCRH must take into account the severity of the cancer at diagnosis. Because the acceptance policy of our institution is based mainly on protocol availability, and because many of our protocols are designed for cancers that are difficult to treat, our study population would be expected to comprise disproportionately large groups of high-risk patients. For example, with the development of the Pediatric Brain Tumor Consortium in 1999, we have not only attracted a higher proportion of patients with brain tumors compared with the SEER program (29% v 21%, P < .01) but we also have an increased referral of patients with high-grade glioma. Among the 254 patients with high-grade glioma in our institution, 109 (42.9%) had brainstem glioma and 77 (30.0%) had glioblastoma multiforme—diseases associated with a dismal prognosis—with survival rates ranging from 10% to 20%.21 The proportion of patients with either subtype of brain tumor is not apparent in the SEER program but is almost certainly lower than that of our patient population because of the relatively high 5-year survival rate. In this regard, brain and other nervous system tumors have been difficult to diagnose pathologically, especially when classified as high-grade glioma.26,27 All of our patients included in the high-grade glioma category had their histologic diagnosis confirmed at SJCRH before treatment, excluding patients with diffuse pontine glioma, for which the diagnosis was made by imaging and clinical criteria, because biopsy was not performed in these patients. The overall survival of our patients with high-grade glioma is consistent with results recently reported by the COG.28,29 Thus, direct comparison of outcome for high-grade gliomas in SEER versus SJCRH patients would not yield reliable results. Similarly, of our 66 patients with nonrhabdomyosarcoma soft tissue sarcoma, 20 (30%) presented with metastatic disease, a proportion substantially higher than that of other reported series.30,31 Of the four black patients, two presented with metastatic disease, and one each had unresectable or high-grade tumor. Hence, the poor outcome is not entirely unexpected in this small cohort of patients.
Why, then, do black and white children with cancer tend to have the same outcome when treated at SJCRH? The most straightforward explanation is that both groups receive the same effective risk-directed therapy and supportive care, which can abolish the prognostic impact of many clinical and biologic variables. This capacity has been clearly demonstrated for patients with ALL, the most common childhood cancer. Despite having a higher frequency of unfavorable prognostic features such as high leukocyte count, T-cell immunophenotype, and chromosomal translocation t(1;19), and a lower frequency of favorable hyperdiploid karyotype, the 68 black patients treated in our Total XIII studies in the 1990s fared as well as the 338 white counterparts (5-year survival rates, 86.2% [95% CI, 77.2% to 95.2%] v 85.0% [95% CI, 80.9% to 89.1%]).18 There was also a parallel improvement in outcome for the 79 black and 340 white patients with ALL enrolled in our Total Therapy XV study between 2000 and 2007, with 5-year survival rates of 88.3% ± 6.2% (SE) and 94.8% ± 2.0%, respectively.32 In this regard, our recent collaborative study with the COG showed that Native American ancestry was associated with an increased risk of relapse in ALL, but this ancestry-related relapse hazard could be abrogated with an additional course of delayed intensification therapy among patients treated on a COG protocol.33 Undoubtedly, recent improvements in the COG studies for ALL have narrowed the survival gap between black and white patients with ALL registered in the SEER program (Table 1), since most of these patients were treated on COG protocols.
There have also been notable improvements in risk-directed treatment and supportive care for patients with acute myeloid leukemia, both at our institution34,35 and in COG studies.36,37 Immunotherapy with the monoclonal antibody anti-GD2 with granulocyte-macrophage colony-stimulating factor, interleukin-2, and isotretinoin has also significantly improved the survival of patients with high-risk neuroblastoma treated in a recent COG study.38 These therapeutic gains have benefited black and white children equally at SJCRH, although among patients registered in the SEER program and treated on COG protocols, their impact has been limited almost exclusively to whites. In fact, the survival gap has widened between black and white patients with acute myeloid leukemia or neuroblastoma in the SEER program. Conceivably, this discrepancy in outcome is due to a lack of equal access to effective treatment and supportive care among some patients represented by the SEER data. For patients without sufficient insurance coverage, access to costly procedures such as stem-cell transplantation may be particularly restricted, even though it is a proven life-saving measure for refractory or relapsed leukemia, non-Hodgkin's lymphoma, and neuroblastoma. In this regard, we have demonstrated recently that white and nonwhite children with high-risk leukemia had similarly high survival rates after transplantation at our institution, where there are no barriers posed by a lack of health insurance or the availability of a matched donor.35 Whether our findings can be generalized to patients treated at other major pediatric cancer centers is unknown.
Our study has several limitations. First, the number of black patients treated at SJCRH is relatively small for certain disease categories, preventing firm conclusions for some types of cancer. Second, specific disease characteristics and follow-up observations were not available for individual patients registered in the SEER program, precluding comparisons based on stratified analyses with adjustment for known risk factors and censoring bias. Third, it is uncertain what proportion of patients in the SEER program received treatment in tertiary medical centers with a multidisciplinary team and extensive psychosocial support, a combination that generally yields superior results.39 Fourth, it is well recognized that protocol-directed therapy is the best treatment for patients with cancer. In this regard, for a variety of reasons, the proportion of children age younger than 20 years in the SEER program who were enrolled in a COG protocol has been low (57%), especially for patients 15 to 19 years of age (24%).40 At SJCRH, 66% to 72% of the patients were enrolled in a therapeutic protocol and 97% in a therapeutic or nontherapeutic protocol. Although there was no substantial difference in the protocol registration rate by race or ethnicity in the SEER program,40 the possibility that the adverse impact of failure of protocol enrollment was more pronounced in blacks cannot be excluded. Finally, the socioeconomic status of patients registered in the SEER program cannot be determined with any degree of certainty. Since this variable clearly affects the outcome of children with cancer, as has been convincingly demonstrated in studies of adults,3,5,6 future research on this topic will need to consider insurance status or ability to pay as potential confounding factors.
Acknowledgment
We thank SEER*Stat technical support for providing data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program; Dr Xueyuan Cao, Catherine Billups, and Valerie McPherson for data collection; and Julie Groff for graphics production. We are indebted to many nurses, nurse practitioners, physician assistants, pharmacists, social workers, nutritionists, and technologists for their contributions to patient care and research, and to the patients and their parents for participation in the research.
Appendix
Table A1.
Comparison of Treatment Outcome by Disease Category: SEER Program
| Diagnosis | 1992-2000 |
2001-2007 |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | 5-Year OS | 95% CI | No. | 5-Year OS | 95% CI | ||
| ALL | 2,164 | 84.5 | 82.9 to 86.1 | 3,058 | 88.2 | 86.4 to 90.0 | < .01 |
| AML | 455 | 49.7 | 45.0 to 54.4 | 712 | 62.4 | 56.9 to 67.9 | < .01 |
| Hodgkin's lymphoma | 969 | 94.0 | 92.4 to 95.6 | 1,318 | 96.4 | 94.8 to 98.0 | .01 |
| NHL | 555 | 79.2 | 75.9 to 82.5 | 890 | 83.1 | 79.2 to 87.0 | .10 |
| Astrocytoma | 1,082 | 85.0 | 82.8 to 87.2 | 1,383 | 85.1 | 82.2 to 88.0 | .58 |
| Ependymoma | 147 | 65.2 | 57.6 to 72.8 | 222 | 71.6 | 62.4 to 80.8 | .13 |
| Medulloblastoma | 274 | 74.5 | 69.2 to 79.8 | 384 | 70.6 | 63.3 to 77.9 | .39 |
| High-grade glioma | 353 | 46.7 | 41.4 to 52.0 | 574 | 50.2 | 43.9 to 56.5 | .19 |
| Other CNS tumors | 262 | 57.1 | 51.0 to 63.2 | 346 | 62.9 | 55.3 to 70.5 | .11 |
| Neuroblastoma | 617 | 67.7 | 64.0 to 71.4 | 887 | 76.8 | 72.3 to 81.3 | < .01 |
| Nephroblastoma | 504 | 90.8 | 88.3 to 93.3 | 581 | 90.1 | 86.2 to 94.0 | .76 |
| Germ cell tumors | 689 | 91.4 | 89.2 to 93.6 | 1,045 | 93.1 | 90.7 to 95.5 | .15 |
| Osteosarcoma | 308 | 65.2 | 59.9 to 70.5 | 494 | 69.8 | 63.7 to 75.9 | .22 |
| Rhabdomyosarcoma | 327 | 65.9 | 60.8 to 71.0 | 484 | 65.6 | 58.7 to 72.5 | .98 |
| NRSTS | 281 | 72.9 | 67.6 to 78.2 | 421 | 68.9 | 62.0 to 75.8 | .24 |
| Ewing sarcoma | 258 | 64.6 | 58.7 to 70.5 | 419 | 69.6 | 62.9 to 76.3 | .16 |
| Retinoblastoma | 219 | 98.6 | 97.0 to 100.0 | 264 | 96.1 | 92.2 to 100.0 | .05 |
| Melanoma | 250 | 88.6 | 84.7 to 92.5 | 463 | 94.5 | 91.2 to 97.8 | .01 |
| Hepatoblastoma | 89 | 57.9 | 47.5 to 68.3 | 137 | 69.3 | 56.4 to 82.2 | .10 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin's lymphoma; NRSTS, nonrhabdomyosarcoma soft tissue sarcoma; OS, overall survival; SEER, Surveillance, Epidemiology, and End Results.
Table A2.
Comparison of Treatment Outcome by Disease Category: St Jude Children's Research Hospital
| Diagnosis | 1992-2000 |
2001-2007 |
P | ||||
|---|---|---|---|---|---|---|---|
| No. | 5-Year OS | 95% CI | No. | 5-Year OS | 95% CI | ||
| ALL | 452 | 84.9 | 81.6 to 88.2 | 456 | 92.6 | 89.7 to 95.5 | < .01 |
| AML | 141 | 46.1 | 37.9 to 54.3 | 211 | 65.0 | 55.2 to 74.8 | < .01 |
| Hodgkin's lymphoma | 219 | 92.5 | 89.0 to 96.0 | 160 | 94.1 | 89.8 to 98.4 | .85 |
| NHL | 157 | 80.0 | 73.7 to 86.3 | 120 | 81.2 | 73.0 to 89.4 | .86 |
| Astrocytoma | 230 | 89.1 | 85.0 to 93.2 | 157 | 94.4 | 90.1 to 98.7 | .07 |
| Ependymoma | 72 | 78.9 | 69.5 to 88.3 | 74 | 80.3 | 69.7 to 90.9 | .87 |
| Medulloblastoma | 106 | 75.4 | 67.2 to 83.6 | 65 | 68.8 | 56.8 to 80.8 | .41 |
| High-grade glioma | 98 | 18.4 | 11.0 to 25.8 | 156 | 15.1 | 7.8 to 22.4 | .27 |
| Other CNS tumors | 63 | 71.2 | 59.8 to 82.6 | 81 | 51.5 | 38.2 to 64.8 | .02 |
| Neuroblastoma | 109 | 59.0 | 49.6 to 68.4 | 86 | 68.0 | 56.6 to 79.4 | .30 |
| Nephroblastoma | 130 | 92.2 | 87.7 to 96.7 | 65 | 95.3 | 89.2 to 100.0 | .38 |
| Germ cell tumors | 51 | 91.7 | 83.7 to 99.7 | 30 | 88.8 | 74.7 to 100.0 | .72 |
| Osteosarcoma | 94 | 63.3 | 53.5 to 73.1 | 63 | 73.9 | 61.2 to 86.6 | .11 |
| Rhabdomyosarcoma | 80 | 65.0 | 54.6 to 75.4 | 55 | 74.3 | 61.2 to 87.4 | .26 |
| NRSTS | 46 | 63.0 | 49.3 to 76.7 | 20 | 60.0 | 34.5 to 85.5 | .95 |
| Ewing sarcoma | 33 | 75.0 | 59.9 to 90.1 | 45 | 81.5 | 68.0 to 95.0 | .36 |
| Retinoblastoma | 42 | 92.9 | 85.3 to 100.0 | 88 | 100.0 | .01 | |
| Melanoma | 24 | 50.0 | 30.8 to 69.2 | 24 | 95.8 | 87.6 to 100.0 | < .01 |
| Hepatoblastoma | 19 | 78.9 | 61.1 to 96.7 | 6 | 50.0 | 1.0 to 99.0 | .21 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin's lymphoma; NRSTS, nonrhabdomyosarcoma soft tissue sarcoma; OS, overall survival.
Footnotes
Supported in part by Grants No. CA21765, CA36401, and U01 GM92666 from the National Cancer Institute and by the American Lebanese and Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Cheng Cheng, Sigma Tau Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ching-Hon Pui, Deqing Pei, Cheng Cheng
Financial support: Ching-Hon Pui, William E. Evans
Administrative support: Ching-Hon Pui, James R. Downing, William E. Evans
Provision of study materials or patients: Ching-Hon Pui, Alberto S. Pappo, Scott C. Howard, John T. Sandlund, Wayne L. Furman, Raul C. Ribeiro, Sheri L. Spunt, Jeffrey E. Rubnitz, Sima Jeha, Melissa M. Hudson, Larry E. Kun, Thomas E. Merchant, Alberto Broniscer, Monika L. Metzger, Wing Leung, Amar Gajjar
Collection and assembly of data: Ching-Hon Pui, Deqing Pei,Amar Gajjar
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD: based on November 2010 SEER data submission, posted to the SEER web site; 2011. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Chornokur G, Dalton K, Borysova ME, et al. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71:985–997. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prout GR, Jr, Wesley MN, McCarron PG, et al. Survival experience of black patients and white patients with bladder carcinoma. Cancer. 2004;100:621–630. doi: 10.1002/cncr.11942. [DOI] [PubMed] [Google Scholar]
- 5.Berndt SI, Carter HB, Schoenberg MP, et al. Disparities in treatment and outcome for renal cell cancer among older black and white patients. J Clin Oncol. 2007;25:3589–3595. doi: 10.1200/JCO.2006.10.0156. [DOI] [PubMed] [Google Scholar]
- 6.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003;21:4200–4206. doi: 10.1200/JCO.2003.01.218. [DOI] [PubMed] [Google Scholar]
- 7.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98:603–609. doi: 10.1002/cncr.11534. [DOI] [PubMed] [Google Scholar]
- 8.Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: A population-based study. Blood. 2010;116:5501–5506. doi: 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekeres MA, Peterson B, Dodge RK, et al. Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood. 2004;103:4036–4042. doi: 10.1182/blood-2003-09-3118. [DOI] [PubMed] [Google Scholar]
- 10.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975-1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadan-Lottick NS, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KA, Aplenc R, Bagatell R. Survival by race among children with extracranial solid tumors in the United States between 1985 and 2005. Pediatr Blood Cancer. 2011;56:425–431. doi: 10.1002/pbc.22825. [DOI] [PubMed] [Google Scholar]
- 13.Children's Oncology Group. Aplenc R, Alonzo TA, et al. Ethnicity and survival in childhood acute myeloid leukemia: A report from the Children's Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: A Children's Oncology Group study. J Clin Oncol. 2011;29:76–82. doi: 10.1200/JCO.2010.29.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pui CH, Boyett JM, Hancock ML, et al. Outcome of treatment for childhood cancer in black as compared with white children: The St. Jude Children's Research Hospital experience, 1962 through 1992. JAMA. 1995;273:633–637. [PubMed] [Google Scholar]
- 16.Rubnitz JE, Lensing S, Razzouk BI, et al. Effect of race on outcome of white and black children with acute myeloid leukemia: The St. Jude experience. Pediatr Blood Cancer. 2007;48:10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- 17.Metzger ML, Castellino SM, Hudson MM, et al. Effect of race on the outcome of pediatric patients with Hodgkin's lymphoma. J Clin Oncol. 2008;26:1282–1288. doi: 10.1200/JCO.2007.14.0699. [DOI] [PubMed] [Google Scholar]
- 18.Pui CH, Sandlund JT, Pei D, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290:2001–2007. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 19.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary M, Krailo M, Anderson JR, et al. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin Oncol. 2008;35:484–493. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pui CH, Gajjar AJ, Kane JR, et al. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. 2011;8:540–549. doi: 10.1038/nrclinonc.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology: ICD-O (ed 3) Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 23.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 25.Scheurer ME, Bondy ML, Gurney JG. Epidemiology of childhood cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. pp. 2–16. [Google Scholar]
- 26.Louis DN, Ohgaki H, Wiestler OD, et al., editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilles FH, Tavaré CJ, Becker LE, et al. Pathologist interobserver variability of histologic features in childhood brain tumors: Results from the CCG-945 study. Pediatr Dev Pathol. 2008;11:108–117. doi: 10.2350/07-06-0303.1. [DOI] [PubMed] [Google Scholar]
- 28.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children's Oncology Group. Neuro Oncol. 2011;13:410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children's Oncology Group. Neuro Oncol. 2011;13:317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus KC, Grier HE, Shamberger RC, et al. Childhood soft tissue sarcoma: A 20-year experience. J Pediatr. 1997;131:603–607. doi: 10.1016/s0022-3476(97)70070-2. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari A, Casanova M, Collini P, et al. Adult-type soft tissue sarcomas in pediatric-age patients: Experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23:4021–4030. doi: 10.1200/JCO.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 32.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43:237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung W, Campana D, Yang J, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118:223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children's Oncology Group phase 3 trial for untreated pediatric acute myeloid leukemia: A report from the Children's Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: A report from the Children's Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 38.Yu AL, Gilman AL, Ozkaynak F, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corrigan JJ, Feig SA. American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics. 2004;113:1833–1835. doi: 10.1542/peds.113.6.1833. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Krailo M, Reaman GH, et al. Childhood cancer patients' access to cooperative group cancer programs: A population-based study. Cancer. 2003;97:1339–1345. doi: 10.1002/cncr.11192. [DOI] [PubMed] [Google Scholar]